Back to Journals » Drug Design, Development and Therapy » Volume 12
GnRH agonist improves pregnancy outcome in mice with induced adenomyosis by restoring endometrial receptivity
Authors Guo S, Li Z, Yan L, Sun Y, Feng Y
Received 14 January 2018
Accepted for publication 6 April 2018
Published 7 June 2018 Volume 2018:12 Pages 1621—1631
DOI https://doi.org/10.2147/DDDT.S162541
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos

Song Guo,1 Zhichao Li,2 Li Yan,1 Yijuan Sun,2 Yun Feng3
1Department of Gynaecology and Obstetrics, Qianfoshan Hospital, Shandong University, Jinan, China; 2Gynecology, Shanghai Ji Ai Genetics & In Vitro Fertilization Institute, Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China; 3Reproductive Medicine Center, Department of Obstetrics and Gynecology, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
Purpose: Adenomyosis has a negative impact on female fertility. GnRH agonist treatment can improve pregnancy outcomes in women with adenomyosis. However, the impact of GnRH agonist upon endometrium receptivity of patients with adenomyosis remains unclear. In this study, endometrial receptivity and pregnancy outcome were investigated using a mouse model of adenomyosis.
Materials and methods: Adenomyosis was induced in 12 female ICR mice, neonatally treated with tamoxifen, while another six female mice (control group) received solvent only. At 75 days, the induced adenomyosis group was randomly divided into two groups: an untreated group and a group treated with GnRH agonist (n = 6 each). Sixty days later, the mice were mated and pregnancy outcomes were observed and compared among the three groups (n = 6 each). In a parallel experiment using the same treatment regimes, uterus samples were collected on day 4 of pregnancy for immunohistochemistry, gene (quantitative polymerase chain reaction) and protein expression (Western blot), and scanning electron microscopy analyses.
Results: We found that the average live litter size was reduced in the adenomyosis compared with control group (8 ± 0.56 versus 13 ± 0.71; P = 0.03). However, the litter size was significantly increased in the treated with GnRH agonist group compared with the untreated group (12 ± 0.35 versus 8 ± 0.56; P = 0.04). The uterine expression levels of Hoxa10, Hoxa11, Lif and integrin b3 mRNA and protein were decreased in the adenomyosis group, and were significantly increased after GnRH agonist treatment. Additionally, pinopodes were reduced in number and poorly developed in mice with induced adenomyosis. However, pinopodes were abundant and well-developed in the GnRH agonist treatment group.
Conclusion: Adenomyosis may have an adverse impact on endometrial receptivity and reduce pregnancy outcomes in mice. However, GnRH agonist may improve the pregnancy outcome by partially restoring endometrial receptivity.
Keywords: adenomyosis, GnRH agonist, mice, endometrial receptivity, pregnancy outcome
Introduction
Adenomyosis is a benign disorder characterized by ectopic endometrial glands and stroma within the myometrium. It frequently occurs in women of childbearing age, and is the main cause of infertility and abortion.1,2 Studies of postoperative pathology suggested that adenomyosis occurs in 20% to 30% of hysterectomy cases.3,4 In assisted reproductive technology (ART), adenomyosis patients had lower implantation, clinical pregnancy and ongoing pregnancy rates with an increased miscarriage rate compared with non-adenomyosis patients undergoing in vitro fertilization (IVF).5 As adenomyosis can impair female fertility, many methods have been used for treatment of adenomyosis. Gonadotropin-releasing hormone (GnRH) agonist is widely used as an effective and non-invasive treatment for uterine adenomyosis.6,7
GnRH is a hypothalamic secretory decapeptide, which is secreted by nerve terminals at the median eminence and binds to the GnRH receptor in pituitary gonadotropes. GnRH stimulation of gonadotropes is required for the biosynthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).8 GnRH agonists are synthetically modeled after the natural GnRH decapeptide with chemical modifications of the sixth and tenth amino acids to improve efficacy.9 They play an important role in the endocrine system by combining with their receptors. GnRH agonists cause a “flare-up effect” during the first week after administration. As their receptors are occupied and depleted, GnRH agonists ultimately lead to loss of FSH and LH secretion, which is called “down-regulation effect”.10 However, the mechanisms of GnRH agonist actions remain unknown.
The window of implantation is defined as the period of optimal synchronization between the embryo and endometrium, and is a receptive state allowing implantation of a blastocyst into the uterus.11 In clinical studies using ART, patients with adenomyosis undergo embryo transfer after GnRH agonist treatment. It is difficult to obtain endometrial samples from patients during the implantation window because of ethical reasons, and mouse models of adenomyosis provide a valuable research platform. In women with regular 28-day cycles, the window of implantation corresponds to menstrual days 21–24.12 However, in mice, the implantation window extends from the morning of day 4 (day of plug = day 1) to the end of day 5 of pregnancy.13
To date, the relationship between GnRH agonist treatment and pregnancy outcome in mice with adenomyosis remains unreported. Endometrial receptivity is a key factor for embryo implantation, and we suspect that GnRH agonist may play a role regulating endometrium receptivity. Assessment of implantation markers, such as homeobox A10 (Hoxa10), homeobox A11 (Hoxa11), integrin b3, leukemia inhibitory factor (Lif) and pinopodes can provide an estimation of endometrium receptivity. They are mainly expressed in endometrium and closely related to the endometrial receptivity. So, they were most widely used to assess endometrial receptivity.14–16
Homeobox genes play an important physiological role in endometrial growth, differentiation, implantation and decidualization through regulating stromal cell responsiveness to progesterone.17 Hoxa10 and Hoxa11 are widely regarded as necessary for the establishment of pregnancy. Hoxa10 is expressed mainly in the developing uterus, and Hoxa11 is expressed in the developing uterus and cervix.18 Lif is another key marker of endometrial receptivity.19 It is a key factor of blastocyst implantation, and its expression increases in the secretory phase.20 Lif was shown to be important for embryo implantation in mice.21 Pinopodes are an important morphological marker of endometrial receptivity. The appearance of pinopodes on the surface of endometrium takes place during the implantation window. Abundant and developed pinopodes correlate with implantation success, whereas many patients with multiple implantation failures fail to produce pinopodes.22 The integrin b3 is expressed on the luminal and glandular epithelium of the endometrium, which facilitates embryo attachment to the surface of the endometrium and early development.17 The integrin b3 plays an important role in reproduction, and is currently being evaluated as a target for unexplained infertility.22
To the best of our knowledge, this is the first study to investigate whether GnRH agonists improve pregnancy outcome through altering uterine receptivity in the adenomyosis mouse model.
Materials and methods
Pregnancy outcome
Ten pregnant Institute of Cancer Research (ICR) mice were used in this study (Shanghai Laboratory Animal Corporation, Shanghai, China). Female pups were then selected for use. We established models of adenomyosis by administering tamoxifen to neonatal mice (2.7 μmol/kg tamoxifen orally in the neonatal period). Prior studies have suggested that this method has the advantage of a high success rate in adenomyosis model.23,24 Twelve neonatal mice were treated orally with 2.7 μmol/kg tamoxifen (Shanghai Fudan Forward Science & Technology Co., Ltd, Shanghai, China) suspended in a peanut oil/lecithin/condensed milk mixture (2.0:0.2:3.0, by volume) once daily on days 2 to 5 after birth (day of birth = day 1). Six female neonatal control mice received vehicle only. All mice were housed in an animal care facility under the same feeding conditions (20°C, 12:12 light–dark cycle with lights on at 06:00). After 75 days, tamoxifen mice were randomly allocated to two groups, with six mice in each group. One group received a single intraperitoneal dose of 8 mg GnRH agonist (Ferring GmbH, Kiel, Germany). The second group received the same amount of solvent, without GnRH agonist. After 28 days, each female was mated by one male mouse from 19:00 to 07:00 and then checked for the presence of a vaginal plug at 08:00 the next day. The female mice remained with the male for 6 days. The day of the vaginal plug was designated gestation day 1. Pregnant mice were individually caged then average litter size was recorded 30 days after mating. The parturition rate was defined as the total number of litters divided by the total number of matings.
Uterine receptivity
Another three groups of mice received the same treatments described above and were used to study uterine receptivity. Mice from the three groups were euthanized at 19:00–20:00 on day 4 of pregnancy. Uterine and endometrial tissue was divided into three sections: one was fixed in 4% formalin and embedded in a paraffin block and cut into 4 mm sections for H&E pathological analysis and immunohistochemical analysis, another was fixed immediately in 2.5% glutaraldehyde solution for electron microscopy, and the remaining tissues were immediately snap frozen and stored at −80°C for further use.
Immunohistochemistry
Fixed uterus samples were cut into 4 μm-thick slices. For immunohistochemistry, dewaxed and rehydrated sections were incubated with 3% H2O2 for 30 min to block endogenous peroxidase activity. Sections were rinsed in PBS, blocked with 10% normal goat serum (Fuzhou Maixin Biotechnology, Fuzhou, China) for 30 min and then incubated with goat anti-mouse Hoxa10 polyclonal antibody (Abcam, Cambridge, UK; 1:100 dilution), rabbit anti-mouse Hoxa11 polyclonal antibody (Abcam; 1:200 dilution), rat anti-mouse Lif monoclonal antibody (Abcam; 1:100 dilution) and rabbit anti-mouse integrin-b3 polyclonal antibody (Abcam; 1:100 dilution) overnight at 4°C. After two rinses in tris-buffered saline, the slides were incubated with secondary biotinylated rabbit anti-goat secondary antibody (Proteintech Group, Inc., Rosemont, IL, USA), goat anti-rabbit secondary antibody (Proteintech Group, Inc.) and goat anti-rat secondary antibody (Proteintech Group, Inc.) for 1 h at room temperature. Positive controls were tissue sections containing the relevant antigens. Negative controls were performed by incubation with PBS instead of primary antibody. Reactivity in the endometrial glands and luminal surface epithelium of the uterine was evaluated (average positive stained area percentage) by two independent investigators blinded to each other.
Scanning electron microscopy
Fixed samples were washed with PBS and then post-fixed in 1% osmium tetroxide for 2 h at 4°C. Samples were then dehydrated in graded alcohols, dried in a critical point drier (HITACHI HCP-2; Hitachi High-Technologies Corp., Tokyo, Japan), coated with palladium gold and examined using a digital scanning electron microscope (Quanta-200JSM; Philips, Amsterdam, Netherlands). Pinopode formation was evaluated by two independent investigators blinded to each other. The semi-quantitative evaluation system was as follows: 0 (0%), 1 (<25%), 2 (25%–50%) and 3 (>50%).
Real-time polymerase chain reaction (RT-PCR)
RNA was prepared using Trizol reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to instructions. For each sample, 1 μg of RNA was converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). The quantitative RT-PCR was performed using SYBR-green PCR Master Mix in a Fast Real-time PCR 7500 System (Thermo Fisher Scientific Inc.). The primers used in this study were designed by Sangon Biotech Co., Ltd (Shanghai, China). Relative gene expression was analyzed according to the 2−ΔΔCt method. All samples were assayed in triplicate reactions. The β-actin gene was used as a housekeeping gene to normalize the expression level. Sequences of the forward (F) and reverse (R) primers for RT-PCR were as follows: Hoxa10 (NM_001122950.2), F-5′-GCCCCTTCAGAAAACAGTAAAG-3′, R-5′-AGGTGGACGCTACGGCTGATCTCTA-3′; Hoxa11(NM_010450.3), F-5′-TCCAGCCTCCCTTCTTTTTTG-3′, R-5′-GTAGCAGTGGGCCAGATTGC-3′; Lif(NM_001039537.2), F-5′-GATGGTCGCATACCTGAGC-3′, R-5′-ACAGACGGCAAAGCACATT-3′; Integrin b3(NM_016780.2), F-5′-GGCGTTGTTGTTGGAGAGTC-3′, R-5′-GCCTCACTGACTGGGAACTC-3′.
Western blotting
Protein extracts from the endometrium of mice were prepared using RIPA lysis buffer (Beyotime Biotechnology, Jiangsu, China) with a protease inhibitor cocktail (EMD Millipore, Billerica, MA, USA) on ice for 15 min. Protein concentrations were calculated using a BCA protein assay kit (Thermo Fisher Scientific Inc.) according to the manufacturer’s instructions. Equal amounts of protein were loaded and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes (EMD Millipore). The membrane was blocked for 1 h using 5% non-fat milk in tris-buffered saline with 0.05% Tween-20 detergent on a shaker for 2 h and then incubated with primary antibody against Hoxa10 (ab191470), Hoxa11 (ab54365), Lif (ab138002) and integrin b3 (ab38460) (1 mg/mL, 1:1,000 dilution; Abcam) and rabbit anti-mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH; AP0063; Bioworld Technology Inc., Minneapolis, MN, USA) as an internal control overnight at 4°C. After washing three times in PBS with Tween 20, the membrane was blotted with the appropriate conjugated secondary antibody for 1 h at room temperature and washed three times in PBS with Tween 20. The protein bands were visualized using the ECL kit (Thermo Fisher Scientific Inc.). The band intensity was quantified using Adobe Photoshop 5.0.
All experiments were performed under the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals25 and approved by the institutional experimental animals review board of Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine.
Statistical analysis
Statistical differences were analyzed using statistical package for the Social Sciences software 17.0 (SPSS Inc., Chicago, IL, USA). The distribution of data was tested using Kolmogorov–Smirnov test. Normally distributed data were compared using one-way analysis of variance (ANOVA). If the data were statistically significant among the three groups, post-hoc analysis was used for comparison between two groups. Unusually distributed data were compared using the Kruskal–Wallis H test. The χ2 test was used to analyze the scores of pinopodes. Results are represented as the mean ± SD. P < 0.05 was considered statistically significant.
Results
Modeling results
The feasibility of modeling method is validated by pathological examination (Figure 1A and B). Among the 21 mice, three died in the experimental progress and one was unsuccessful in the model establishment, suggesting that the treatment of neonatal mice with tamoxifen is an effective method of modeling adenomyosis.
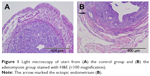  | Figure 1 Light microscopy of uteri from (A) the control group and (B) the adenomyosis group stained with H&E (×100 magnification). |
Pregnancy outcome
Using one-way ANOVA, we found that the average live litter size was statistically significant among the three groups (F = 6.37, P = 0.02); post-hoc analysis was used for comparison between two groups. We found that the average live litter size was significantly reduced in the adenomyosis group than the control group (8 ± 0.56 versus 13 ± 0.71; P = 0.03). However, average litter size was increased in the GnRH agonist treatment group (12 ± 0.35 versus 8 ± 0.56; P = 0.04) as shown in Table 1. There was no significant difference in parturition rates among the three groups (83.33% versus 50% versus 66.67%, P = 0.73).
Uterine receptivity
Immunocytochemistry
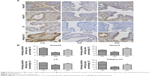
As shown in Figure 2A, Hoxa10 and Hoxa11 immunostaining was detected in the nucleus of glandular epithelial cells and endometrial luminal epithelial cells. Additionally, Lif and Integrin b3 were mainly expressed in the cytoplasm of glandular epithelial cells and endometrial luminal epithelial cells. Compared with the control group, the expression levels of Hoxa10, Hoxa11, Lif and integrin b3 were significantly lower in the adenomyosis group (9.81 ± 0.23 versus 23.34 ± 0.62, P = 0.02; 9.51 ± 0.24 versus 14.67 ± 0.05, P = 0.03; 4.13 ± 0.55 versus 5.97 ± 0.04, P = 0.04; 11.37 ± 0.31 versus 32.11 ± 0.34, P = 0.01). However, their expression levels were significantly increased after GnRH agonist treatment (18.81 ± 0.57 versus 9.81 ± 0.23, P = 0.03; 17.89 ± 0.17 versus 9.51 ± 0.24, P = 0.02; 5.63 ± 0.12 versus 4.13 ± 0.53, P = 0.04; 21.34 ± 0.45 versus 11.37 ± 0.31, P = 0.04; Figure 2B).
Hoxa10, Hoxa11, Lif and integrin b3 mRNA and protein expression during the implantation window
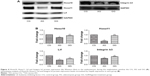
When compared with the control group, the expression levels of Hoxa10, Hoxa11, Lif and integrin b3 mRNA were significantly decreased in mice with adenomyosis (0.25 ± 0.01 versus 1.00 ± 0.00, P = 0.03; 0.21 ± 0.02 versus 1.00 ± 0, P = 0.02; 0.11 ± 0.01 versus 1.00 ± 0, P = 0.001; 0.45 ± 0.02 versus 1.00 ± 0, P = 0.03; Figure 3). However, after GnRH agonist treatment, endometrial expression levels of Hoxa10, Hoxa11, Lif and integrin b3 mRNA were significantly upregulated in mice with adenomyosis (0.47 ± 0.01 versus 0.25 ± 0.01, P = 0.02; 0.47 ± 0.03 versus 0.21 ± 0.02, P = 0.02; 0.64 ± 0.03 versus 0.11 ± 0.01, P = 0.01; 0.68 ± 0.01 versus 0.45 ± 0.02, P = 0.04; Figure 3).
We further examined the protein expression levels of Hoxa10, Hoxa11, Lif and integrin b3 in the three groups. The results were similar to the expression levels of mRNA (0.67 ± 0.02 versus 1.00 ± 0, P = 0.04; 0.27 ± 0.02 versus 1.00 ± 0, P = 0.02; 0.53 ± 0.01 versus 1.00 ± 0, P = 0.03; 0.75 ± 0.01 versus 1.00 ± 0, P = 0.03). As shown in Figure 4A and B, protein expression levels of Hoxa10, Hoxa11, Lif and integrin b3 were significantly higher in the GnRH agonist treatment group compared with the adenomyosis group (0.89 ± 0.01 versus 0.67 ± 0.02, P = 0.03; 0.97 ± 0.01 versus 0.27 ± 0.02, P = 0.01; 0.68 ± 0.02 versus 0.53 ± 0.01, P = 0.04; 0.83 ± 0.02 versus 0.75 ± 0.01, P = 0.03).
Expression of pinopodes during the implantation window
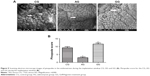
Scanning electron micrographs of pinopodes were compared in tissue samples (Figure 5A). We observed many well-formed pinopodes in the control group. However, only a few small and scattered pinopodes were found distributed over the endometrial surface in the adenomyosis group. There were significant differences in the proportion of pinopodes exhibiting different morphologies and pinopode coverage between the two groups (Figure 5B, P = 0.001). Pinopode morphology and coverage were significantly improved after GnRH agonist treatment (Figure 5B, P = 0.003).
Discussion
Adenomyosis is an important factor leading to infertility and abortion, and has a detrimental effect on IVF clinical outcomes.26 Its pathogenic mechanism includes disrupting normal uterine contraction and disturbing embryo implantation with aggregation of macrophages, cytokines and immunological factors within the superficial endometrial glands,5 damaging fertilized eggs and inhibiting embryo development via abnormal concentrations of intrauterine free radicals.27 Other possible biological mechanisms for this effect include alterations of adhesion molecules, cell proliferation and apoptosis.28
Because of the negative effects on female fertility and its high incidence in women of childbearing age, the treatment of adenomyosis has been widely studied in the reproductive medicine field. The non-invasive and reliable GnRH agonist treatment of adenomyosis is widely used.29,30 GnRH agonist provides an advantage in the treatment of diffuse adenomyosis, which unlike focal adenomyosis cannot be easily excised.26 At present, it is generally thought that GnRH agonist administration suppresses ovulation and the production of estrogen, and then causes atrophy of the ectopic endometrium. Other research showed that GnRH agonist therapy significantly reduced the inflammatory reaction and angiogenic response and induced considerable apoptosis in different tissues of patients with adenomyosis.31,32 Additionally, our previous study revealed that reduced levels of autophagy may be one pathogenic factor for adenomyosis, and GnRH agonist treatment may improve the impaired autophagy level and pregnancy outcome.33 However, opposing evidence in one retrospective study suggested that GnRH agonist treatment did not improve the pregnancy outcome of patients with adenomyosis or endometriosis, and the researchers argued against its usefulness in the treatment of adenomyosis-induced infertility.34 As the effects of GnRH agonist on fertility remain debatable, we studied whether GnRH agonist could improve the pregnancy outcome in a mouse model of adenomyosis and its effects on endometrial receptivity.
Litter size is one important parameter in the assessment of female reproduction in mice. In the current study, we demonstrated that the average litter size was reduced in the adenomyosis compared with the control group. Furthermore, we observed a significant increase in the average litter size in the adenomyosis group undergoing GnRH agonist therapy, suggesting that GnRH agonist improved the pregnancy outcome in mice. These results are also consistent with previous experimental studies in humans.35
The implantation window is a crucial period when the uterus is receptive for the implantation of blastocysts, so it is important to understand the mechanisms of GnRH agonist actions in this period. In humans, blastocyst implantation occurs about 9 days after ovulation, ranging between 6 and 12 days.36 In mice, blastocyst implantation into the uterus occurs on the evening of day 4 post-coitum.11 We chose this time point to study the expression of endometrial receptivity markers, which reflect the functional characteristics of endometrial receptivity.
Our current research revealed a significant decrease in Hoxa10, Hoxa11, Integrin b3 and Lif mRNA and protein expression levels in the adenomyosis compared with the control group. In addition, we observed a significant increase in Hoxa10, Hoxa11, Integrin b3 and Lif mRNA and protein expression levels after GnRH agonist therapy. This observation is in agreement with the results shown in the previous mouse studies. They found that compared with the pregnant mare serum gonadotropin (PMSG) alone protocol, PMSG + GnRH agonist co-treatment can elevate the expression levels of integrin b3 and Lif and improve the implantation rate, suggesting that PMSG + GnRH agonist co-treatment may potentially improve uterine receptivity through the restoration of physiologically endometrial secretion in the ovarian stimulation cycle.37
Hoxa-10 and Hoxa-11 are the members of the homeobox gene family. They play an important role in murine uterine receptivity for implantation.14,38 In the secretory phase of the implantation period, endometrial Hoxa-10 and Hoxa-11 mRNA expression levels are upregulated and improve endometrial receptivity. Mutations of these two genes in mice can lead to female infertility.14,18 Lif, an IL-6-family cytokine, influences endometrial receptivity during the early implantation window by regulating the Lif receptor on both the embryo and endometrium, and affects trophoblast function and vascular formation in the placenta.22,39 Moreover, the knockout of Lif in mice leads to infertility.40 Other findings showed that Lif may maintain the proper development of the endometrium and implantation receptivity by regulating downstream target genes. One study suggested that uterine receptivity and implantation was regulated by the Lif-Lifr/gp130-JAK/STAT3 signaling pathway.41
Disruptive changes during the implantation period in mice may arise from changes in the distribution or level of expression of genes regulating endometrium receptivity, including Hoxa10, Hoxa11 and Lif.14 However, it is unknown if the present finding of increased endometrial Hoxa10, Hoxa11, Lif and integrin b3 mRNA expression after GnRH agonist treatment was associated with a pituitary downregulation effect or a consequence of other factors associated with tamoxifen-induced adenomyosis. Xiao-xia et al found that GnRH agonist may increase the apoptotic ratio of cultured adenomyosis endometrial cells. In addition, they also found that GnRH agonist can directly suppress the survival and growth of ectopic endometrial cell by suppressing the secretion of vascular endothelial growth factor (VEGF).42 In work by Lee et al, altered endometrial gene expression was reported to be associated with altered epigenetic programming in women with endometriosis.43 In another endometriosis study, methylation patterns of the Hoxa10 gene were analyzed in baboons with induced endometriosis. In this research, the promoter F1 region was found to be more significantly methylated in animals with endometriosis, and was proposed to be related to decreased Hoxa10 expression.44 Therefore, changes in the expression of Hoxa10 and Hoxa11 after GnRH agonist therapy maybe correlated with changes to repressive histone and DNA methylation in the F1 promoter regions.
We also investigated a key morphological marker (pinopodes) during the implantation window to determine more accurately the endometrial receptivity. Our study showed that pinopodes were reduced in number and poorly developed in the adenomyosis compared with the control group during the implantation window. Moreover, pinopodes were abundant and well-developed in the GnRH agonist therapy group. This morphological finding indicated that GnRH agonist therapy may improve endometrial receptivity. Pinopodes may influence the concentration of endometrial fluids near the implantation site, thus facilitating the process of adhesion and invasion. Pinopodes may also elevate the implantation surface toward the embryo45 or release implantation-facilitating or promoting molecules.46 Quinn et al suggested that blastocysts attach to pinopodes during the initial processes of implantation in vitro.47 So a large number of fully developed pinopodes favor embryo implantation and early development. It is consistent with the results obtained from our study. GnRH agonist could also improve the pregnancy outcome in adenomyosis by increasing the number of fully developed pinopodes in vivo. In addition, it has been illustrated that there is a positive correlation between Hoxa10 and pinopodes. The upregulation of Hoxa10 can increase the number of pinopodes.48 This suggested that they are a cause-and-effect relationship rather than a paralleled relationship. In addition to all of the above mentioned factors, a considerable amount of research has shown that pinopode formation is progesterone dependent.47 Progesterone level rises after ovulation, so the number of pinopodes increase during luteal phase. Based on this view, we thought that luteum insufficiency could hinder pinopode development causing spontaneous abortion, which could be the pathophysiologic mechanism of some spontaneous abortion patients.
The main limitations of this study were the small number of animals studied and the lack of confirmation of these results using human samples. Although humans and mice are both mammals, the adenomyosis mouse model will not entirely reflect the natural course of the human disease. Therefore, future experiments should examine the current findings in patients with adenomyosis. In addition, the downstream signaling pathways of these implantation period genes should be further determined, such as the JAK/STAT3 signaling pathway.
Conclusion
In summary, we propose that GnRH agonist treatment may improve the pregnancy outcome in mice by improving uterine receptivity. These results provide insights into the potential molecular mechanisms of GnRH agonist actions in adenomyosis therapy. Embryo implantation is a complicated process likely to involve many molecular mechanisms working together, which will require consideration for further study into the actions of GnRH agonist therapy for adenomyosis.
Acknowledgments
This work was supported by the Shanghai Municipal Health and Family Planning Commission Fund (Grant number: 201640367) and the Key Technology Research and Development Programme of Shandong Province (2017G006022).
The authors thank Charles Allan, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
Song Guo: data collection, manuscript writing. Zhichao Li: data analysis. Li Yan, Yijuan Sun, Yun Feng: protocol/project development. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Graziano A, Lo Monte G, Piva I, Caserta D, Karner M, Engl B, Marci R. Diagnostic findings in adenomyosis: a pictorial review on the major concerns. Eur Rev Med Pharmacol Sci. 2015;19(7):1146–1154. | ||
Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98(3):572–579. | ||
Parazzini F, Vercellini P, Panazza S, Chatenoud L, Oldani S, Crosignani PG. Risk factors for adenomyosis. Hum Reprod. 1997;12(6):1275–1279. | ||
Vercellini P, Parazzini F, Oldani S, Panazza S, Bramante T, Crosignani PG. Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod. 1995;10(5):1160–1162. | ||
Salim R, Riris S, Saab W, Abramov B, Khadum I, Serhal P. Adenomyosis reduces pregnancy rates in infertile women undergoing IVF. Reprod Biomed Online. 2012;25(3):273–277. | ||
Mijatovic V, Florijn E, Halim N, Schats R, Hompes P. Adenomyosis has no adverse effects on IVF/ICSI outcomes in women with endometriosis treated with long-term pituitary down-regulation before IVF/ICSI. Eur J Obstet Gynecol Reprod Biol. 2010;151(1):62–65. | ||
Streuli I, Dubuisson J, Santulli P, de Ziegler D, Batteux F, Chapron C. An update on the pharmacological management of adenomyosis. Expert Opin Pharmacother. 2014;15(16):2347–2360. | ||
Stojilkovic SS, Bjelobaba I, Zemkova H. Ion channels of pituitary gonadotrophs and their roles in signaling and secretion. Front Endocrinol (Lausanne). 2017;8:126. | ||
Li X, Kang X, Deng Q, Cai J, Wang Z. Combination of a GnRH agonist with an antagonist prevents flare-up effects and protects primordial ovarian follicles in the rat ovary from cisplatin-induced toxicity: a controlled experimental animal study. Reprod Biol Endocrinol. 2013;11:16. | ||
Hodgen GD. GnRH analogs in reproductive medicine. Keio J Med. 1991;40(1):25–32. | ||
Chu B, Zhong L, Dou S, et al. miRNA-181 regulates embryo implantation in mice through targeting leukemia inhibitory factor. J Mol Cell Biol. 2015;7(1):12–22. | ||
Navot D, Scott RT, Droesch K, Veeck LL, Liu HC, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril. 1991;55(1):114–118. | ||
Yoshinaga K. A sequence of events in the uterus prior to implantation in the mouse. J Assist Reprod Genet. 2013;30(8):1017–1022. | ||
Xu B, Geerts D, Bu Z, et al. Regulation of endometrial receptivity by the highly expressed HOXA9, HOXA11 and HOXD10 HOX-class homeobox genes. Hum Reprod. 2014;29(4):781–790. | ||
Bourdiec A, Ahmad SF, Lachhab A, Akoum A. Regulation of inflammatory and angiogenesis mediators in a functional model of decidualized endometrial stromal cells. Reprod Biomed Online. 2016;32(1):85–95. | ||
Makker A, Goel MM, Nigam D, Bhatia V, Mahdi AA, Das V, Pandev A. Endometrial expression of homeobox genes and cell adhesion molecules in infertile women with intramural fibroids during window of implantation. Reprod Sci. 2017;24(3):435–444. | ||
Celik O, Unlu C, Otlu B, Celik N, Caliskan E. Laparoscopic endometrioma resection increases peri-implantation endometrial HOXA-10 and HOXA-11 mRNA expression. Fertil Steril. 2015;104(2):356–365. | ||
Chau YM, Pando S, Taylor HS. HOXA11 silencing and endogenous HOXA11 antisense ribonucleic acid in the uterine endometrium. J Clin Endocrinol Metab. 2002;87(6):2674–2680. | ||
Senturk LM, Arici A. Leukemia inhibitory factor in human reproduction. Am J Reprod Immunol. 1998;39(2):144–151. | ||
Unlu C, Celik O, Celik N, Otlu B. Expression of endometrial receptivity genes increase after myomectomy of intramural leiomyomas not distorting the endometrial cavity. Reprod Sci. 2016;23(1):31–41. | ||
Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. 1992;359:76–79. | ||
Xu B, Sun X, Li L, Wu L, Zhang A, Feng Y. Pinopodes, leukemia inhibitory factor, integrin-b3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertil Steril. 2012;98(2):389–395. | ||
Green AR, Edwards RE, Greaves P, White IN. Comparison of the effect of oestradiol, tamoxifen and raloxifene on nerve growth factor-alpha expression in specific neonatal mouse uterine cell types using laser capture microdissection. J Mol Endocrinol. 2003;30(1):1–11. | ||
Koike N, Tsunemi T, Uekuri C, Akasaka J, Ito F, Shigemitsu A, Kobayashi H. Pathogenesis and malignant transformation of adenomyosis (review). Oncol Rep. 2013;29(3):861–867. | ||
National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press; 1996. | ||
Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil Steril. 2017;108(3):483.e3–490.e3. | ||
Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17(3):261–264. | ||
Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014;29(5):964–977. | ||
Lin J, Sun C, Zheng H. Gonadotropin-releasing hormone agonists and laparoscopy in the treatment of adenomyosis with infertility. Chin Med J (Engl). 2000;113(5):442–445. | ||
Silva PD, Perkins HE, Schauberger CW. Live birth after treatment of severe adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril. 1994;61(1):171–172. | ||
Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod. 2010;25(3):642–653. | ||
Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Ishimaru T, Masuzaki H. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod. 2010;25(11):2878–2890. | ||
Song Guo, Xiaowei Lu, Ruihuan Gu, Di Zhang, Yijuan Sun, Yun Feng. GnRH agonist enhances autophagy in a mouse model of adenomyosis. Int J Clin Exp Pathol. 2016;9(12):12559–12566. | ||
Sõritsa D, Saare M, Laisk-Podar T, et al. Pregnancy rate in endometriosis patients according to the severity of the disease after using a combined approach of laparoscopy, GnRH agonist treatment and in vitro fertilization. Gynecol Obstet Invest. 2015;79(1):34–39. | ||
Niu Z, Chen Q, Sun Y, Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol. 2013;29(12):1026–1030. | ||
Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–1799. | ||
Ruan HC, Zhu XM, Luo Q, et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum Reprod. 2006;21(10):2521–2529. | ||
Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374(6521):460–463. | ||
Marwood M, Visser K, Salamonsen L, Dimitriadis E. Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology. 2009;150(6):2915–2923. | ||
Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141(12):4365–4372. | ||
Cheng J, Rosario G, Cohen TV, Hu J, Stewart CL. Tissue-specific ablation of the LIF receptor in the murine uterine epithelium results in implantation failure. Endocrinology. 2017;158(6):1916–1928. | ||
Xiao-xia W, Jia-li K, Xue-fei S, Jia Y, Ling-hong D. Effect of GnRHa on apoptosis and release of VEGF in endometrial cell cultures from patients with adenomyosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012;28(1):72–73. | ||
Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80(1):79–85. | ||
Kim JJ, Taylor HS, Lu Z, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13(5):323–332. | ||
Aplin JD. Embryo implantation: the molecular mechanism remains elusive. Reprod Biomed Online. 2006;13(6):833–839. | ||
Kabir-Salmani M, Nikzad H, Shiokawa S, Akimoto Y, Iwashita M. Secretory role for human uterodomes (pinopods): secretion of LIF. Mol Hum Reprod. 2005;11(8):553–559. | ||
Quinn C, Ryan E, Claessens EA, et al. The presence of pinopodes in the human endometrium does not delineate the implantation window. Fertil Steril. 2007;87(5):1015–1021. | ||
Quinn CE, Casper RF. Pinopodes: a questionable role in endometrial receptivity. Hum Reprod Update. 2009;15(2):229–236. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.