Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Glycemic Control Among People Living with Diabetes and Human Immunodeficiency Virus in Ethiopia: Leveraging Clinical Care for the Looming Co-Epidemics
Authors Melaku T , Chelkeba L , Mekonnen Z , Kumela K
Received 22 August 2020
Accepted for publication 28 October 2020
Published 17 November 2020 Volume 2020:13 Pages 4379—4399
DOI https://doi.org/10.2147/DMSO.S266105
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Tsegaye Melaku,1 Legese Chelkeba,1 Zeleke Mekonnen,2 Kabaye Kumela1
1Department of Clinical Pharmacy, School of Pharmacy, Institute of Health, Jimma University, Jimma, Ethiopia; 2School of Medical Laboratory Sciences, Institute of Health, Jimma University, Jimma, Ethiopia
Correspondence: Tsegaye Melaku
Jimma University, Jimma, Ethiopia
Tel +251 913765609
Email [email protected]
Background: Antiretroviral therapy has decreased human immunodeficiency virus related mortality. However, the incidence of diabetes mellitus is increasing among people living with human immunodeficiency virus and adds complexity to the standards of care.
Objective: The study was aimed to determine the glycemic control and delivery of clinical care among people living with diabetes and human immunodeficincy virus in Ethiopia.
Methods: A comparative prospective cohort study was conducted among patients living with diabetes at follow-up clinics of Jimma Medical Center in two study arms. The first arm was people living with diabetes and human immunodeficiency virus. The second arm was human immunodeficiency virus negative patients living with diabetes. The expanded English version of the summary of diabetes self-care activities scale was used to measure self-care behaviors. In order to identify the predictors of glycemic control, multivariable Cox regression analysis was used. Statistical significance at p-value ≤ 0.05 was considered.
Results: A total of 297 eligible participants were followed for one year, with a mean age of 44.35± 12.55 years. Males accounted for 55.9%. After one year of follow-up, 61.9% of diabetes people living with human immunodeficiency virus, and 49% of human immunodeficiency virus-negative patients with diabetes poorly met blood glucose target (p=0.037). Female gender [AHR: 2.72; 95% CI (1.21– 5.72)], age > 31 years [AHR: 2.48; 95% CI (1.34– 11.01)], increased waist circumference [AHR: 3.64; 95% CI (2.57– 16.12)], overweight [AHR: 3.63; 95% CI (1.65– 22.42)], chronic disease comorbidity [AHR: 2.02; 95% CI (1.44– 2.84)], human immunodeficiency virus infection [AHR: 3.47; 95% CI (2.03– 23.75)], living longer with diabetes (> 5 years) [AHR: 3.67; 95% CI (3.26– 4.14)] showed a higher risk of blood sugar control failure and were independent predictors of uncontrolled glycemia. Tuberculosis infection increased the risk of uncontrolled blood sugar among people living with diabetes and human immunodeficency virus[AHR:3.82;95% CI(2.86-5.84].
Conclusion: Significant gaps were observed in achieving the recommended glycemic target and involvement of patients on self-care care behavior in the study area. The co-occurrence of tuberculosis, human immunodeficiency virus, and diabetes is triple trouble needing special attention in their management. It is high time to leverage the clinical care of the looming co-epidemics through chronic comprehensive care clinic.
Keywords: human immunodeficiency virus, glycemic control, treatment outcome, self-care behavior
Introduction
The unequivocal success of antiretroviral therapy (ART) in the management of human immunodeficiency virus (HIV) has been tempered by the recognition that metabolic disorders, such as diabetes mellitus (DM), are increasing in incidence among people living with HIV (PLHIV). Studies from developed countries have reported that the incidence of diabetes in HIV-infected adults receiving ART is between 1% and 10%1 and also in other studies from European, North American, and African cohorts showed the prevalence of diabetes in PLHIV ranges from 2% to 14%.2–4 In Ethiopia prevalence of DM and pre-diabetes (pre-DM) in HIV-infected patients reach up to 8.8% and19.6%, respectively.5–8 Traditional risk factors, such as overweight, age and gender, are significant determinants of diabetes.9 However, particular antiretrovirals (ARVs)10 and ARV-related weight gain and lipodystrophy11 are known risk factors. The major contributor to hyperglycemia is thought to be iatrogenic, with protease inhibitors being most commonly associated with insulin resistance. High pre-ART viral loads and low baseline CD4+ counts can also raise the risk of insulin resistance and accelerate diabetes pathogenesis.12
As the access to ARVs increases globally and survival improves, the main contributors to morbidity and mortality in HIV-infected individuals are DM and other cardiometabolic comorbidities.13,14 In contemporary health-care services, a substantial proportion of PLHIV dies not from opportunistic infections or other AIDS-defining illnesses, but from vascular events.15 In patients with coexisting diabetes mellitus, greater focus is important on the management of conventional risk factors for cardiovascular diseases. In order to improve clinical treatment and performance for chronic diseases, the World Health Organization (WHO) includes self-management as a best practice. Programs that inform and assist patients in managing their conditions have been effective in achieving better health outcomes.16,17 In order to update HIV care policies, these changes need to be considered.18
One cohort study evaluating glycemic regulation in HIV-infected patients and HIV-infected controls showed that HIV-infected patients achieved a substantially lower reduction in glycated hemoglobin (HbA1c) relative to the overall population.19 This suggests that diabetes PLHIV have more difficulty controlling hyperglycemia. The evaluation and treatment of diabetes are included in the WHO list of priority interventions in the HIV continuum of care in the health-care settings.20 The diagnosis criteria for diabetes in PLHIV are the same as the general population with one caveat. It has been reported that HbA1clevels underestimate glycemic levels by 10% to 15% in PLHIV.21–23 In the absence of treatment strategies specific to PLHIV, current international and national guidelines for the general population served as the guiding tool for the treatment of diabetes in PLHIV.24 Recommendations specific to PLHIV relate to drug interactions between ARVs and antidiabetics medication and the use of HbA1c for screening and diagnosis of diabetes. In some studies, people living with HIV have the same response to treatment as their HIV non-infected counterparts.25,26
In patients with diabetes, the significance of glycemic control in preventing both microvascular and macrovascular complications are well known. While several major studies have estimated the prevalence of poor glycemic regulation in the general population, relatively few studies have estimated this prevalence in HIV-infected patients.27–29 In order to ensure that patients are screened routinely for diabetes, HIV programs may need to adapt comprehensive patient evaluation. Routine screening for complications associated with diabetes should take place after a diagnosis of diabetes is made, and attempts should be made to improve glycemic control. How this is delivered depends on the number of variables in any particular healthcare system (eg, manpower, patient involvement into the care decision and the need for a multidisciplinary approach).
Furthermore, while age, number of years with diabetes, dyslipidemia, body mass index (BMI) and poor self-care have been related to inadequate glycemic control in the general diabetes population, no study in Ethiopia has investigated these associations in HIV-infected diabetes patients. In addition, HIV-infected patients with diabetes have particular possible co-factors for poor glycemic regulation, including the use of specific antiretroviral drugs. Therefore, the objective of this prospective cohort study was to determine the glycemic control, provision of diabetes-related clinical service, and associated factors among HIV-negative patients living with diabetes and diabetes PLHIV in Ethiopia.
Methods
Study Design and Setting
A facility-based prospective cohort study design was conducted at chronic care (ART and diabetes) clinics of Jimma Medical Center (JMC), Ethiopia. Adult HIV-infected individuals on HAART and HIV-negative individuals with diabetes who met the inclusion criteria were recruited into the study. With a bed size of 660, JMC is the only teaching and referral hospital in the South-West part of Ethiopia. It is located 352 km south-west of Addis Ababa, the capital. It delivers care to about 9000 inpatients and 80,000 outpatients each year with a catchment population of about 20 million people. The present study is in accordance with the STROCS (strengthening the reporting of cohort studies) criteria.30
Study Population
This current study was a comparative prospective cohort study assessing diabetes mellitus (DM) related clinical care and level of glycemic control between two groups of the diabetes adult population. The first group was adult PLHIV and diabetes on follow-up at the JMC ART clinic (Arm-1). The second group was adult HIV negative patients living with diabeteson follow-up at JMC diabetes clinic (Arm-2). All patients were followed for a 12-months following 3 months of enrollment period. The inclusion criteria were; diabetes (Type 1 and Type 2 DM) patients, age greater or equal to 18 years, on follow-up for either at DM and/or ART chronic care clinics on a monthly basis. Pregnant women and those patients unwilling to give participation consent were excluded from the study.
Participants Inclusion and Enrollments
The number of individuals living with HIV/AIDS and diagnosed with DM is unknown in the study area and had a different follow-up clinic. They have separate clinic for prescription refill and consultation. To trace and enroll patients with diabetes, all PLHIV coming to ART clinic for follow-up were interviewed and their medical records were reviewed by trained nurses. In order to enroll all potential study participants, it took 3 months (December 2018 to February 2019) of enrollment period. Finally, 97 adult diabetes PLHIV were included in the study and followed for 12 months for their glycemic status and clinical care which was assessed at a baseline. To increase the power of the study, we enrolled 200 HIV negative patients living with diabetes (~ratio 1:2.06). Finally, 297 patients with diabetes [ie, HIV negative (HIV-) and positive (HIV+)] were included in the final analysis. All the participants were followed for 12 months starting from their enrollment date (Figure 1).
 |
Figure 1 Study participants’ enrollment flow chart. |
Data Collection Tool and Procedure
Based on the study objective, a standardized data collection questionnaire produced by the World Health Organization (WHO) on STEPs (stepwise approach to chronic disease risk factor surveillance) in developing countries31 was used. We assessed self-care behaviors using the expanded version of the summary of diabetes self-care activities (SDSCA).32,33 The SDSCA measure is a brief self-report questionnaire of diabetes self-management. On the previous psychometric validation34,35 SDSCA had a content validity index of 0.83 and a Cronbach’s alpha reliability of 0.69. Two trained data collectors (both trained ART nurses) interviewed the participants in the study and checked patient charts and medical history at the ART clinic for the pertinent information. At the JMC diabetes clinic, two trained research assistants (nurses in the profession) gathered specific information from HIV-negative patients with diabetes for 12 months. All pertinent information such as sociodemographic information, clinical and laboratory data, and behavioral characteristics were recorded each patient. One (1) year monthly measurements of fasting blood sugar (FBS) were recorded for all study participants. CD4+cell count of PLHIV were recorded. Waist circumference (WC) was measured with a flexible inelastic tape placed in a perpendicular plane to the long axis of the body at the midpoint between the lower rib margin and the iliac crest. Using a portable stadiometer, height was measured without shoes. A Tanita scale was used to measure weight; patients were fully dressed, without heavy clothing or shoes.
Data Processing and Analysis
Data were entered into the computer using EpiData version 3.1 and exported to the Statistical Package for Social Science (SPSS) version 22.0 for analysis. Differences between mean values were evaluated using Student’s t-test while proportions were compared using Pearson’s Chi-square test. In order to assess the crude and adjusted effects of factors affecting glycemic control predictors, Cox regression analysis were used. Variables that had p-value≤0.25 on univariate analysis were eligible for multivariate Cox regression. Categorical and continuous data were expressed as percentages and mean ± standard deviation, respectively. The proportion of patients, who had good, fair, and poor self-care behaviors and practices was calculated for the individual dimensions SDSCA and the overall self-care practice. Significance was considered at p-value≤0.05.
Outcome Measures
Glycemic Control
In the two clinics (diabetes and ART), the FBS of all patients with diabetes [HIV (+) and HIV (-)] was measured on a monthly basis for 12 months according to the standard institutional treatment protocol. On the basis of the existing American Diabetes Association (ADA) guideline for treating diabetes mellitus,36 the quarterly (consecutive three months) average of each record was taken and status was categorized into good glycemic control or poor glycemic control.
Self-Care Behavior
To assess self-care behavior and practice, the summary of diabetes self-care practices (SDSCA) questionnaire was used. There are 10 items in the questionnaire for five sub-scale domains. A general diet, specific diet, physical activity, blood glucose testing, and foot-care are the five sub-scales. This self-care scale measures the frequency of performing diabetes self-care activities in the last 7 days. Responses ranged from 0 to 7 days for each of the items in the six domains, and responses to item-4 were reverse coded.32 The score was presented for each self-care action in terms of the mean number of days, which was determined by summing the number of days of self-care practice measured by the total number of patients. We took the average of the mean values in each of the domains (ie, diet, foot care, exercise, blood glucose test, and medication-taking divided by the sum of the number of questions under each scale) for the overall diabetes self-care practice value. As the suggested cut-off point for SDSCA was not available in the biomedical literature, we defined a score less than or equal to the 25th percentile as a poor self-care behavior and practices. Values between the 25thpercentile and the 75th percentile as fair self-care behavior and practices and values greater than or equal to 75th percentile as a good self-care behavior and practices.
Definition and Explanations of Terms
- Co-morbidity: Diseases or disorders that exist together with an index disease or co-occurrence of two or more diseases or disorders in an individual.37
- Multi-morbidity: Living with two or more types of chronic non-communicable diseases.37
- Self‑care practices: refer to behaviors such as following a diet plan, increased exercise, self-blood glucose testing, and foot care.32
- Controlled glycemia: average fasting blood glucose measurement 80–130 mg/dl (4.4–7.2 mmol/L).36
- Uncontrolled glycemia: average blood glucose measurements on three consecutive visits is >130 mg/dL (>7.2 mmol/L).36
- Waist circumference: Waist circumferences >102 cm in males and >88 cm in females were considered elevated.38
- Underweight: Body mass index (BMI) less than 18 kg/m2.39
- Normal body weight: BMI between 18 and 24.9 kg/m2.39
- Overweight: BMI between 25 and 29.9 kg/m2.39
- Obese: BMI between ≥30 kg/m2.39
Results
Socio-Demographic Characteristics of Study Participants
From the total 297 participants in this study, 162 (54.5%) were in the age group of 31–60years and the mean age was 44.35±12.55 years. The majority of the participants were male 166 (55.9%). About 63.3% of them were residing in a rural area. The majority (68.4%) of the study participants attended some level of formal education. There were statistically significant differences with respect to family support (p=0.019), marital status (p=<0.001) and body mass index (p=<0.001) among HIV positive [HIV (+)] and HIV negative [HIV (-)] participants. On the other assessed socio-demographic variables, there were no statistically significant differences between the HIV (+) and HIV (-) groups (Table 1).
 |
Table 1 Baseline Socio-Demographic Characteristics of Study Participants |
Behavioral and Clinical Characteristics of Study Participants
Concerning behavior measures, about 16.5% of diabetes PLHIV reported taking alcohol regularly and 11.8% of them were current smokers. Only 5.1% of HIV (+) and 7.5% of HIV (-) patients with diabetes were consuming fruits and vegetables every day. Limited numbers (7.1%) of participants were involved in daily physical activity. Prevalence rates of comorbid hypertension and heart failure in our study population were 26.3%, and 8.8%, respectively. There were no statistical differences in diabetes-related complications between diabetes PLHIV compared to DM controls. However, on diabetes-related health education and advice given by health-care providers, there was a statistically significant difference on advice related to losing weight (p=0.012), stopping and/or not to try smoking (p=0.037), starting or doing more exercise (p=0.001) and eye care/ophthalmology consultation (p=0.029) (Table 2).
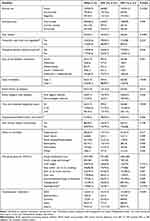 |
Table 2 Baseline Clinical and Behavioral Characteristics of Study Participants |
From a total of 97 diabetes PLHIV, about 88 (90.7%) were actively engaged in their daily activity. About 81.4% of them had greater than 5 cumulative years of HIV exposure. At baseline, about 683.5% of them were within the WHO clinical stage III and IV. More than half (54.6%) of diabetes PLHIV had a previous history of TB treatment. Concerning prophylactic medication use, about 4.1% and 78.4% were on isoniazid preventive therapy (IPT) and cotrimoxazole preventive therapy (CPT), respectively. About 31.9% of PLHIV were diagnosed with diabetes before HIV infection. The hepatitis infection status of 68% of HIV (+) participants was unknown (yet not tested) (Table 3).
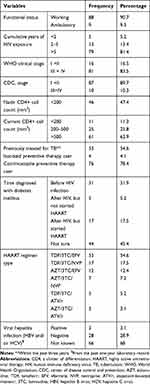 |
Table 3 Baseline Clinical Characteristics of Diabetes Human Immune Deficiency Virus-Positive Participants |
Glycemic Control Status
Of a total of 297 patients included in the study, 158 met the American Diabetes Association (ADA) definition of uncontrolled blood glucose, yielding a prevalence of uncontrolled glycemia of 53.2%. Overall, the mean±SD fasting blood sugar (FBS) of the total participants was 142±14.64 g/dL at the baseline. One hundred twenty-three (41.4%) of the patients reached the glycemic control target of FBS <130g/dL. After 12 months of follow-up, the mean FBS decreased to 141.25±14.06 g/dL and 44.4% of the patients reached FBS<130g/dL. There were statistically significant differences in the achievement of the ADA blood glucose target between HIV (+) and HIV (-) diabetes participants during the follow-up periods, except in the fourth quarter (Table 4).
 |
Table 4 Follow-Up Glycemic Status of Study Participants |
Diabetes-Related Clinical and Self-Care Management
On the overall self-care behaviors and practices of PLHIV, about 31 (31.9%) had a good level, 27 (27.9%) had a fair level, and 39 (40.2%) had a poor level of self-care practices. The difference in overall self-care practices between HIV (+) and HIV (-) patients was not statistically significant (p=0.097). More than 50% of diabetes PLHIV patients had poor adherence to activities related to specific diet management, foot care, physical exercise, and blood glucose testing domains. There was a statistically significant difference in foot care (p=0.0003), specific diet (p=0.008), medication adherence (p=0.016) between HIV (-), and HIV (+) patients with diabetes (Figure 2).
 |
Figure 2 Domain-specific and the overall diabetes self-care behaviors and practices of study participants. |
Predictors of Poor Glycemic Control
Sex, age, job/occupation, waist circumference, body mass index, smoking history, consumption of fruits and vegetables, physical activity, chronic disease comorbidity, serostatus, family history of diabetes, a period after the diagnosis of diabetes was found to be associated with uncontrolled glycemia in bivariable Cox regression. These variables were entered into the multivariate Cox regression analysis, in addition to variables with a p-value of less than 0.25. Univariate analysis showed the female gender [CHR: 3.67; 95% CI (1.16–6.17); p<0.001], older patients (61 years) [CHR: 2.07; 95% CI (1.03–9.43); p=0.003], increased waist circumference [CHR: 4.14; 95% CI (1.11–32.19); p<0.001] and appeared to have higher risks of uncontrolled glycemia. In addition, self-employed employees [CHR: 1.68; 95% CI (1.52, 5.34); p<0.001], unemployed person [CHR: 1.13; 95% CI (1.04–11.40); p=0.003] had marginally higher potential for failure in glycemic control.
Blood glucose control was more likely to fail in overweight patients [CHR: 2.62; 95% CI (1.20–5.72); p<0.001]. In terms of health behavior, active smokers were more likely to fail in glycemic control than non-smokers and ex-smokers [CHR: 1.76; 95% CI (1.09–9.68); p=0.044]. In addition, those who did not participate in frequent exercise [CHR: 2.60; 95% CI (1.96–7.45); p=0.022] and regularly did not consume fruit and vegetables [CHR: 2.17; 95% CI (1.79–8.63); p<0.001] showed a higher risk of uncontrolled blood sugar.
Participants with a family history of diabetes [CHR: 1.59; 95% CI (1.06–2.38); p=0.047], with a longer history of diabetes [greater than 5 years] [CHR: 4.21; 95% CI (1.54–8.19) p=<0.001] and chronic disease comorbidity [CHR: 1.63; 95% CI (1.19–4.24); p=0.037] had a greater risk of uncontrolled glycaemia. In this study, patients with diabetes living with HIV had a greater risk of failure to meet the ADA blood sugar goal [CHR: 4.36; 95% CI (1.16–22.19); p=0.039]. However, there was no statistical significance for the impact of other health habits (alcohol consumption, chat chewing), diabetes types, and antidiabetic regimes on glycemic control.
On multivariable model, female gender [AHR: 2.72; 95% CI (1.21–5.72); p=0.036], age between 31 and 60 years [AHR: 2.48; 95% CI (1.34–11.01); p=0.047], age ≥61 years [AHR: 3.13; 95% CI (1.71–9.51); p=0.026]; self-employed [AHR: 2.19; 95% CI (1.84,11.60); p=0.014], unemployed [AHR: 1.04; 95% CI (1.01–7.01); p=0.036]; waist circumference above the normal [AHR: 3.64; 95% CI (2.57–16.12); p=0.007], overweight [AHR: 3.63; 95% CI (1.65–22.42); p<0.001], chronic disease comorbidity [AHR: 2.02;95% CI (1.44–2.84); p=0.047]; HIV infection [AHR: 3.47; 95% CI (2.03–23.75); p= 0.021]; living longer with diabetes (0.5 years) [AHR: 3.67; 95% CI (3.26–4.14); p<0.001] showed higher hazards of failure in blood sugar control and were independent predictor of uncontrolled glycaemia (Table 5).
 |
Table 5 Factors Associated with Uncontrolled Blood Glucose Among Study Participants |
Subgroup Analysis
We performed a subgroup analysis for factors associated with poor glycemic control for participants living with human immune deficiency virus. On the univariate Cox regression, female gender (p=0.001), age of participants (p=0.001), body mass index, baseline WHO clinical stage of HIV (p=0.028), nadir baseline CD4+ cell count (p=0.020), tuberculosis (TB) co-infection (p=0.009) were found to be associated with uncontrolled glycemia. The risk of uncontrolled glycemia was about 3.24 times more likely to occur in female patients as compared to male counterparts (p=0.029) (Table 6).
 |
Table 6 Factors Associated with Glycemic Control Among Diabetes People Living with HIV/AIDS |
Discussion
Treatment of diabetes mellitus (DM) is now an increasingly essential part of the long-term management of PLHIV. Among PLHIV, metabolic disorders, such as DM, are likely to evolve at the intersection of typical risk factors and HIV and ART-specific contributors, such as chronic inflammation and immune activation. To our knowledge, this is the first study examining differences in phenotype, glycemic control, and clinical care delivery between diabetes mellitus patients with and without HIV in sub-Saharan Africa.
More than half [158 (53.2%)] of the study participants were not at the target glycemic control level. This higher than the study done by the National Health and Nutrition Examination Survey (NHANES) (44%).27 The follow-up glycemic control status in our study finding was lower than the findings from Jordan,40 Malaysia,41 and India42 ranging from 65.1% to 78.6%. The difference is maybe related to a large number of participants who were older in those studies and live longer with diabetes than in our study.
HIV infection and diabetes mellitus (DM) represent a collision of deadly chronic conditions. Within the diabetes PLHIV, about 61.9% of them did not achieve the glycemic target set at fasting blood sugar (FBS) of <130mg/dL, which was higher than previous reports from the USA,29,43,44 Netherlands,45 and Chicago28 ranging from 33% to 46%. These three research studies reported the rates of glycemic control using HbA1C. However, we used the three months average of the fasting blood sugar measurement in this study. There was the involvement of clinical pharmacists who will provide patient-focused diabetes education and screening recommendations for providers on the latter study. However, a study from South Africa46 showed that about 85.23% of patients with diabetes living HIV displayed suboptimal glycemic control, which was higher than the current study. Our study was nearly equivalent to a report from the USA47 and Australia,48 where (54% and 59%, respectively) of diabetes PLHIV did not achieve the ADA glycemic target. There are multiple risk factors for the occurrence of diabetes and uncontrolled blood sugar among PLHIV. These are ART medications, the HIV itself, pill burden, multiple opportunistic infections, which will increase oxidative stress, insulin resistance, from lipodystrophy, non-comprehensive clinical care for both patients with diabetes only and patients with diabetes living with HIV.1,3,4,15,49 Indeed, HIV-infected patients are mostly treated in most settings by infectious disease clinicians or HIV treatment qualified professionals with less expertise in endocrinology or diabetology, who may be more focused on HIV control rather than metabolic sequelae.
In this study, we found that the hazard of uncontrolled glycemia in diabetes PLHIV was more than three-time higher than HIV-negative patients with diabetes (AHR=3.47; p=0.021). As from the records of our follow-up blood glucose data, the average quarterly blood glucose measurement showed a statistically significant difference, when compared with HIV-negative patients with diabetes. Owing to the high incidence of diabetes in HIV-infected patients, the European Aids Clinical Society recommends that all patients be tested for elevated glucose levels at the time of HIV diagnosis, prior to and periodically following initiation of therapy.50 Infection-related disorders, the combined influence of ART on lipid metabolism, seem to stand out.51 From different epidemiological studies, the incidence of metabolic syndrome in HIV-infected people reaches up to 50%,52–54 which will affect the glycemic outcome. In our study also the prevalence of hypertension, abnormal waist circumference, and increased body weight were seen. These will have a significant impact on the glycemic outcome of study participants.
In the body of biomedical literature, there were no researches done on self-care behavior and practices of diabetes PLHIV using the SDSCA scale. Therefore, we used researches done on the general diabetes population for comparison. Self-care practices are main components of DM care and help to regulate blood sugar levels effectively and avoid risks associated with diabetes. About 28.5% of the diabetes PLHIV negative and 40.2% of HIV negative patients with diabetes had poor self-care behaviors toward Diabetes mellitus management. This was statistically significant between the two groups of patients. As compared to other studies from Ethiopia55,56 and Kenya,57 our study showed a lower percentage of patients in the category of poor self-care practice. The difference in these studies may be related to the instrument used for measuring the self-care practice. However, the reported figure for poor self-care practice was still high and denoting diabetes as a significant public health problem. From SDSCA domains medication adherence was the most commonly achieved domain-for both groups, which is consistent with previous reports.58,59 In the current study, a higher percentage of patients reported a poor level of self-care behavior in engagement in physical exercise and self-monitoring their blood sugar which was also consistent with previous study.59 This is maybe related to the information gap on the domain and inaccessibility of self-glucose measuring devices on a routine basis in the settings. The difference across the SDSCA domains between diabetes PLHIV and HIV-negative patients with diabetes implicate the need to integrate between the two cares and further research on the area.
In this study, abnormal waist circumference had a statistically significant association with uncontrolled blood sugar. This may be due to the influence of adipose tissue on insulin resistance. This can be from the lipodystrophy syndrome (increased adipose tissue), the main side effects of ART.60 As a result, the waist circumference increases, which will lead to insulin resistance.
Concerning a healthy eating plan, in this study, less frequent use of fruits and vegetable use was significantly associated with uncontrolled glycemia. The hazards of having uncontrolled blood sugar were more than 2 times higher among frequent users of fruits and vegetables compared to their counterparts. This is supported by studies from the United Arab Emirates,61 China,62 and the United Kingdom63 where daily intake of fresh fruit was shown to be protective. Except for some of the studies,64–66 large studies across the globe67 showed that increased fruit intake was linked with a lower risk of diabetes, hence blood sugar control. One randomized controlled trial from Denmark68 showed no difference in glycemic control among fruit consumers and non-consumers. However, they recommended intake of fruit in patients with diabetes. The American Diabetes Association (ADA) advises the consumption of carbohydrates from whole grains, vegetables, fruits, legumes and dairy products, with an emphasis on foods rich in fiber and lower in glycemic load.69 Fruits and vegetables have antioxidants that may inhibit harmful effects of the reactive oxygen species involved in the development and progression of diabetes.70 There is a fear that there are sugars in fruit (ie, glucose and fructose) that could have a detrimental effect on blood sugar. However, sugars in fruit and vegetables are natural sugars and will not be metabolized in the same manner as refined sugars.71 The multitude of effects of fruits and vegetables such as anti-oxidative, anti-inflammatory, anti-hypertensive, anti-dyslipidaemic, anti-hyperglycaemic, and modulation of the composition and metabolic activity of gut microbiota72,73 may be implicated in controlling the blood sugar in patients with diabetes. Therefore, as optimum nutrition has an integral role in glycemic control of patients with diabetes decreasing the intake of fizzy drinks and fast food, increasing daily intake of fresh fruits and vegetables will have a clinically relevant protective effect and aid in achieving the recommended glycemic target.
Furthermore, in terms of health behavior, this study revealed that patients with higher body mass index (overweight and obese) had higher risks of uncontrolled glycemia. Overweight patients had higher risks of glucose control failure (AHR=3.63; p=<0.001). Similarly, on a subgroup analysis of diabetes PLHIV, overweight (AHR=1.61; p=0.043) and obesity (AHR=2.09; p=0.044) had statistically significant hazards of uncontrolled blood sugar. At the same time, those engaged in physical exercise less frequently were less likely to meet glycemic control than their counterparts. Our study was in line with studies from China,74 the USA,75,76 and Portugal.77 Less frequent engagement in physical exercise, as in the case of our study, will contribute to uncontrolled blood sugar. Body mass index and infrequent engagement in physical exercise have a direct relationship and may similarly affect the blood sugar. Overweight or obesity from a sedentary life (such as lack of physical exercise) have a significant role in the pathophysiology of diabetes and its macrovascular complications,78 expected to affect the glycemic outcome. Physical exercise increases insulin action and sensitivity and glucose uptake by skeletal muscles and higher BMI is highly correlated with insulin resistance.79 While lifestyle habits, such as diet balance, physical exercise, need to be maintained in patients with diabetes, it was expected that it will often be difficult for them to improve and maintain a healthier lifestyle. Therefore, physical exercise and/or dietary education programs should become important diabetes management packages to prevent diabetes-related complications and meet the recommended target blood sugar.
We found that patients who had chronic disease comorbidity had a higher hazard of having poor glycemic control. The hazard of uncontrolled blood sugar was two times more likely to occur among patients living with other chronic diseases. This was similar to studies from the USA43,80,81 India,82 and done by Sanal et al83 where disease comorbidity showed a significant association with poor blood glucose control. This may be related to poor adherence to drug regimens of HIV, diabetes, and the comorbid disease(s) due to the cocktail of pills. At the same time, these comorbidities will affect adherence to healthy lifestyle modifications such as diet plan, exercise, and quality of life.
Uncontrolled blood sugar was significantly associated with the duration of the first diagnosis of for diabetes. Those patients lived with diabetes for more than five (5) years showed impaired glycemic control, which is consistent with previous studies40,84,85 that reported living long duration with diabetes was associated with poor glycemic control. This may be related to adherence to medication and recommended therapeutic life changes, including diets in early diabetes diagnosis. Residual ß-cell activity can improve glycemic regulation in the early stages of diabetes. In the long run due to progressive deficiency of insulin secretion, increased insulin resistance, and resulting decline in insulin secretion would have a detrimental effect on blood glucose regulation.86,87 Therefore, due emphasis should be given to patients with longstanding diabetes in adherence support and dose adjustments especially for patients with multiple disease conditions, such as HIV/AIDS, as in this study.
Multiple studies showed the bidirectional relationship between tuberculosis (TB) and diabetes. Diabetes mellitus is increasingly recognized as an independent risk factor for infection of tuberculosis, and both sometimes coexist.88–92 This was recognized as early as 1000AD. Up to one-third patients with TB have co-occurring diabetes mellitus.93 The two are separate disease entities that negatively affect the treatment outcome of each other94 TB can lead to, new-onset diabetes, impaired glucose tolerance) and poor uncontrolled blood sugar.92,95 In our study, the hazard of uncontrolled blood sugar was higher among diabetes PLHIV who had TB infection. In univariate analysis, the hazard of uncontrolled glycemia was more than four times among TB infected patients. It had the same direction on multivariate Cox regression, with TB infection carries almost four times the hazards of uncontrolled blood sugar (AHR=3.82; p=0.022). This was similar to studies from Nigeria,91 Taiwan,96,97 China,98 Australia99 and, India.100 This is linked with some widely used antituberculosis medications, such as rifampicin and isoniazid, which can worsen glycemic regulation in newly diagnosed patients with diabetes.101 Insulin resistance from the inflammatory stress response from TB infection, pancreatic endocrine hypofunction, and TB involving the pancreatitis is often implicated in hyperglycemia.88 Among the cocktail of anti-TB drugs, rifampin stimulates the metabolism of sulphonylureas and biguanides, lower their plasma levels and thereby contributing to higher blood sugar.88 Isoniazid antagonizes the effect of sulphonylureas and affects glycemic regulation.60 Therefore, the treatment of diabetes with concomitant TB infection requires proper assessment and choosing of anti-glycemic medications. It warrants regular glucose monitoring, the adjustment in doses of anti-glycemic agents, or a complete switch to insulin therapy. This will be very critical in drug regimen optimization in the deadly looming co-epidemics of TB, diabetes, and HIV infection, due to polypharmacy and significant drug–drug interaction from the HAART regimen too.
In this study, female patients had higher hazards of uncontrolled blood glucose in the overall cohort (AHR=2.72; p=0.036), and also within the diabetes PLHIV (AHR=3.24; p=0.029). It is in agreement with studies.82,102,103 Differences in glucose and energy homeostasis (eg, hormones and visceral adipose distribution), drug response (eg, side effects), and psychological influences (eg, condition acceptance) may be correlated with the disparity.104,105
Strength and Limitations of the Study
The key strength of this study was the prospective nature and the continuous monthly follow-up of each participant for the result that would minimize bias and missing results. The prospective collection of data helped us to gather reliable relevant data from our participants. It is also the first study in sub-Saharan Africa, which focuses on glycemic regulation of PLHIV diabetes and HIV-negative counterparts.
There are some limitations to this report. First, it is small sample size study from a single university hospital. Secondly, as an observational study with 12 months follows up, this follow-up time is not adequate to provide us detailed information about health results such as macrovascular and microvascular problems linked to HIV infection and collision with diabetes. Thus, a multiple-year longitudinal cohort study will be further needed. And also, we cannot get information on TB treatment among HIV negative patients with diabetes.
Conclusion
In summary, we found that the prevalence of uncontrolled glycemia in this comparative cohort study was high in the study area. Gaps were observed between real-world diabetes management and the recommendations for the treatment targets in this study especially in the diabetes PLHIV in achieving recommended glycemic level and involvement of patients on his/her self-care care behavior and practices. In the achievement of recommended targets, it is high time to leverage the clinical care of chronic comorbid disease into HIV care packages through chronic comprehensive care clinic.
The co-occurrence of TB-HIV and diabetes is triple trouble needing special attention in their clinical care. Successfully addressing diabetes-TB-HIV therefore requires a coordinated response to both diseases at all levels of the health system—from the crafting and implementation of national policies to the management of disease control programs to the delivery of services to individual patients. Health services for HIV and diabetes have common features since both require health systems that can provide for people’s chronic care needs. Therefore, long-term care for HIV/AIDS and diabetes present an opportunity to coordinate efforts and synergies between both programs and their integration can be used to strengthen health systems.
We noted that patients with higher levels of baseline BMI, female gender, TB infection, older than 31 years of age, patients with less frequent involvement exercise, chronic comorbidities, those who consume fruits and vegetables infrequently, unemployment, self-employment, living longer with diabetes (>5 years), concomitant HIV infection, increased waist circumference had higher hazards of uncontrolled blood glucose level based on ADA recommendation and were independent predictors of uncontrolled glycemia.
Abbreviations
ADA, American Diabetes Association; AHR, adjusted hazard ratio; ART, antiretroviral therapy; BMI, body mass index; CD4, cluster of differentiation 4; CHR, crude hazard ratio; CI, confidence interval; DM, diabetes mellitus; HIV, human immunodeficiency virus; HR, hazard ratio; HAART, highly active antiretroviral therapy; JMC, Jimma Medical Center; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PLHIV, people living with HIV; PI, protease inhibitor; NRTIs, nucleoside reverse transcriptase inhibitors; SD, standard deviation; SDSCA, summary of diabetes self-care activities; TB, tuberculosis; WC, waist circumference; WHO, World Health Organization.
Data Sharing Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
Ethical clearance and approval were obtained from the institution review board (IRB) of Jimma University with the reference number of IHRPGC/2098/10. It was based on the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All study participants provided written informed consent.
Consent
Not applicable. No person’s details, images, or videos were used in this study.
Acknowledgments
We thank Jimma University for supporting the study. We are grateful to staff members of chronic care clinics of JMC, data collectors, and study participants for their cooperation in the success of this study.
Author Contributions
All authors made a significant contribution to the work reported; in the conception, study design, execution, acquisition of data, analysis, and interpretation, they all took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
The only funder for the study was Jimma University. The funding body did not have any role in study design, data collection, and data analysis, interpretation of data, or in writing the manuscript.
Disclosure
The authors declared that they have no competing interests.
References
1. Yoon C, Gulick RM, Hoover DR, Vaamonde CM, Glesby MJ. Case-control study of diabetes mellitus in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;37(4):1464–1469. doi:10.1097/01.qai.0000137373.26438.18
2. Guariguata L, de Beer I, Hough R, Mulongeni P, Feeley FG, de Wit TFR. Prevalence and knowledge assessment of HIV and non-communicable disease risk factors among formal sector employees in Namibia. PLoS One. 2015;10(7):e0131737. doi:10.1371/journal.pone.0131737
3. Steiniche D, Jespersen S, Erikstrup C, et al. Diabetes mellitus and impaired fasting glucose in ART-naive patients with HIV-1, HIV-2 and HIV-1/2 dual infection in Guinea-Bissau: a cross-sectional study. Trans R Soc Trop Med Hyg. 2016;110(4):219–227. doi:10.1093/trstmh/trw017
4. Hadigan C, Kattakuzhy S. Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus. Endocrinol Metab Clin. 2014;43(3):685–696. doi:10.1016/j.ecl.2014.05.003
5. Njuguna B, Kiplagat J, Bloomfield GS, Pastakia SD, Vedanthan R, Koethe JR. Prevalence, risk factors, and pathophysiology of dysglycemia among people living with HIV in Sub-Saharan Africa. J Diabetes Res. 2018;2018:1–12. doi:10.1155/2018/6916497
6. Fiseha T, Belete AG. Diabetes mellitus and its associated factors among human immunodeficiency virus-infected patients on antiretroviral therapy in Northeast Ethiopia. BMC Res Notes. 2019;12(1):372. doi:10.1186/s13104-019-4402-1
7. Abebe SM, Getachew A, Fasika S, Bayisa M, Demisse AG, Mesfin N. Diabetes mellitus among HIV-infected individuals in follow-up care at University of Gondar Hospital, Northwest Ethiopia. BMJ Open. 2016;6(8):e011175. doi:10.1136/bmjopen-2016-011175
8. Ataro Z, Ashenafi W, Fayera J, Abdosh T. Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV-positive individuals receiving highly active antiretroviral therapy at Jugal hospital, Harar, Ethiopia. HIV/AIDS. 2018;10:181.
9. Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. Aids. 2012;26(3):303–314. doi:10.1097/QAD.0b013e32834e8776
10. De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the data collection on adverse events of anti-HIV drugs (D: A: D) study. Diabetes Care. 2008;31(6):1224–1229. doi:10.2337/dc07-2013
11. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32(1):130–139. doi:10.1086/317541
12. Samaras K. The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV/AIDS Rep. 2012;9(3):206–217. doi:10.1007/s11904-012-0124-x
13. Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–220. doi:10.1016/j.amjcard.2015.10.030
14. Miller CJ, Baker JV, Bormann AM, et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One. 2014;9(4):e95061. doi:10.1371/journal.pone.0095061
15. Palella FJ
16. Epping-Jordan J, Bengoa R, Kawar R, Sabaté E. The challenge of chronic conditions: WHO responds: the sooner governments act, the better. BMJ. 2001;323(7319):947–948. doi:10.1136/bmj.323.7319.947
17. Melaku T, Chelkeba L, Mekonnen Z. Clinical care & blood pressure control among hypertensive people living with human immune deficiency virus: prospective cohort study. Ann Med Surg. 2020;54:114–124. doi:10.1016/j.amsu.2020.04.017
18. Saag MS. HIV Now Firmly Established in the Middle Ages. Oxford University Press; 2011.
19. Carr A. HIV protease inhibitor-related lipodystrophy syndrome. Clin Infect Dis. 2000;30(Supplement_2):S135–S42.
20. Opwora A, Waweru E, Toda M, et al. Implementation of patient charges at primary care facilities in Kenya: implications of low adherence to user fee policy for users and facility revenue. Health Policy Plan. 2014;30(4):508–517.
21. Eckhardt BJ, Holzman RS, Kwan CK, Baghdadi J, Aberg JA. Glycated hemoglobin A1C as screening for diabetes mellitus in HIV-infected individuals. AIDS Patient Care STDS. 2012;26(4):197–201. doi:10.1089/apc.2011.0379
22. Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32(9):1591–1593. doi:10.2337/dc09-0177
23. Glesby MJ, Hoover DR, Shi Q, et al. Glycated hemoglobin in diabetic women with and without HIV infection: data from the women’s interagency HIV study. Antivir Ther. 2010;15(4):571. doi:10.3851/IMP1557
24. Venables E, Edwards JK, Baert S, Etienne W, Khabala K, Bygrave H. “ They just come, pick and go.” The acceptability of integrated medication adherence clubs for HIV and non-communicable disease (NCD) patients in Kibera, Kenya. PLoS One. 2016;11(10):e0164634.
25. Organization WH. Prevention and Control of Noncommunicable Diseases: Guidelines for Primary Health Care in Low Resource Settings. World Health Organization; 2012.
26. Organization WH. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization; 2016.
27. Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in US adults? Diabetes Care. 2008;31(1):81–86. doi:10.2337/dc07-1572
28. Adeyemi O, Vibhakar S, Max B. Are we meeting the American diabetes association goals for HIV-infected patients with diabetes mellitus? Clin Infect Dis. 2009;49(5):799–802. doi:10.1086/605286
29. Bury JE, Stroup JS, Stephens JR, Baker DL, editors. Achieving American diabetes association goals in HIV-seropositive patients with diabetes mellitus. In: Baylor University Medical Center Proceedings. Taylor & Francis; 2007.
30. Agha RA, Borrelli MR, Vella-Baldacchino M, et al. The STROCSS statement: strengthening the reporting of cohort studies in surgery. Int J Surg. 2017;46:198–202. doi:10.1016/j.ijsu.2017.08.586
31. Riley L, Guthold R, Cowan M, et al. The World Health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health. 2016;106(1):74–78. doi:10.2105/AJPH.2015.302962
32. Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi:10.2337/diacare.23.7.943
33. Sharoni SKA, Wu SFV. Self‐efficacy and self‐care behavior of Malaysian patients with type 2 diabetes: a cross-sectional survey. Nurs Health Sci. 2012;14(1):38–45. doi:10.1111/j.1442-2018.2011.00658.x
34. Choi EJ, Nam M, Kim SH, et al. Psychometric properties of a Korean version of the summary of diabetes self-care activities measure. Int J Nurs Stud. 2011;48(3):333–337. doi:10.1016/j.ijnurstu.2010.08.007
35. Adarmouch L, Sebbani M, Elyacoubi A, Amine M. Psychometric properties of a moroccan version of the summary of diabetes self-care activities measure. J Diabetes Res. 2016;2016:1–6. doi:10.1155/2016/5479216
36. Association AD. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl1):S61.
37. Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–363. doi:10.1370/afm.983
38. Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world‐wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–480. doi:10.1111/j.1464-5491.2006.01858.x
39. Obesity N, Heart N, Lung Institute B, Initiative NOE. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and obesity in Adults. National Institutes of Health, National Heart, Lung, and Blood Institute; 2000.
40. Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications. 2010;24(2):84–89. doi:10.1016/j.jdiacomp.2008.12.008
41. Eid M, Mafauzy M, Faridah A. Glycaemic control of type 2 diabetic patients on follow up at Hospital Universiti Sains Malaysia. Malays J Med Sci. 2003;10(2):40.
42. Gopinath B, Sri Sai Prasad M, Jayarama N, Prabhakara K. Study of factors associated with poor glycemic control in type 2 diabetic patients. Glob J Med Public Health. 2013;2(2):1–5.
43. Satlin MJ, Hoover DR, Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American diabetes association management guidelines. AIDS Patient Care STDS. 2011;25(1):5–12. doi:10.1089/apc.2010.0237
44. Chu C, Umanski G, Blank A, Meissner P, Grossberg R, Selwyn PA. Comorbidity-related treatment outcomes among HIV-infected adults in the Bronx, NY. J Urban Health. 2011;88(3):507–516. doi:10.1007/s11524-010-9540-7
45. Roerink M, Meijering R, Bosch M, de Galan B, van Crevel R. Diabetes in patients with HIV: patient characteristics, management and screening. Neth J Med. 2015;73(7):310–315.
46. Pillay S, Aldous C, Mahomed F. A deadly combination–HIV and diabetes mellitus: where are we now? S Afr Med J. 2016;106(4):378–383. doi:10.7196/SAMJ.2016.v106i4.9950
47. Davies ML, Johnson MD, Brown JN, Bryan WE, Townsend ML. Predictors of glycaemic control among HIV-positive veterans with diabetes. Int J STD AIDS. 2015;26(4):262–267. doi:10.1177/0956462414535207
48. Fazekas-Lavu M, Tonks KT, Samaras K. Benchmarks of diabetes care in men living with treated HIV-infection: a tertiary center experience. Front Endocrinol (Lausanne). 2018;9:634. doi:10.3389/fendo.2018.00634
49. Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV cohort study. Clin Infect Dis. 2007;45(1):111–119. doi:10.1086/518619
50. Lundgren JD, Battegay M, Behrens G, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9(2):72–81. doi:10.1111/j.1468-1293.2007.00534.x
51. da Cunha J, Maselli LMF, Stern ACB, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol. 2015;4(2):56. doi:10.5501/wjv.v4.i2.56
52. Adeyemi O, Rezai K, Bahk M, Badri S, Thomas-Gossain N. Metabolic syndrome in older HIV-infected patients: data from the CORE50 cohort. AIDS Patient Care STDS. 2008;22(12):941–945. doi:10.1089/apc.2008.0119
53. Alencastro PR, Fuchs SC, Wolff FH, Ikeda ML, Brandão AB, Barcellos NT. Independent predictors of metabolic syndrome in HIV-infected patients. AIDS Patient Care STDS. 2011;25(11):627–634. doi:10.1089/apc.2010.0360
54. Alvarez C, Salazar R, Galindez J, et al. Metabolic syndrome in HIV-infected patients receiving antiretroviral therapy in Latin America. Braz J Infect Dis. 2010;14(3):256–263. doi:10.1016/S1413-8670(10)70053-2
55. Feleke SA, Alemayehu CM, Adane HT, Onigbinde A, Akindoyi O, Faremi F. Assessment of the level and associated factors with knowledge and practice of diabetes mellitus among diabetic patients attending at FelegeHiwot hospital, Northwest Ethiopia. Clin Med Res. 2013;2(6):110. doi:10.11648/j.cmr.20130206.11
56. Ayele K, Bisrat Tesfa LA, Tilahun T, Girma E. Self care behavior among patients with diabetes in Harari, Eastern Ethiopia: the health belief model perspective. PLoS One. 2012;7(4).
57. Maina WK, Ndegwa ZM, Njenga EW, Muchemi EW. Knowledge, attitude and practices related to diabetes among community members in four provinces in Kenya: a cross-sectional study. Pan Afr Med J. 2010;7(1).
58. Bonger Z, Shiferaw S, Tariku EZ. Adherence to diabetic self-care practices and its associated factors among patients with type 2 diabetes in Addis Ababa, Ethiopia. Patient Prefer Adherence. 2018;12:963. doi:10.2147/PPA.S156043
59. Tewahido D, Berhane Y. Self-care practices among diabetes patients in Addis Ababa: a qualitative study. PLoS One. 2017;12(1). doi:10.1371/journal.pone.0169062
60. Rao P. Persons with type 2 diabetes and co-morbid active tuberculosis should be treated with insulin. Int J Diabetes Dev Ctries. 1999;19:79–86.
61. Sadiya A, Mnla R. Impact of food pattern on glycemic control among type 2 diabetic patients: a cross-sectional study in the United Arab Emirates. Diabetes Metab Syndr Obes. 2019;12:1143.
62. Du H, Li L, Bennett D, et al. Fresh fruit consumption in relation to incident diabetes and diabetic vascular complications: a 7-y prospective study of 0.5 million Chinese adults. PLoS Med. 2017;14(4):e1002279. doi:10.1371/journal.pmed.1002279
63. Carter P, Gray L, Talbot D, Morris D, Khunti K, Davies M. Fruit and vegetable intake and the association with glucose parameters: a cross-sectional analysis of the let’s prevent diabetes study. Eur J Clin Nutr. 2013;67(1):12–17. doi:10.1038/ejcn.2012.174
64. Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347(aug28 1):f5001. doi:10.1136/bmj.f5001
65. Montonen J, Järvinen R, Heliövaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr. 2005;59(3):441–448. doi:10.1038/sj.ejcn.1602094
66. Tanaka S, Yoshimura Y, Kawasaki R, et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology. 2013;204–211.
67. Wang PY, Fang JC, Gao ZH, Zhang C, Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta‐analysis. J Diabetes Investig. 2016;7(1):56–69. doi:10.1111/jdi.12376
68. Christensen AS, Viggers L, Hasselström K, Gregersen S. Effect of fruit restriction on glycemic control in patients with type 2 diabetes–a randomized trial. Nutr J. 2013;12(1):29. doi:10.1186/1475-2891-12-29
69. Association AD. Standards of medical care in diabetes—2015: summary of revisions. Am Diabetes Assoc. 2015.
70. Maritim A, Sanders A, Watkins J
71. Sievenpiper JL, de Souza RJ, Jenkins DJ. Sugar: fruit fructose is still healthy. Nature. 2012;482(7386):470.
72. Sinclair AJ, Armes DG, Randhawa G, Bayer AJ. Caring for older adults with diabetes mellitus: characteristics of carers and their prime roles and responsibilities. Diabet Med. 2010;27(9):1055–1059. doi:10.1111/j.1464-5491.2010.03066.x
73. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi:10.1136/gutjnl-2015-309990
74. Cai X, Hu D, Pan C, et al. The risk factors of glycemic control, blood pressure control, lipid control in Chinese patients with newly diagnosed type 2 diabetes a nationwide prospective cohort study. Sci Rep. 2019;9(1):1–14. doi:10.1038/s41598-019-44169-4
75. Bae J, Lage M, Mo D, Nelson D, Hoogwerf B. Obesity and glycemic control in patients with diabetes mellitus: analysis of physician electronic health records in the US from 2009–2011. J Diabetes Complications. 2016;30(2):212–220. doi:10.1016/j.jdiacomp.2015.11.016
76. El-Kebbi IM, Cook CB, Ziemer DC, Miller CD, Gallina DL, Phillips LS. Association of younger age with poor glycemic control and obesity in urban African Americans with type 2 diabetes. Arch Intern Med. 2003;163(1):69–75. doi:10.1001/archinte.163.1.69
77. Martins RA, Jones JG, Cumming SP, e Silva MJC, Teixeira AM, Veríssimo MT. Glycated hemoglobin and associated risk factors in older adults. Cardiovasc Diabetol. 2012;11(1):13. doi:10.1186/1475-2840-11-13
78. Babikr WG, Alshahrani ASA, Hamid HGM, Abdelraheem AHMK, Shalayel MHF. The Correlation of HbA1c with Body Mass Index and HDL-Cholesterol in Type 2 Diabetic Patients. 2016.
79. Wojtaszewski J, Hansen BF, Kiens B, Markuns J, Goodyear L, Richter E. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49(3):325–331. doi:10.2337/diabetes.49.3.325
80. El-Kebbi IM, Ziemer DC, Cook CB, Miller CD, Gallina DL, Phillips LS. Comorbidity and glycemic control in patients with type 2 diabetes. Arch Intern Med. 2001;161(10):1295–1300. doi:10.1001/archinte.161.10.1295
81. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725–731. doi:10.2337/diacare.29.03.06.dc05-2078
82. Haghighatpanah M, Nejad ASM, Haghighatpanah M, Thunga G, Mallayasamy S. Factors that correlate with poor glycemic control in type 2 diabetes mellitus patients with complications. Osong Public Health Res Perspect. 2018;9(4):167. doi:10.24171/j.phrp.2018.9.4.05
83. Sanal T, Nair N, Adhikari P. Factors associated with poor control of type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetol. 2011;3(1):1–10.
84. Sasi ST, Kodali M, Burra KC, Muppala BS, Gutta P, Bethanbhatla MK. Self care activities, diabetic distress and other factors which affected the glycaemic control in a tertiary care teaching hospital in South India. J Clin Diagn Res. 2013;7(5):857.
85. Kakade AA, Mohanty IR, Sandeep R. Assessment of factors associated with poor glycemic control among patients with type II diabetes mellitus. Integr Obes Diabetes. 2018;4(3).
86. Ali T, Sindhu Kaitha SM, Ftesi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79. doi:10.2147/DHPS.S28801
87. Pamungkas RA, Hadijah S, Mayasari AN, Nusdin N. Factors associated with poor glycemic control among type 2 diabetes mellitus in Indonesia. Belitung Nurs J. 2017;3(3):272–280. doi:10.33546/bnj.61
88. Niazi AK, Kalra S. Diabetes and tuberculosis: a review of the role of optimal glycemic control. J Diabetes Metab Disord. 2012;11(1):28. doi:10.1186/2251-6581-11-28
89. Baghaei P, Marjani M, Javanmard P, Tabarsi P, Masjedi MR. Diabetes mellitus and tuberculosis facts and controversies. J Diabetes Metab Disord. 2013;12(1):58. doi:10.1186/2251-6581-12-58
90. Young F, Wotton C, Critchley J, Unwin N, Goldacre M. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health. 2012;66(6):519–523. doi:10.1136/jech.2010.114595
91. Oluboyo P, Erasmus R. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle. 1990;71(2):135–138. doi:10.1016/0041-3879(90)90010-6
92. Basoglu O, Bacakoglu F, Cok G, Saymer A, Ates M. The oral glucose tolerance test in patients with respiratory infections. Monaldi Arch Chest Dis. 1999;54:307–310.
93. Ruslami R, Aarnoutse RE, Alisjahbana B, Van Der Ven AJ, Van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15(11):1289–1299. doi:10.1111/j.1365-3156.2010.02625.x
94. Holman R, Turner R, Pickup J, Williams G. Textbook of Diabetes. Oxford: Blackwell; 1991:467–469.
95. Jeon CY, Harries AD, Baker MA, et al. Bi‐directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Health. 2010;15(11):1300–1314. doi:10.1111/j.1365-3156.2010.02632.x
96. Wang M-C, Cervantes J. Glycemic control in tuberculosis: lessons learned from Taiwan. Asian Pac J Trop Med. 2019;12(10):438.
97. Lee P-H, Fu H, Lai T-C, Chiang C-Y, Chan -C-C, Lin -H-H. Glycemic control and the risk of tuberculosis: a cohort study. PLoS Med. 2016;13(8):e1002072. doi:10.1371/journal.pmed.1002072
98. Martinez L, Zhu L, Castellanos ME, et al. Glycemic control and the prevalence of tuberculosis infection: a population-based observational study. Clin Infect Dis. 2017;65(12):2060–2068. doi:10.1093/cid/cix632
99. Dobler CC, Flack JR, Marks GB. Risk of tuberculosis among people with diabetes mellitus: an Australian nationwide cohort study. BMJ Open. 2012;2(1):e000666. doi:10.1136/bmjopen-2011-000666
100. Gurukartick J, Murali L, Shewade HD, et al. Glycemic control monitoring in patients with tuberculosis and diabetes: a descriptive study from programmatic setting in Tamil Nadu, India. F1000Research. 2019;8(1725):1725. doi:10.12688/f1000research.20781.1
101. Amrit G, Ashok S. Tuberculosis and diabetes: an appraisal. Indian J Tuberc. 2000;47(1):3–8.
102. Demoz GT, Gebremariam A, Yifter H, et al. Predictors of poor glycemic control among patients with type 2 diabetes on follow-up care at a tertiary healthcare setting in Ethiopia. BMC Res Notes. 2019;12(1):207. doi:10.1186/s13104-019-4248-6
103. Kautzky‐Willer A, Kosi L, Lin J, Mihaljevic R. Gender‐based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient‐level pooled data of six randomized controlled trials. Diabetes Obes Metab. 2015;17(6):533–540. doi:10.1111/dom.12449
104. Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20–23. doi:10.1016/j.physbeh.2017.08.016
105. Arnetz L, Ekberg NR, Alvarsson M. Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab Syndr Obes. 2014;7:409. doi:10.2147/DMSO.S51301
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
