Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Glucagon-Like Peptide 1 Attenuates Lipotoxicity-Induced Islet Dysfunction in ApoE–/– Mice
Authors Liu F , Gong L, Qin W, Cui C, Chen L, Zhang M
Received 14 May 2020
Accepted for publication 8 July 2020
Published 28 July 2020 Volume 2020:13 Pages 2701—2709
DOI https://doi.org/10.2147/DMSO.S262479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Fuqiang Liu,1– 4 Lei Gong,1– 4 Weidong Qin,5 Chen Cui,1– 4 Li Chen,1– 4 Mingxiang Zhang5
1Department of Endocrinology, Qilu Hospital, Shandong University, Jinan 250012, People’s Republic of China; 2Institute of Endocrine and Metabolic Diseases, Shandong University, Jinan 250012, People’s Republic of China; 3Key Laboratory of Endocrine and Metabolic Diseases, Shandong Province Medicine and Health, Jinan, People’s Republic of China; 4Jinan Clinical Research Center for Endocrine and Metabolic Diseases, Jinan, People’s Republic of China; 5Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Public Health, Qilu Hospital, Shandong University, Jinan Shandong 250012, People’s Republic of China
Correspondence: Li Chen
Department of Endocrinology, Qilu Hospital of Shandong University, 107 Wen Hua Xi Road, Jinan, Shandong 250012, People’s Republic of China
, Tel +86-531-8216-9255
Fax +86-531-86169356
Email [email protected]
Mingxiang Zhang
Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Public Health, Qilu Hospital, Shandong University, 107 Wen Hua Xi Road, Jinan, Shandong 250012, People’s Republic of China
, Tel +86-531-8216-9255
Fax +86-531-86169356
Email [email protected]
Aim: Glucagon-like peptide-1 (GLP1) is known to decrease glucagon release and may be beneficial for the reduction of elevated blood glucose. However, its role and mechanism of action in diabetes remain elusive. This study aimed to examine the function of GLP1 and analyze the mechanism of effect that GLP1exerts on inducible nitric oxide synthase (iNOS) in diabetic mice.
Methods: A diabetes model was established in ApoE–/– mice fed a high-fat diet and treated with GLP1 and/or lentivirus-expressing PARP1. PARP1, iNOS, and inflammatory factors in islets were detected by Western blot and ELISA. Islet α cells and β cells and CD8+ T lymphocytes were detected by immunostaining. Islet-cell apoptosis was detected by TUNEL.
Results: GLP1 inhibited the expression of PARP1 and iNOS in islets, alleviated decrease in β cells, and suppressed cell apoptosis induced by the high-fat diet. Moreover, GLP1 recovered the decline in insulin sensitivity and glucose tolerance in ApoE–/– mice fed the high-fat diet, and the effects of GLP1 were related to the inhibition of COX2 and NFκB expression.
Conclusion: GLP1 significantly alleviated the decrease in β-cell numbers, suppressed β-cell apoptosis induced by the high-fat diet, inhibited the expression of iNOS, and alleviated inflammatory islet injury via inhibiting the COX2–NFκB pathway.
Keywords: GLP1, islet function, PARP1
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and impaired insulin response to glucose.1–3 Impaired function of islet β cells is one of the major causes of DM.4 Studies have shown that high glucose, high lipids, and inflammatory factors contribute to the apoptosis of islet β cells in T2DM patients, mainly due to the imbalance of the reactive oxygen species (ROS)- and reactive nitrogen species (RNS)-production and -clearance system.5,6 Nitric oxide (NO) is an oxygen-free species synthesized by the action of NO synthetase (NOS).7,8 It is generally believed that NO produced by constitutive NOS (cNOS) mainly plays a physiological regulatory role, while the NO produced by inducible NOS (iNOS) causes cell damage and plays a cytotoxic role. In addition, NO can directly activate Fas and induce apoptosis through p53.2,9
PARP1 is a member of the PARP family, which are nuclear proteins and serve as DNA-break sensors.10 PARP1 can be activated via binding to DNA-strand breaks and facilitates damage repair through poly(ADP-ribosyl)ation of target proteins, such as histones, transcription factors, and PARP1 itself.11 Moreover, PARP1 can regulate the expressions of inflammatory factors, including iNOS, ICAM1, and VCAM1.12 Accumulated evidence shows that exposure of islets to high lipids induces PARP1 expression, concomitant with reduced insulin response to glucose.13,14
Nutrient ingestion stimulates the secretion of gut hormones, including GLP1 and gastric inhibitory polypeptide, to amplify glucose-stimulated insulin release. GLP1 is known to decrease glucagon release and may be beneficial in the reduction of elevated blood glucose.15–17 We recently showed that GLP1 can restore impairment of glucose-stimulated insulin release in the islets of lipid-infused rats, which may be mediated by increased cyclic AMP levels and the suppression of both neuronal cNOS and iNOS.13 The exposure of islets to high glucose in healthy animals resulted in enhanced production of iNOS-derived NO in a short time.14,15 However, the molecular mechanism by which GLP1 exerts its effects remains unclear. In the current research, our purpose was to investigate how the abnormal expression of iNOS in insulin and glucagon cells of ApoE–/– mice and GLP1 works on insulin secretion, and to analyze the mechanism that GLP1 exerts on iNOS in ApoE–/– mice, so as to find a new treatment target for T2DM.
Methods
Reagents
Monoclonal antibodies for PARP1, glucagon, insulin, and iNOS were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) or Cell Signaling Technology (Beverly, MA, USA). Primary antibodies of nitrotyrosine were purchased from Cayman Biochemicals (Ann Arbor, MI, USA). Primary antibodies for COX2 and NFκB p65 were purchased from Abcam. Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Recombinant human GLP1 (rhGLP1; 7–36) was obtained from Huayi Bio-Lab (Shanghai). PARP1 lentivirus or control lentivirus was obtained from Genechem (Shanghai). All other drugs and chemicals were purchased from Merck (Darmstadt, Germany). Radioimmunoassay kits for insulin and glucagon were obtained from Diagnostika (Falkenberg, Sweden) and Euro Diagnostica (Malmö, Sweden), respectively.
Animals
Animal experiments were approved by the Experimental Animal Welfare and Ethics Committee, Shandong University (approval DWLL-2015-006, 30 July, 2015), and conducted in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments)guidelines). Laboratory animals underwent all operations under anesthesia, and every effort was made to minimize pain and death. ApoE–/– mice were from Jackson Laboratories (Bar Harbor, ME, USA) and housed in a pathogen-free animal-care facility with free access to water and food. Mice were divided into six treatment groups: normal diet (n=15), high-fat diet (n=15), high-fat diet treated with GLP1 (n=15), high-fat diet infected with PARP1 lentivirus (n=15), high-fat diet infected with control lentivirus (n=15), and high-fat diet infected with PARP1 lentivirus and treated with rhGLP1group (n=15). The normal diet-group were fed a normal diet for 32 weeks and subcutaneously injected with saline. The remaining mice were fed high-fat diet for 32 weeks. Diabetic mice in the high-fat diet treated with GLP1 group were then subcutaneously injected with 80 μg/kg rhGLP1 for 8 weeks (rhGLP1 was diluted with 10 mL sterile saline). Diabetic mice in the high-fat diet with PARP1 lentivirus–infected group and high-fat diet infected with control-lentivirus group were subcutaneously injected with 5 μL PARP1 lentivirus or control lentivirus (109 TU/mL) for 8 days. After PARP1-lentivirus infection, mice serving as the high-fat diet infected with PARP1 lentivirus and treated with rhGLP1 were then subcutaneously injected with 80 μg/kg rhGLP1 for 8 weeks. Blood samples were collected by angular venipuncture in mice fasted overnight to measure total cholesterol (TC), triglycerides (TGs), low-density-lipoprotein cholesterol (LDL-C), and high-density-lipoprotein cholesterol (HDL-C).
Histology and Immunostaining
Pancreata were dissected from mice and fixed in 10% methanol, deparaffinized, rehydrated, and washed in PBS, then stained for 5 minutes with hematoxylin and 2 minutes with eosin. For immunostaining, sections were stained overnight at 4°C with antinitrotyrosine primary antibodies(1:100), then stained by appropriate secondary antibodies at 37°C for 30 minutes. Then, coverslips were sealed using Prolong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA). Images were acquired by laser scanning confocal microscopy (LSM710; Carl Zeiss, Germany) and analyzed using Image-Pro Plus 6.0.
TUNEL Assays
The number of apoptotic cells was evaluated with a TUNEL-assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. In brief, sections were washed with xylene twice, then dipped in ethanol (100%, 95%, 90%, 80%, and 70%) for 3 minutes in each concentration and then incubated in a TUNEL reaction mixture in the dark for 1 hour at 37°C. Labeled samples were visualized under fluorescence microscopy (Olympus, Tokyo, Japan) and analyzed with Zen 2011 software (Carl Zeiss).
ELISAs
Pancreatic tissue was collected and concentrations of IL1β, (ELH-IL1β; RayBiotech), MCP-2, (ELH-MCP2; RayBiotech),IL6 (ELH-IL6; RayBiotech, and TGFβ measured using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. In addition, plasma samples were collected and CRP levels measured using the ELISA kits according to the manufacturer’s instructions.
Western Blot Analysis
Protein was extracted from the pancreata and equal amounts of protein separated with 10% SDS-PAGE and transferred onto nitrocellulose membrane. Membranes were blocked in 5% nonfat milk powder for 2 hours at room temperature and washed three times in TBS-T. Then, membranes were probed with primary antibodies overnight at 4°C. Primary antibodies included anti–β-actin (1:1,000), anti-glucagon (1:2,000), anti-insulin (1:2,000), anti-PARP1 (1:500), anti-iNOS (1:250), COX2 (1:500), and NFκB p65 (1:1,000). After being washed in TBS-T, membranes were incubated with horseradish peroxidase–conjugated secondary antibodies for 2 hours at room temperature. Signals were detected by enhanced chemiluminescence (Millipore) and analyzed using Image-Pro Plus 6.0.
Determination of Glucose-Tolerance, Blood-Glucose, and Blood-Insulin Levels
After 10 hours’ fasting, venous blood glucose in mice was measured with a blood-glucose meter from the tail. Blood-insulin levels were measured using ELISA. Glucose solution (2 mg glucose/g body weight) and insulin (0.5 U/kg body weight) was injected intraperitoneally into the mice and intraperitoneal glucose-tolerance testing (IPGTT) and intraperitoneal insulin-tolerance testing (IPITT) were performed.
Statistical Analysis
Data are expressed as means ± SEM, and were analyzed with Graph-Pad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). Statistical analyses were performed using one-way ANOVA. Differences with p<0.05 were considered statistically significant.
Results
High-Fat Diet Aggravates Islet Injury
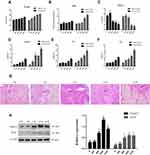
First, we measured fasting blood sugar, weight, HDL-C, LDL-C, TC, and TGs in diabetic mice. The mice fed a high-fat diet showed significant increases in body weight compared to the normal-diet group at 8, 16, and 32 weeks (Figure 1A) and significant increases in fasting blood-sugar levels compared to the normal-diet group at 16 and 32 weeks (p<0.05) (Figure 1B). Moreover, the mice fed the high-fat diet had significantly higher TC, LDL-C, and TG levels, but significantly lower HDL-C levels than the control group at 16 and 32 weeks (p<0.05, Figure 1C–F).
Next, we examined histology of the islets in ApoE–/– mice fed the high-fat diet. H&E staining showed that the morphology of the islets was round or oval in normal mice. In contrast, with increased duration of the high-fat diet, the islets became small, atrophic, and distorted, and had increased inflammatory injury (Figure 1G). Furthermore, we analyzed PARP1 and iNOS expression in islets of ApoE–/– mice fed the high-fat diet. PARP1 and iNOS protein had levels increased significantly at 8, 16, and 32 weeks compared to 0 and 4 weeks (p<0.05, Figure 1H)
GLP1 Recovers Declines in Insulin Sensitivity and Glucose Tolerance in ApoE–/– Mice Fed High-Fat Diet
Next, we investigated blood-glucose tolerance and insulin sensitivity in each group of mice. IPITT values increased significantly in the high-fat diet group compared to the normal-diet group, but GLP1 decreased IPITT values in the high-fat diet group and high-fat diet group with PARP1 treatment (Figure 2A). Similarly, IPGTT values increased significantly in the high-fat diet group compared to the normal-diet group, but GLP1 decreased IPGTT values in the high-fat diet group and high-fat diet group with PARP1 treatment (Figure 2B). Consistently, PARP1 and iNOS expression increased significantly in the high-fat diet group compared to the normal-diet group, but GLP1 decreased PARP1 and iNOS expression in the high-fat diet group and high-fat diet group with PARP1 treatment (Figure 2C).
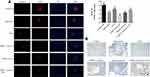
Since iNOS can cause tissue damage via NO radicals, we detected 3-nitrotyrosine (3-NT), a marker for RNS in the damaged pancreas.18,19 Immunohistochemistry staining showed that 3-NT levels increased significantly in the high-fat diet group compared to the normal-diet group, but GLP1 decreased 3-NT levels in thehigh-fat diet group and high-fat diet group with PARP1 treatment (Figure 2D). Taken together, these data indicate that GLP1 may alleviate high-fat diet–induced declines in insulin sensitivity and glucose tolerance, and this is related to increased expression of iNOS and increased production of 3-NT in the islets.
GLP1 Reduces Islet-Cell Apoptosis
Islet β cells and α cells were identified by insulin antibody and glucagon antibody, respectively. Immunostaining showed that the high-fat diet significantly decreased the number of β cells, but increased the number of α cells compared to the control group (1.163±0.145 vs 2.062±0.266, p<0.05). GLP1 inhibited glucagon and improved insulin secretion in islets of high-fat diet–fed mice (1.583±0.125 vs 1.163±0.145, p<0.05). In high-fat diet–fed mice infected with PARP1 lentivirus, the number of β cells had further decreased and the number of α cells further increased compared to high-fat diet–fed mice (0.764±0.121 vs 1.163±0.145, p<0.05), while GLP1 reversed these results (1.470±0.104 vs 0.764±0.121, p<0.05) (Figure 3A). TUNEL assays showed that islet-cell apoptosis was aggregated in mice fed the high-fat diet for 16 weeks compared to the control group, while GLP1 reduced islet-cell apoptosis. Moreover, PARP1 lentiviral infection further increased islet-cell apoptosis compared to the high-fat diet group, while GLP1 inhibited the further increase of apoptosis (Figure 3B).
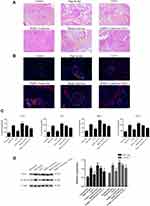
GLP1 Inhibits Islet Inflammation
H&E staining showed that the islets in the high-fat diet group were irregular and had inflammation, while GLP1 alleviated islet injury. Whenhigh-fat diet–fed mice were infected with the PARP1 lentivirus, inflammation in islets was more severe the high-fat diet group, and GLP1 reversed the inflammation (Figure 4A). To confirm pancreatic inflammation, CD8+ T lymphocytes were stained and mononuclear cell infiltration into islet was observed, as previously described. Glucagon was stained to outline a residual islet.20 We found large numbers of CD8+ T lymphocytes inislets of the high-fat diet group, while GLP1 decreased the number of CD8+ T lymphocytes. Infection with PARP1 lentivirus increased the number of CD8+ T lymphocytes infiltrating the islets, and GLP1 reversed the increase in CD8+ T lymphocytes due to PARP1 overexpression (Figure 4B).
Furthermore, we detected concentrations of inflammatory factors in the islets. Compared with the control group, the high-fat diet increased concentrations of IL1β, IL6, TNFα, and MIP2, while GLP1 treatment significantly decreased their concentrations. Infection with PARP1 lentivirus further increased concentrations of inflammatory factors compared with the high-fat diet group, and GLP1 inhibited the increase due to PARP1 overexpression (Figure 4C). To explore the mechanism by which GLP1 inhibits inflammation, we detected expression of COX2 and NFκB. We found that expression of COX2 and NFκB p65 had significantly increased in the islets of the high-fat diet group compared to the control group, but decreased after GLP1 was applied. Infection with PARP1 lentivirus further increased the expression of COX2 and NFκB p65 compared with the high-fat diet group, and GLP1inhibited increased expression of COX2 and NFκB p65 due to PARP1 overexpression (Figure 4D). Collectively, these data indicate that GLP1 protects the islets by inhibiting inflammation.
Discussion
The prevalent hypothesis is that damage to islet function and insulin resistance are the main pathological aspects of T2DM. Recent studies have shown that GLP1 improves glycemic control and reduces islet damage in patients with T2DM.21–23 However, the molecular mechanism by which GLP1 reverses fat-induced islet injury remains poorly understood. In the current study, we found that GLP1 inactivated PARP1 and suppressed islet damage via inhibiting COX2–NFκB pathway–mediated inflammation.
A distinctive feature of T2DM is the dysfunction of insulin-secreting pancreatic β cells. Under hyperglycemic conditions, islet β cells will initially enhance the secretion of insulin in a compensatory way, but after a long period they will develop decompensation and eventually fail, leading to loss of secretion function and DM.24–26 Therefore, increasing the number of islet β cells is an important aspect in effective treatment of T2DM. In the current study, we found that GLP1 alleviated decreases in β cells by inhibiting cell apoptosis induced by the high-fat diet. Consequently, GLP1 recovered the decline in insulin sensitivity and glucose tolerance. Our results are consistent with a previous study reporting that GLP1 protected β-cell viability against streptozocin-induced toxicity, inhibited weight gain, and relieved symptoms of polydipsia.27
Oxidized LDL is a harmful cholesterol produced when LDL-C interacts with free radicals generated by macrophages. Uptake of oxidized LDL by islet β cells leads to oxidative damage, ROS production, and PARP1 activation.28–31 PARP1 is known to activate iNOS expression and enhance NO production, and is involved in a variety of diseases, including chronic obstructive pulmonary disease, atherosclerotic lesions, and DM.32,33 We found that the application of GLP1 suppressed the expression of PARP1 and iNOS and inhibited RNS generation mediated by PARP1. Moreover, the severity of mononuclear cell infiltration was alleviated and the expression of inflammatory factors decreased after GLP1 had been applied, suggesting that GLP1 protected the islets by inhibiting inflammation.
COX2 functions as an inducible enzyme with low or undetectable levels in most tissue types, and its expression can be markedly increased by a number of inflammatory, mitogenic, and physical stimuli.34–36 In the primary stage of inflammation, NFκB is activated and upregulates the expression of COX2 and iNOS, thereby promoting NO production. iNOS also enhances the activation of NFκB, which further promotes inflammation effects.37 In this study, we found that GLP1 suppressed the expression of COX2 and NFκB induced by ahigh-fat diet. These results indicate that GLP1 attenuates islet-inflammation damage by inhibiting COX2 and NFκB pathways. In conclusion, we found that GLP1 significantly alleviated decreased β-cell numbers and suppressed β-cell apoptosis induced by ahigh-fat diet, inhibited the expression of iNOS, and alleviated inflammatory-islet injury via inhibiting the COX2–NFκB pathway.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Tang SM, Yang, ZM, Chen, WQ, et al. Acad J Sec Milit Med Univ. Astragalus polysaccharide improves type 2 diabetes mellitus in rats by protecting islet β cells. 2017;38(4):482–487.
2. Wang WH, Guo, D.Z., Yang, S.J, et al. Activity of iNOS and cNOS in serum and lung of broilers with pulmonary hypertension syndrome. Acta Veterinaria Et Zootechnica Sinica. 2006;37(7):711–716.
3. Bellinger DA, Merricks EP, Nichols TC. Swine models of type 2 diabetes mellitus: insulin resistance, glucose tolerance, and cardiovascular complications. ILAR J. 2006;47(3):243–258. doi:10.1093/ilar.47.3.243
4. Haffner SM. Lipoprotein disorders associated with type 2 diabetes mellitus and insulin resistance. Am J Cardiol. 2002;90(8–supp–S):55–61. doi:10.1016/S0002-9149(02)02634-6
5. Ahmad W, Ijaz B, Shabbiri K, et al. Oxidative toxicity in diabetes and Alzheimer’s disease: mechanisms behind ROS/RNS generation. J Biomed Sci. 2017;24(1):76. doi:10.1186/s12929-017-0379-z
6. Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation. Cardiovasc Diabetol. 2004;3(1):1. doi:10.1186/1475-2840-3-1
7. Husebye ES, Winqvist, O, Sundkvist, G, et al. Autoantibodies against adrenal medulla in type 1 and type 2 diabetes mellitus: no evidence for an association with autonomic neuropathy. Acta Physiol. 1996;239(2):139–146.
8. Soskic SS. Regulation of Inducible Nitric Oxide Synthase (iNOS) and its Potential Role in Insulin Resistance, Diabetes and Heart Failure. Open Cardiovasc Med J. 2011;5(1):153–163. doi:10.2174/1874192401105010153
9. Hallemeesch MM, Janssen, B.J., de Jonge, W.J, et al. NO production by cNOS and iNOS reflects blood pressure changes in LPS-challenged mice. Am J Physiol. 2003;189(9):161–166.
10. Diefenbach J, Bürkle A. Introduction to poly(ADP-ribose) metabolism. Cell mol life sci. 2005;62(7–8):721–730.
11. Sharp C, Warren A, Oshima T, et al. Poly ADP ribose-polymerase inhibitors prevent the upregulation of ICAM-1 and E-selectin in response to Th1 cytokine stimulation. Inflammation. 2001;25(3):157–163. doi:10.1023/A:1011032313445
12. Salehi A, Ekelund M, Henningsson R, et al. Total parenteral nutrition modulates hormone release by stimulating expression and activity of inducible nitric oxide synthase in rat pancreatic islets. Endocrine. 2001;16(2):97–104. doi:10.1385/ENDO:16:2:097
13. Jimenez-Feltstrom J, Lundquist I, Salehi A. Glucose stimulates the expression and activities of nitric oxide synthases in incubated rat islets: an effect counteracted by GLP-1 through the cyclic AMP/PKA pathway. Cell Tissue Res. 2005;319(2):221–230. doi:10.1007/s00441-004-1013-4
14. Henningsson R, Salehi A, Lundquist I. Role of nitric oxide synthase isoforms in glucose-stimulated insulin release. Am J Physiol Cell Physiol. 2002;283(1):C296–C304. doi:10.1152/ajpcell.00537.2001
15. Drucker DJ. Glucagon-like peptide-1 and the islet β-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144(12):5145–5148. doi:10.1210/en.2003-1147
16. Kuhre RE, Wewer Albrechtsen NJ, Hartmann B, et al. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J Diabetes Complications. 2015;29(3):445–450. doi:10.1016/j.jdiacomp.2014.12.006
17. Lim DM, Park, K.Y., Hwang, W.M, et al. Difference in protective effects of GIP and GLP?1 on endothelial cells according to cyclic adenosine monophosphate response. Exp Ther Med. 2017;13(5):2558–2564.
18. Ito T, Nagaoka K, Nagaoka K, et al. Relationship of particulate matter and ozone with 3-nitrotyrosine in the atmosphere. Environ Pollut. 2018;236:948. doi:10.1016/j.envpol.2017.10.069
19. Hui YWM, Zhao SS, Love JA, Ansley DM, Chen DD, Chen DDY. A simple and robust LC-MS/MS method for quantification of free 3-nitrotyrosine in human plasma from patients receiving on-pump CABG surgery. Electrophoresis. 2012;33(4):697–704. doi:10.1002/elps.201100368
20. Itoh N, Hanafusa, T., Miyazaki, A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J clin invest. 1993;92(5):2313–2322.
21. Mather KJ, Considine RV, Hamilton L, et al. Combination GLP-1 and insulin treatment fails to alter myocardial fuel selection vs. insulin alone in type 2 diabetes. J Clin Endocrinol Metab. 2018;103(9):3456–3465. doi:10.1210/jc.2018-00712
22. Nystrom T. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. AJP. 2004;287:E1209.
23. Isabel DO-GM, Francisco M-TJ. GLP-1 Receptor Agonists and Cardiovascular Disease in Patients with Type 2 Diabetes. J Diabetes Res. 2018;1–12.
24. Tomita T. Apoptosis in pancreatic β-islet cells in Type 2 diabetes. Bosnian J Basic Med Sci. 2016;16:162.
25. Klöppel G, Löhr, M., Habich, K, et al. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Survey synthesis pathol res. 1985;4(2):110–125.
26. Porte D. β-cells in type II diabetes mellitus. Diabetes. 1991;40(2):166–180. doi:10.2337/diab.40.2.166
27. Sun L, et al. Novel pentapeptide GLP‐ 1(32‐36)amide inhibits β‐cell apoptosis in vitro and improves glucose disposal in streptozotocin‐induced diabetic mice. Chem Biol Drug Design. 2016;86(6):1482–1490.
28. Unger RH. The banting memorial lecture 1975: diabetes and the alpha cell. Diabetes. 1976;25(2):136–151. doi:10.2337/diab.25.2.136
29. Cieślar-Pobuda A, Saenko Y, Rzeszowska-Wolny J. Rzeszowska-Wolny, PARP-1 inhibition induces a late increase in the level of reactive oxygen species in cells after ionizing radiation. Mutat Res. 2012;732(1–2):9–15. doi:10.1016/j.mrfmmm.2012.01.005
30. Yang W, Lu J, Weng J, et al. Prevalence of Diabetes among Men and Women in China. N Eng J Med. 2010;362(12):1090–1101. doi:10.1056/NEJMoa0908292
31. Nakhjavani M, Khalilzadeh, O., Khajeali, L, et al. Serum Oxidized-LDL is Associated with Diabetes Duration Independent of Maintaining Optimized Levels of LDL-Cholesterol. Lipids. 2018;454:321–327.
32. Yu Z, Kuncewicz T, Dubinsky WP, et al. Nitric Oxide-dependent Negative Feedback of PARP-1 trans-Activation of the Inducible Nitric-oxide Synthase Gene. J Biol Chem. 2006;281(14):9101–9109. doi:10.1074/jbc.M511049200
33. Perrotta I, Brunelli, E., Sciangula, A, et al. iNOS induction and PARP-1 activation in human atherosclerotic lesions: an immunohistochemical and ultrastructural approach. Cardiovasc Pathol. 2018;20(4):0–203.
34. Harris RC, et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J clin invest. 1994;94(6):2504–2510.
35. Tetsuka T, Daphna-Iken D, Miller BW, et al. Nitric oxide amplifies interleukin 1 induced cyclooxygenase 2 expression in rat mesangial cells. J Clin Invest. 1996;97(9):2051–2056. doi:10.1172/JCI118641
36. Fletcher BS, Kujubu, D.A., Perrin, D.M, et al. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J Biol Chem. 1992;267:4338.
37. Wang Z-Q, Chen, M.T., Zhang, R, et al. Docosahexaenoic acid attenuates doxorubicin-induced cytotoxicity and inflammation by suppressing NF-κB/iNOS/NO signaling pathway activation in H9C2 cardiac cells. J Cardiovasc Pharmacol. 2015;67(4):1.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.