Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Ginkgo biloba Extract EGb 761 in the Treatment of Patients with Mild Neurocognitive Impairment: A Systematic Review
Authors Hort J , Duning T, Hoerr R
Received 7 January 2023
Accepted for publication 4 March 2023
Published 23 March 2023 Volume 2023:19 Pages 647—660
DOI https://doi.org/10.2147/NDT.S401231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Jakub Hort,1 Thomas Duning,2 Robert Hoerr3
1Memory Clinic, Department of Neurology, Charles University, 2nd Faculty of Medicine and Motol University Hospital, Prague, Czech Republic; 2Department of Neurology, Klinikum Bremen-Ost, Bremen, Germany; 3Research and Development, Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany
Correspondence: Robert Hoerr, Research and Development, Dr. Willmar Schwabe GmbH & Co. KG, Willmar-Schwabe-Str. 4, Karlsruhe, 76227, Germany, Tel +49 721 4005 429, Email [email protected]
Background: Many clinical trials testing Ginkgo biloba extract EGb 761 in patients with mild forms of cognitive impairment were conducted before widely accepted terms and diagnostic criteria for such conditions were available. This makes it difficult to compare any results from earlier and more recent trials. The objective of this systematic review was to provide a descriptive overview of clinical trials of EGb 761 in patients who met the diagnostic criteria for mild neurocognitive disorder (mild NCD) according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5).
Methods: MEDLINE, PubMed and EMBASE were searched for randomized, placebo-controlled double-blind trials of EGb 761 in mild impairment of cognitive functioning. All trials involving patients who met retrospectively applied diagnostic criteria for mild NCD were included. Trials of primary prevention of dementia and trials of combinations of medical treatments were excluded.
Results: Among 298 records found in databases and 76 further records related to EGb 761 in references of systematic reviews, 9 reports on clinical trials involving 946 patients met the pre-specified criteria for inclusion. Beneficial effects of EGb 761 were seen in neuropsychological tests (8 of 9 studies), scales for neuropsychiatric symptoms (3 of 3 studies), geriatric rating scales (1 of 2 studies) and global ratings of change (1 of 1 study). Significant effects were found in several domains of cognition (memory, speed of processing, attention and executive functioning). Among the neuropsychiatric symptoms, depression (2 of 3 studies) and anxiety (1 of 1 study) were significantly improved. No differences between EGb 761 treatment and placebo were seen with regard to the rates of adverse events.
Discussion: The included studies demonstrate treatment benefits of Ginkgo biloba extract EGb 761, mainly on cognitive deficits and neuropsychiatric symptoms, in patients with mild NCD. The drug was safe and well tolerated.
Keywords: Ginkgo biloba, EGb 761, mild neurocognitive disorder, NCD, mild cognitive impairment, systematic review
Introduction
The Ginkgo biloba extract EGb 761 has been assessed in more than 40 clinical trials involving patients with a variety of clinical conditions. Data from older trials in cognitive disorders may be difficult to interpret, because diagnostic terms for cognitive impairment and diagnostic criteria are different from those commonly used today. In this review, we summarize the evidence for clinical efficacy of EGb 761 in mild neurocognitive disorder (mild NCD) while taking into account most recent guideline developments and diagnostic criteria.
Cognitive impairment is a condition frequently seen in the elderly. Detailed diagnostic criteria for dementia syndromes and Alzheimer’s disease (AD) have been available and commonly used since 1980s (Diagnostic and Statistical Manual of Mental Disorders, third edition, DSM-III)1 (National Institute of Neurological and Communicative Disorders/Alzheimer’s Disease and Related Disorders Association, NINCDS-ADRDA).2 On the other hand, until the end of 1990s, a variety of terms were used for milder forms of aging-associated or early cognitive impairment related to a specific pathology (eg amyloid-related early stages of AD). Scientists coined terms such as impairment of cerebral functions, cerebrovascular insufficiency or organic brain syndrome, and they introduced other terms representing more or less different concepts, such as benign senescent forgetfulness,3 age-associated memory impairment (AAMI),4 aging-associated cognitive decline (AACD)5 and cognitive impairment – no dementia (CIND).6 The term “mild cognitive impairment” (MCI), although previously used to denote pre-dementia conditions of cognitive impairment,7 became prominent when defined by Petersen et al in the late 1990s.8,9 The concept of the MCI syndrome and the diagnostic criteria were developed further by Petersen and an international working group10,11 and later integrated into the modern systematology of the Alzheimer’s disease continuum.12 By contrast, an International Working Group (IWG) led by B. Dubois referred to the MCI patient population using the label of prodromal AD.13,14 With the taxonomy of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) of 2013, the concept of mild neurocognitive disorder (mild NCD) was introduced, with additional criteria for sub-classification by etiology.15
Cholinesterase inhibitors, which are first-line treatments in Alzheimer’s disease dementia, have shown minor symptomatic effects in patients with MCI,16 whereas in ApoE4 carriers 3-year treatment with donepezil reduced the risk of progression to dementia.17 Studies of anti-amyloid substances have yielded more or less inconsistent results.18,19 Concerns have been expressed with regard to safety and tolerability of such treatments in patients with mild symptoms.16,19 Patients whose ailments are still mild and not too distressing are likely to be more compliant, if a treatment with proven efficacy is safe and well tolerable.
In neuropathology studies of MCI patients, mixed pathology was observed most frequently, predominantly including features of Alzheimer’s disease and cerebro-vascular lesions.20 This ties in with findings from clinically healthy older adults followed longitudinally in the Harvard Aging Brain Study. Patients with both amyloid pathology on positron emission tomography (PET) scans and a high vascular risk score showed faster cognitive decline than those with a single pathology.21 In summary, the view that considers mainly mono-etiological pathologies is outdated. In addition to cerebrovascular pathologies, other neurodegenerative comorbidities (eg synucleinopathies, tauopathies, Lewy bodies, etc.) are also common.20
Ginkgo biloba extract EGb 761 (a proprietary extract of Dr. Willmar Schwabe GmbH & Co. KG) has shown vaso-regulatory, vaso-protective and blood flow-enhancing properties in clinical trials.22–25 In animal models, it inhibited the formation of toxic amyloid-oligomers, attenuated amyloid toxicity and improved neurogenesis and synaptogenesis.26–28 It also showed antioxidant activity and protection of mitochondrial function against various toxicities,23,29 a process that is strongly linked to Alzheimer’s disease pathophysiology.30
Clinical trials assessing the effects of EGb 761 on cognitive functions were conducted long before internationally or even globally recognized terms, concepts and diagnostic criteria for the condition now called MCI or mild NCD existed. Most of these studies demonstrated improvements in cognitive performance that were comparable to later studies, which enrolled patients with criteria-based diagnoses of MCI or dementia. Based on these results, the Herbal Medicinal Products Committee (HMPC) of the European Medicines Agency (EMA) recommended the use of EGb 761 for “the improvement of (age-associated) cognitive impairment and of quality of life in mild dementia.”31 Expert consensus papers and practice guidelines of several countries also recommend EGb 761 for the treatment of MCI.32,33 The beneficial effects of EGb 761 in mild-to-moderate dementia have been summarized by several systematic reviews and meta-analyses.34,35 However, in older studies of patients with MCI, the variability of previous inclusion criteria and the lack of precisely defined and widely accepted diagnostic procedures were difficult to interpret. With the implementation of the DSM-5 and the retrospective application of its diagnostic criteria,36 a systematic review of the older studies together with those conducted in the early 2000s became possible.
Both the DSM-537 and the core diagnostic criteria of the National Institute on Aging – Alzheimer’s Association workgroups (NIA-AA)12 for MCI require the independence in functional abilities to be preserved, although subtle difficulties with complex functional tasks are acceptable. Acknowledging that the clinical characteristics of patients with MCI (due to AD) may overlap with those at the milder end of the (AD) dementia spectrum and that these two stages of the disease are not clearly demarcated, the “Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease” issued recently by the European Medicines Agency permits subjects with MCI and mild dementia to be studied together.38 This has, in fact, already been done in trials of anti-amyloid agents.39,40
Objectives
The objective of this paper is to review the data on efficacy and safety of EGb 761 in the treatment of mild NCD, also encompassing studies dating back to 1980s which enrolled only patients with mild NCD as well as those which admitted patients with mild NCD and mild stages of major NCD. Outcomes of interest are cognitive performance, neuropsychiatric symptoms, geriatric assessments, and global impression of change.
Methods
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement41,42 was taken into account.
Eligibility Criteria
We included randomized, placebo-controlled, double-blind clinical trials in patients diagnosed with mild NCD who were treated with the Ginkgo biloba extract EGb 761 as the only drug treatment for cognitive deficits for at least 8 weeks. Patients had to meet the mild NCD criteria of the DSM-5.37 Studies that enrolled mainly patients with mild NCD but did not strictly exclude mild dementia (ie, mild stage of major NCD) were also included. Studies were only considered eligible if the outcome measures were appropriate to assess therapeutic efficacy in patients with mild NCD, ie, standardized neuropsychological tests, established scales to assess neuropsychiatric symptoms, geriatric rating scales (eg, Sandoz Clinical Assessment Geriatric, SCAG) and/or Clinical Global Impression (CGI) scales.
The following were deemed ineligible: cohort studies, studies assessing primary preventive effects, studies of Ginkgo biloba products not containing EGb 761 as active substance, studies of patients with diagnoses not compatible with mild NCD (eg, aging-associated memory impairment as defined by Crook et al,4 subjective cognitive decline), studies of EGb 761 as add-on treatment for cognitive deficits (eg, EGb 761 and cholinesterase inhibitors) or studies in which the outcome measures were not appropriate to assess therapeutic efficacy in patients with mild NCD. We also excluded studies published in languages other than English, French, German, Czech, Spanish, Italian or Portuguese.
Information Sources and Search Strategy
We searched the electronic databases Embase (between 1947 and 12 May 2022), Medline (between 1946 and 06 May 2022) and PubMed (from the beginning until 06 May 2022) for eligible studies. Briefly, the search strategy included the terms “ginkgo” (as well as common abbreviations and brand names for ginkgo extracts), “maidenhair tree”, “mild neurocognitive disorder”/“mild NCD”, “mild cognitive impairment”/“MCI”, “preclinical AD”, “randomized controlled trial” and common variations of these terms. A full search strategy is presented in Online Supplementary Content.
We adopted two further approaches to identify relevant studies: 1) screening the references of the systematic reviews retrieved through the searches above, and 2) screening the references of a former publication,36 in which the diagnoses for inclusion in randomized clinical trials of EGb 761 were retrospectively classified according to the DSM-5.
Selection Process
Two reviewers independently screened the retrieved records but used a common approach: Firstly, they eliminated duplicates by manual screening; secondly, they screened the titles of all articles for relevance, including systematic reviews. If titles did not reveal relevance or irrelevance, abstracts were then screened; if the abstracts did not provide enough information to ascertain eligibility, the reviewers screened the full text. Finally, the reviewers’ ratings of the articles were compared, then disagreements were discussed to reach a consensus.
Data Collection Process, Data Items Collected and Effect Measures
Data were extracted from published study reports as presented. The following outcomes were considered eligible: cognitive function, behavioral and psychological symptoms of dementia (BPSD), global assessments (CGI, geriatric rating scales, dementia rating scales), functional measures (activities of daily living, ADL), quality of life, adverse events (AEs) and serious adverse events (SAEs). We considered changes from baseline (for each group or difference between groups), response rates or both, as reported in the published study reports.
In addition to the outcome measurements, the following data were also collected: author and year of publication, diagnosis according to the DSM-5 and original diagnosis reported in the publication, main inclusion criteria, treatment dose and duration, number of patients included in the analyses.
Due to the heterogeneity of the study designs and outcome measures, the methodological criteria to perform meta-analyses were not met.
Study Risk of Bias Assessment
We used the Jadad score43 to assess the risk of bias in the included studies. This instrument addresses bias in three domains: randomization, blinding and withdrawals/dropouts. Scores can range between 0 (high risk of bias) to 5 (low risk of bias).
Results
Study Selection
Of 395 records identified from databases, 106 duplicates were removed, and 289 records were screened for eligibility. Of these, 269 could be excluded due to the information in the title or abstract, for 20 records the published reports were retrieved. Based on the information provided by the reports, two studies were identified as meeting our eligibility criteria and 18 had to be excluded. See PRISMA flow diagram (Figure 1) for details. The reference lists of the 12 systematic reviews identified by database searches and of the former publication focusing on DSM-5 criteria36 yielded 148 further records, of which 72 duplicates were removed and 67 could be excluded due to the information given in the reviews and in the titles. The published reports were retrieved for nine records. Seven studies meeting our eligibility criteria were identified using this approach (Figure 1). Altogether, nine studies involving 946 patients could be included in this review.
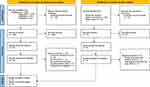 |
Figure 1 PRISMA flow diagram of the literature search of this systematic review. Adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.41 |
Study Characteristics and Outcome Measures
Nine studies that met the selection criteria had been published between 1987 and 2019. Detailed study characteristics are provided in Table 1. Daily doses of EGb 761 ranged between 120 mg and 240 mg, and treatment periods were between 8 weeks and 52 weeks. Daily doses of 240 mg were applied in 3 studies (2 of which were conducted after the year 2000), whereas daily doses of 120 mg or 160 mg were used in 6 studies (5 of which were conducted before the year 2000). Seven studies followed a parallel group design with two groups, one treated with EGb 761, the other one receiving placebo. In one study,44 patients were randomly assigned to one of four parallel groups, two of which were treated with EGb 761, while the other two received placebo. One of the EGb 761 groups and one of the placebo groups underwent additional standardized memory training. Another study45 was run with three parallel groups, receiving a traditional Chinese medicine (TCM) treatment, EGb 761 or placebo.
 |
Table 1 Randomized, Placebo-Controlled, Double-Blind Clinical Trials of EGb 761 in Patients with Mild NCD – Description of Included Trials |
The trials were heterogeneous with respect to the outcome measures. Neuropsychological tests were administered in all trials. In two trials, tests or test batteries were used for which summary scores were reported.45,46 Seven trials used specific tests for single cognitive domains.44,47–52 Geriatric assessment scales were administered in two trials;51,52 one used the clinician’s global impression of change (CGI-C) rating.47 In one trial that included MCI patients with clinically significant neuropsychiatric symptoms (NPS), a rating scale for NPS (Neuropsychiatric Inventory, NPI) was administered;47 two trials focused on depressive symptoms in patients with mild NCD using the Hamilton Depression Scale (HAMD).51 In one trial, a computerized test battery consisting of six validated tests of information processing49,53 was administered and scores were combined for the domains speed and accuracy.52 One trial focused on memory, using an elaborate “memory battery” to assess four different aspects of memory.44 In a one-year trial, the progression from MCI to dementia was analyzed.45 One study included patients with very mild (borderline to slightly abnormal cognitive function) and mild (clearly abnormal cognitive function) cognitive impairment. Ceiling effects were therefore expected (and found) in patients with very mild impairment, due to the limited sensitivity of available tests. Therefore, the conduct and reporting of subgroup analyses, which included patients with more pronounced cognitive impairment (ie clearly abnormal cognitive function) were specified in advance by the protocol.48
Risk of Bias in Studies
Jadad scoring supports the assumption of a low risk of bias for most of the studies included. Six studies achieved a score of 5, one study a score of 4 and two studies a score of 3; recent studies tended to score higher (Table 1). The Jadad rating does not take into account concealment of treatment allocation. However, with identical appearance of drug and placebo tablets, identical packaging and indistinguishable labelling, effective concealment was assured in the seven trials for which the double-blinding procedures were described appropriately, and it was likely for the two trials, which were merely described as double-blinded.
Efficacy Results of Individual Studies
The published results of the nine studies are shown in detail in Table 2. Superiority of EGb 761 compared to placebo in cognitive tests was reported in all studies, except in one that mainly focused on depression scales.49 Treatment effects were reported for composite neuropsychological test scores (Alzheimer’s Disease Assessment Scale – cognitive subscale, ADAS-cog; Mini-Mental Status Examination, MMSE; SKT short cognitive performance test), tests of working memory, short- and long-term memory (short-term retention capacity,54 Appointments Test, Wechsler Memory Scale (WMS-III) Faces I and II; Israël’s Memory Battery), neuropsychological tests of attention (Trail-Making Test – Form A, TMT-A, Vienna Test System – Attention Performance Series, WTS-ALS) and executive functioning (TMT-B). In the three studies which enrolled patients with mild NCD and symptoms of depression or other significant neuropsychiatric symptoms at baseline, changes in specific rating scales (NPI, HAMD) indicated significant benefits of EGb 761 treatment. A geriatric rating scale (SCAG), an observer global assessment (CGIC), and a rating of treatment satisfaction also found superiority of EGb 761 over placebo. A rating scale for dementia severity (Clinical Dementia Rating – Sum of Boxes, CDR-SB) used in patients with mild NCD showed a trend in favor of EGb 761, whereas an ADL scale for patients with MCI (Alzheimer’s Disease Cooperative Study ADL Scale for MCI, ADCS-ADL-MCI-24) showed a significant treatment effect of EGb 761. In a trial with a 52-week treatment period, 1 of 104 patients (0.96%) treated with EGb 761, but 7 of 70 patients (10%) of the placebo group progressed to dementia (p = 0.0076).
 |
Table 2 Randomized, Placebo-Controlled, Double-Blind Clinical Trials of EGb 761 in Patients with Mild NCD – Results |
Safety Results
Numbers and percentages of patients who experienced AEs or SAEs were reported only for the most recent trials.45,47,48 In all three trials, there was no difference between EGb 761 and placebo groups regarding the proportions of patients with AEs. During 52-week treatment, 41% and 43% of patients taking 160 mg per day of EGb 761 or placebo, respectively, reported AEs.45 In studies of EGb 761 at 240 mg per day, in 46% of those randomized to active drug and in 45% of those on placebo, AEs were observed during a 24-week treatment period,47 whereas 15% of patients of each treatment group experienced AEs during a 12-week period.48 In two trials, no SAEs were observed;47,48 only SAEs from active treatment groups were reported from the third trial,45 but none of these SAEs was considered to be causally related to EGb 761. Most frequently reported AEs were headache, gastrointestinal symptoms (eg dyspepsia, diarrhea and constipation), respiratory tract infections and urinary tract infections. AE information from all older trials is limited, because events were reported only for active treatment groups, or only if they were suspected to be side effects or if they led to study discontinuation. In more recent studies, numbers for specific AEs were reported at different levels of precision47,48 or not at all,45 so that a representation across studies is not possible.
Discussion
Overall, nine randomized, placebo-controlled, double-blind trials involving 946 patients demonstrated significant clinical effects of the Ginkgo biloba extract EGb 761 in patients with mild NCD. Significant improvements in cognitive function, neuropsychiatric symptoms, overall geriatric assessments, and global ratings of change were reported. Consistent positive effects were found for more specified neuropsychological tests in several cognitive domains including memory, attention, processing speed and executive functioning. Improvements in neuropsychiatric symptoms including depression and anxiety were also reported.
The limitations of this review are the retrospective application of the DSM-5 diagnostic criteria and the heterogeneity of the trials with respect to design and outcome measures. No widely recognized scientific recommendations or guidelines were available when the early trials were performed. This is due to the lack of clear diagnostic guidelines or diagnostic criteria at that time. The US Food and Drug Administration (FDA) published a draft guideline for the development of drugs for the treatment of early-stage Alzheimer’s disease55 only in 2013, and the European Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease first included a short section on studies in patients with MCI in 2018.38 This, to some extent, explains the heterogeneity of study designs, phrasing of inclusion diagnoses and choice of endpoints in the EGb 761 trials.
The review was restricted to studies in patients who met the criteria for a defined diagnostic entity, ie mild NCD. This may, however, weaken the power of the data presented, because some studies included similar patients and their findings could corroborate the favorable effects of EGb 761 on factors such as neuropsychological performance.56–58 However, these studies were excluded, since it could not be ascertained that the patients who were enrolled fulfilled the diagnostic criteria for mild NCD.
When the early trials of EGb 761 were conducted, most of the tests to evaluate cognitive functioning were designed to detect, characterize and assess the severity of cognitive deficits. Thus, many of these tests had a rather low sensitivity as well as a statistical ceiling effect regarding treatment-related changes over time or measurement of smaller differences between treatment groups. Measurements of minor cognitive or neuropsychological changes in patients with very mild symptoms in the early stages of the disease are still subject to further discussion, eg, in the context of current disease-modifying antibody therapies for the treatment of Alzheimer’s disease.40,59 Here, too, many authors have discussed the pronounced ceiling effect of eg the CDR-SB or the MMSE as the primary endpoint.
While cognitive assessments for clinical drug trials in dementia due to Alzheimer’s disease were available in the early 1980s (eg ADAS-cog;60 Brief Cognitive Rating Scale, BCRS),61 the development of neuropsychological tests focused on treatment trials in MCI began only in the late 1990s. At this time, Mohs et al extended the ADAS-cog by adding two items, which addressed cognitive abilities that are typically impaired at the MCI stage of dementia disorders.62 Even later, with the advent of potentially disease-modifying therapies, further instruments were designed for the assessment of neuropsychological changes in early stages of Alzheimer’s disease (eg Alzheimer’s Disease Cooperative Study – Preclinical Alzheimer Cognitive Composite, ADCS-PACC;63 Alzheimer’s Disease Composite Score, ADCOMS).64 The availability of these new assessments may lead to more homogeneous trials with better comparability of results in the future but are still significantly less established than the well-known tests, such as the MMSE or the CDR.
In terms of neuropsychological domains, the most consistent effects of EGb 761 treatment were seen for memory (eg Appointments Test, WMS-III Faces I/II and Israël’s Memory Battery); clearcut effects were also found for attention (WTS-ALS), processing speed (TMT-A) and executive functioning (TMT-B). This is in line with findings reported by Kaschel65 in a review of clinical trials of Ginkgo extracts in patients with less well-specified cognitive deficits. Interestingly, memory impairment is characteristic for MCI and dementia due to Alzheimer’s disease,12 whereas impaired processing speed, executive functioning and attention are considered typical for vascular NCD.66 In fact, composite scores of neuropsychiatric tests (ADAS-cog, SKT) showed superiority of EGb 761 over placebo in patients with Alzheimer’s disease and vascular dementia.35,67 The specific cognitive domains mainly covered by these tests are attention, memory, speed of processing and executive functioning.
Some domain-specific neuropsychological tests apparently were not sensitive enough to clearly distinguish between treatments in patients with borderline or very mildly impaired cognitive functioning. One reason may be that at the very early stages of neurocognitive disorders, many patients have deficits only in one or two domains, so the numbers of patients with deficits in specific domains and therefore the statistical power may be too low. This renders the demonstration of between-group differences in tests for individual domains difficult. Composite scores from test batteries and rating scales, which assess cognitive abilities together with performance in daily life and neuropsychiatric symptoms (eg SCAG) may therefore be more useful in studies of patients with mild NCD.
In mild NCD, cognitive deficits have no major impact on activities of daily living (ADL) but may render instrumental activities more difficult to perform. This is in line with the lack of effect of EGb 761 on the number of activities still possible or still practiced,52 whereas a significant improvement was found in an ADL scale specifically designed for use in mild NCD.45 The observed improvement in neuropsychiatric symptoms concurs with the results of trials in dementia, as shown in a meta-analysis by Savaskan.68 This is noteworthy, because patients with MCI have a higher risk of progressing to dementia and a lower chance for reverting to normal, if they have neuropsychiatric symptoms.69
As expected, EGb 761 was well tolerated. The rates of adverse events and suspected adverse drug reactions were similar in the placebo groups, the rates of serious adverse events were very low, and clinical examinations, ECG and lab tests did not reveal significant changes.
Conclusion
Overall,36,45 the randomized, placebo-controlled trials included in this systematic review provide evidence of efficacy of Ginkgo biloba extract EGb 761 in the treatment of patients with mild neurocognitive disorder. Improvements in cognitive performance, neuropsychiatric symptoms, geriatric rating scales and clinical global impression were demonstrated. The drug was safe and well tolerated.
Acknowledgments
We thank René Lattmann, M.Sc., Department of Psychology, Faculty for Medicine and Health Sciences, Carl von Ossietzky University Oldenburg, for independently screening search results and scrutinizing retrieved records for eligibility.
Funding
The work was supported by Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany.
Disclosure
Jakub Hort received consulting fees and speaker honoraria from Dr. Willmar Schwabe GmbH & Co. KG, grants from the National Institute for Neurological Research (Programme EXCELES, ID Project No. LX22NPO5107) – Funded by the European Union – Next-Generation EU and IPE2 2. LF UK Grant No. 6980382; he also owns stock options for and is an advisory board member of Alzheon. This company is developing a treatment for Alzheimer's disease. Thomas Duning received honorariums and travel expenses from Genzyme, Shire, Bristol-Myers Squibb, Boehringer-Ingelheim Pharma, Sanofi Aventis, Eisai, Novartis, Bayer Vital, Merz Pharma, Actelion, Roche, Schwabe and Amicus to serve as a speaker and consultant. Thomas Duning received research support from Genzyme, Shire, Amicus and Actelion. To conduct studies on dementia, Thomas Duning received grants from Novartis, Merz Pharma, Roche and Biogen. Robert Hoerr is a full-time employee of Dr. Willmar Schwabe GmbH & Co. KG and receives a fixed salary. The authors report no other conflicts of interest in this work.
References
1. American Psychiatric Association. Neurocognitive Disorders. Diagnostic and Statistical Manual of Mental Disorders (DSM-3).
2. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi:10.1212/wnl.34.7.939
3. Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J. 1962;86(6):257–260.
4. Crook T, Larrabee GJ. Age-associated memory impairment: diagnostic criteria and treatment strategies. Psychopharmacol Bull. 1988;24(4):509–514.
5. Levy R. Aging-associated cognitive decline. Int Psychogeriatr. 1994;6(1):63–68. doi:10.1017/s1041610294001626
6. Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52(6):612–619. doi:10.1001/archneur.1995.00540300086018
7. Zaudig M. A new systematic method of measurement and diagnosis of “Mild Cognitive Impairment” and dementia according to ICD-10 and DSM-III-R criteria. Int Psychogeriatr. 1992;4(Suppl. 2):203–219. doi:10.1017/S1041610292001273
8. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9(Suppl 1):65–69. doi:10.1017/s1041610297004717
9. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi:10.1001/archneur.56.3.303
10. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi:10.1111/j.1365-2796.2004.01388.x
11. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi:10.1111/j.1365-2796.2004.01380.x
12. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi:10.1016/j.jalz.2011.03.008
13. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi:10.1016/s1474-4422(07)70178-3
14. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi:10.1016/s1474-4422(14)70090-0
15. American Psychiatric Association. Diagnostic and Statistical Manual of mental disorders (DSM-5).
16. Matsunaga S, Fujishiro H, Takechi H. Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis. 2019;71(2):513–523. doi:10.3233/JAD-190546
17. Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi:10.1056/NEJMoa050151
18. Jeremic D, Jiménez-Díaz L, Navarro-López JD. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: a systematic review. Ageing Res Rev. 2021;72:101496. doi:10.1016/j.arr.2021.101496
19. Haddad HW, Malone GW, Comardelle NJ, Degueure AE, Kaye AM, Kaye AD. Aducanumab, a novel anti-amyloid monoclonal antibody, for the treatment of Alzheimer’s disease: a comprehensive review. Health Psychol Res. 2022;10(1):31925. doi:10.52965/001c.31925
20. Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171–186. doi:10.1007/s00401-017-1717-7
21. Rabin JS, Schultz AP, Hedden T, et al. Interactive associations of vascular risk and beta-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the Harvard aging brain study. JAMA Neurol. 2018;75(9):1124–1131. doi:10.1001/jamaneurol.2018.1123
22. Erdincler DS, Karakoc Y, Toplan S, et al. The effect of Ginkgo biloba glycoside on the blood viscosity and erythrocyte deformability. Clin Hemorheol. 1996;16(3):271–276.
23. Lang F, Hoerr R, Noeldner M, Koch E. Ginkgo biloba extract EGb 761: from an ancient asian plant to a modern european herbal medicinal product. In: Wagner H, Ulrich-Merzenich G, editors. Evidence and Rational Based Research on Chinese Drugs. Springer Vienna; 2013:431–470.
24. Huang SY, Jeng C, Kao SC, Yu JJ, Liu DZ. Improved haemorrheological properties by Ginkgo biloba extract (Egb 761) in type 2 diabetes mellitus complicated with retinopathy. Clin Nutr. 2004;23(4):615–621. doi:10.1016/j.clnu.2003.10.010
25. Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? A systematic review and meta-analysis. Pharmacotherapy. 2011;31(5):490–502. doi:10.1592/phco.31.5.490
26. Luo Y, Smith JV, Paramasivam V, et al. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci U S A. 2002;99(19):12197–12202. doi:10.1073/pnas.182425199
27. Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21(10):2400–2408. doi:10.1096/fj.06-7649com
28. Tchantchou F, Lacor PN, Cao Z, et al. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J Alzheimers Dis. 2009;18(4):787–798. doi:10.3233/JAD-2009-1189
29. Muller WE, Eckert A, Eckert GP, et al. Therapeutic efficacy of the Ginkgo special extract EGb761 within the framework of the mitochondrial cascade hypothesis of Alzheimer’s disease. World J Biol Psychiatry. 2019;20(3):173–189. doi:10.1080/15622975.2017.1308552
30. Jurcău MC, Andronie-Cioara FL, Jurcău A, et al. The link between oxidative stress, mitochondrial dysfunction and neuroinflammation in the pathophysiology of Alzheimer’s disease: therapeutic implications and future perspectives. Antioxidants. 2022;11(11):2167. doi:10.3390/antiox11112167
31. European Medicines Agency - Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Ginkgo Biloba L., Folium. European Medicines Agency; 2015.
32. Kasper S, Bancher C, Eckert A, et al. Management of mild cognitive impairment (MCI): the need for national and international guidelines. World J Biol Psychiatry. 2020;21(8):579–594. doi:10.1080/15622975.2019.1696473
33. Kandiah N, Chan YF, Chen C, et al. Strategies for the use of Ginkgo biloba extract, EGb 761, in the treatment and management of mild cognitive impairment in Asia: expert consensus. CNS Neurosci Ther. 2021;27:149–162. doi:10.1111/cns.13536
34. Hashiguchi M, Ohta Y, Shimizu M, Maruyama J, Mochizuki M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J Pharm Health Care Sci. 2015;1:14. doi:10.1186/s40780-015-0014-7
35. Gauthier S, Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761 in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging. 2014;9::2065–77. doi:10.2147/CIA.S72728
36. Hoerr R, Zaudig M. A retrospective classification of diagnoses in terms of DSM-5 for patients included in randomized controlled trials of Ginkgo biloba extract EGb 761. Eur Arch Psychiatry Clin Neurosci. 2016;266(3):249–259. doi:10.1007/s00406-015-0632-y
37. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; 2013.
38. European Medicines Agency - Committee for Medicinal Products for Human Use (CHMP). Guideline on the Clinical Investigation of Medicines for the Treatment of Alzheimer’s Disease. European Medicines Agency; 2018.
39. Aisen PS, Cummings J, Doody R, et al. The future of anti-amyloid trials. J Prev Alzheimers Dis. 2020;7(3):146–151. doi:10.14283/jpad.2020.24
40. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210. doi:10.14283/jpad.2022.30
41. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
42. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi:10.1136/bmj.n160
43. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4
44. Israel L, Dell’accio E, Martin G, Hugonot R. Extrait de Ginkgo biloba et exercices d’entrainement de la mémoire. Évaluation comparative chez des personne âgées ambulatoires. [Ginkgo biloba extract and memory training programs. Comparative assessment on elderly outpatients living in the community]. Psychol Med. 1987;19(8):1431–1439. French.
45. Tian J, Shi J, Wei M, et al. Chinese herbal medicine Qinggongshoutao for the treatment of amnestic mild cognitive impairment: a 52‐week randomized controlled trial. Alzheimers Dement. 2019;5(1):441–449. doi:10.1016/j.trci.2019.03.001
46. Halama P, Bartsch G, Meng G. Hirnleistungsstörungen vaskulärer Genese. Randomisierte Doppelblindstudie zur Wirksamkeit von Gingko–biloba–Extrakt. [Disorders of brain performance of vascular origin. Randomized double-blind study of the effectiveness of Ginkgo biloba extract]. Fortschr Med. 1988;106(19):408–412. German.
47. Gavrilova SI, Preuss UW, Wong JW, et al. Efficacy and safety of Ginkgo biloba extract EGb 761® in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int J Geriatr Psychiatry. 2014;29(10):1087–1095. doi:10.1002/gps.4103
48. Grass-Kapanke B, Busmane A, Lasmanis A, Hoerr R, Kaschel R. Effects of Ginkgo biloba special extract EGb 761 in very mild cognitive impairment (vMCI). Neurosci Med. 2011;02(01):48–56. doi:10.4236/nm.2011.21007
49. Stocksmeier U, Eberlein M. Depressive Verstimmung bei Hirnleistungsstörungen. [Depressive mood in cerebral dysfunction. The effects of a Ginkgo biloba extract tested in a double-blind study]. TW Neurologie Psychiatrie. 1992;6:74–76. German.
50. Gräßel E. Einfluß von Ginkgo-biloba-Extrakt auf die geistige Leistungsfähigkeit. Doppelblindstudie unter computerisierten Meßbedingungen bei Patienten mit Zerebralinsuffizienz [The influence of Ginkgo biloba extract on mental performance. A double blind study under computerized measurement conditions in patients with cerebral insufficiency]. Fortschr Med. 1992;110(5):73–78. German.
51. Schubert H, Halama P. Primär therapieresistente depressive Verstimmung älterer Patienten mit Hirnleistungsstörungen: Wirksamkeit der Kombination von Ginkgo-biloba-Extrakt EGb-761 mit Antidepressiva. [Primary therapy-resistant depressive instability in elderly patients with cerebral disorders: efficacy of a combination of Ginkgo biloba extract EGb 761 with antidepressants]. Geriatrie Forschung. 1993;3(1):45–53. German.
52. Wesnes K, Simmons D, Rook M, Simpson P. A double-blind placebo-controlled trial of Tanakan in the treatment of idiopathic cognitive impairment in the elderly. Hum Psychopharmacol. 1987;2:159–169. doi:10.1002/hup.470020305
53. Wesnes K. A fully automated psychometric test battery for human psychopharmacology.
54. Lehrl S, Fischer B. The basic parameters of human information processing: their role in the determination of intelligence. Person Individ Diff. 1988;9(5):883–896. doi:10.1016/0191-8869(88)90006-2
55. Food and Drug Administration. U.S. Department of Health and Human Services. Alzheimer’s Disease: Developing Drugs for the Treatment of Early Stage Disease, DRAFT GUIDANCE for Industry. Food and Drug Administration; 2013.
56. Dieli GLM, Saetta V, Costanzo M. Studio clinico in doppio cieco del Tanakan nell’ insufficienza cerebrale cronica. [Double-blind clinical trial with Tanakan in chronic cerebral insufficiency]. Il Lavoro Neuro Psichiatrico. 1981;68(1/2):3–15. Italian.
57. Taillandier JA, Rabourdin JP, Ribeyre JP, Pichon J, Niddam S, Pierart H. Traitement des troubles du vieillissement cérébral par l’extrait de Ginkgo biloba. [Treatment of cerebral aging disorders with Ginkgo biloba extract]. Presse Med. 1986;15:1583–1587. French.
58. Hofferberth B. Einfluss von Ginkgo biloba-Extrakt auf neurophysiologische und psychometrische Messergebnisse bei Patienten mit hirnorganischem Psychosyndrom. Eine Doppelblindstdie gegen Placebo. [The effect of Ginkgo biloba extract on neurophysiological and psychometric measurement results in patients with psychotic organic brain syndrome. A double-blind study against placebo]. Arzneim-Forsch. 1989;39(8):918–922. German.
59. Cohen S, Cummings J, Knox S, Potashman M, Harrison J. Clinical trial endpoints and their clinical meaningfulness in early stages of Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(3):507–522. doi:10.14283/jpad.2022.41
60. Mohs RC, Rosen WG, Davis KL. The Alzheimer’s disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull. 1983;19(3):448–450.
61. Reisberg B, London E, Ferris SH, Borenstein BA, Scheier L, de Leon MJ. The brief cognitive rating scale: language, motoric, and mood concomitants in primary degenerative dementia. Psychopharmacol Bull. 1983;19(4):702–708.
62. Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S13–S21. doi:10.1097/00002093-199700112-00003
63. Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71(8):961–970. doi:10.1001/jamaneurol.2014.803
64. Wang J, Logovinsky V, Hendrix SB, et al. ADCOMS: a composite clinical outcome for prodromal Alzheimer’s disease trials. J Neurol Neurosurg Psychiatry. 2016;87(9):993–999. doi:10.1136/jnnp-2015-312383
65. Kaschel R. Ginkgo biloba: specificity of neuropsychological improvement – a selective review in search of differential effects. Hum Psychopharmacol. 2009;24(5):345–370. doi:10.1002/hup.1037
66. Sachdev PS, Lipnicki DM, Crawford JD, Brodaty H. The vascular behavioral and cognitive disorders criteria for vascular cognitive disorders: a validation study. Eur J Neurol. 2019;26(9):1161–1167. doi:10.1111/ene.13960
67. von Gunten A, Schlaefke S, Überla K. Efficacy of Ginkgo biloba extract EGb 761 in dementia with behavioural and psychological symptoms: a systematic review. World J Biol Psychiatry. 2016;17(8):622–633. doi:10.3109/15622975.2015.1066513
68. Savaskan E, Mueller H, Hoerr R, von Gunten A, Gauthier S. Treatment effects of Ginkgo biloba extract EGb 761 on the spectrum of behavioral and psychological symptoms of dementia: meta-analysis of randomized controlled trials. Int Psychogeriatr. 2018;30(3):285–293. doi:10.1017/S1041610217001892
69. McGirr A, Nathan S, Ghahremani M, Gill S, Smith EE, Ismail Z. Progression to dementia or reversion to normal cognition in mild cognitive impairment as a function of late-onset neuropsychiatric symptoms. Neurology. 2022;98(21):e2132–e2139. doi:10.1212/WNL.0000000000200256
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
