Back to Journals » OncoTargets and Therapy » Volume 13
Gigantol Attenuates the Metastasis of Human Bladder Cancer Cells, Possibly Through Wnt/EMT Signaling
Authors Zhao M, Sun Y , Gao Z, Cui H, Chen J, Wang M, Wang Z
Received 6 July 2020
Accepted for publication 14 October 2020
Published 4 November 2020 Volume 2020:13 Pages 11337—11346
DOI https://doi.org/10.2147/OTT.S271032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Meili Zhao,1,2 Yangyang Sun,3 Zhen Gao,3 Hongqiu Cui,1,2 Jianbin Chen,1,2 Meina Wang,1,2 Zhicai Wang1– 3
1Key Laboratory of National Forestry and Grassland Administration for Orchid Conservation and Utilization (Shenzhen), Shenzhen 518114, People’s Republic of China; 2Shenzhen Key Laboratory for Orchid Conservation and Utilization, The National Orchid Conservation Center of China and the Orchid Conservation & Research Center of Shenzhen, Shenzhen 518114, People’s Republic of China; 3Key Laboratory of Medical Reprogramming Technology, Shenzhen Second People’s Hospital, The First Affiliated Hospital of Shenzhen University, Shenzhen 518035, People’s Republic of China
Correspondence: Zhicai Wang; Meina Wang
The National Orchid Conservation Center of China and the Orchid Conservation & Research Center of Shenzhen, Shenzhen 518114, People’s Republic of China
Email [email protected]; [email protected]
Background: Bladder cancer has long been recognized as one of the most common and aggressive human malignant carcinomas due to the increased invasiveness and metastasis. The discovery and development of natural compounds from Dendrobium species for cancer therapy have garnered increasing attention in recent years. Among those natural elements, the bibenzyl compound gigantol has promising therapeutic potential against several cancer cell lines; however, its roles on bladder tumor metastasis have not been investigated.
Materials and Methods: Here in this in vitro study, we utilized viability tests, cell migration, cell invasion and apoptosis assays to evaluate the anti-tumor activity of gigantol on three human bladder cancer cell lines (SW780, 5637, and T24) and a normal human bladder cell line (SVHUC-1). Cells were treated with different concentrations of gigantol (0, 40, 80, and 160 μM) for 24, 48 and 72 h.
Results: Here in this study, we showed that gigantol suppressed cancer cell proliferation but not normal SVHUC-1 cells. The inhibitory effect of the compound on cell migration and invasion was also exhibited in the cancer cell lines. Cell apoptosis assay by flow cytometry revealed enhanced apoptotic effects of gigantol on cancer cells. Gene expression analysis revealed that Wnt/EMT signaling might involve in the response of bladder cancer cells to gigantol.
Conclusion: Therefore, the present data demonstrate gigantol as a strong anticancer reagent against bladder cancer possibly through Wnt/EMT signaling.
Keywords: bladder cancer, tumor metastasis, Dendrobium, gigantol
Introduction
Bladder cancer, the most predominant genitourinary malignancy is the 9th most common cancer and the 13th most common cause of cancer death worldwide, representing the 7th most prevalent in men and the 17th most prevalent in women.1 Cigarette smoking is the greatest established risk factor for bladder cancer with the highest incidence rates in Europe, the United States, and Egypt,2 while other risk factors include aging, genetic variation, Schistosoma haematobium infection, occupational exposure to aromatic amines and hydrocarbons.3,4 The most common symptom in 85% patients is haematuria, while other clinical presentations may include urinary urgency and painful urination.5 Localized urothelial carcinoma of the bladder is broadly classified as non-muscle invasive bladder cancer (80% cases, do not typically pose threats to survival but easily recur), muscle-invasive bladder cancer (15% cases, clinically aggressive and usually fatal), and the remaining 5% presenting with metastases.6 It is evidenced that the patient experience for people with bladder cancer is worse than affected individuals with other cancers,7 and that the most bladder cancer patients have a chronic disease that requires continued surveillance and regular, long-term follow-up, making it one of the most expensive tumors to manage on a per-patient basis and imposing heavy economic burden to the patients and healthcare system.8 Failure is usually due to occult metastatic diseases present at the time of diagnosis. Metastasis is characterized as a dissemination of primary tumors to secondary sites, consisting of several sequential steps, cell migration, invasion, intravasation, extravasation, and establishment of secondary tumors. Cancer migration and invasion are the initial steps and important prerequisites for successful metastasis; inhibition of cancer motility can cause metastasis suppression.9
Recently, natural compounds from herbal plants have attracted increasing attention as major parts of alternative medicines because of the plants’ abundance, compound diversity, and cost-effectiveness.10,11 Several classes of such bioactive compounds including bibenzyl have been identified in medicinal Dendrobium plants previously.12,13 Dendrobium in the family Orchidaceae has more than 1100 species which are widely distributed throughout Asia and Australia.14 As a representative of small molecule, Gigantol (3′,4-dihydroxy-3,5′-dimethoxy-bibenzyl), a bibenzyl phenolic compound frequently found in the stem of medicinal Dendrobium,15 has been shown to have a wide range of pharmacological activities without adverse effects, eg, anti-inflammation,16,17 anti-osmosis,18 anti-oxidant,18 anti-spasm,19 anti-nociception,17 anti-diabetic cataracts,20 anti-platelet aggregation,21 anti-mutagenesis,22 and immune modulation.16 In addition, gigantol has significant anti-tumor activity as evidenced in several cancer cell lines, including lung cancer,23,24 liver cancer,25 and breast cancer.26
Based on our previous works, which revealed that recurrence and death rates of human bladder cancer remained high,27 and that gigantol induced growth inhibition and apoptosis of human breast cancer cells via Wnt/β-catenin signaling,26 we speculated that gigantol as representatives of small molecule from Dendrobium species, might also have inhibitory effects on bladder cancer tumorigenesis. However, certain information regarding the tumor growth attenuation and the underlying mechanism are still required. The present study investigated the regulatory role of gigantol on bladder cancer cell proliferation, migration, invasion and apoptosis using CCK-8 cell counting, wound healing, transwell assays, and flow cytometry and further explored the possible molecular mechanism through qRT-PCR and Western blot analysis of Wnt signaling and epithelial–mesenchymal transition (EMT)-related genes. Our results demonstrated that gigantol was effective to attenuate the metastatic behavior of human bladder cancer cells. The findings gained from the present study may support the development and further investigation of gigantol for cancer therapy.
Materials and Methods
Reagents and Equipment
Dimethyl sulfoxide (DMSO) was purchased from Amresco (OH, USA), reverse transcription system kit and quantitative real-time PCR (qRT-PCR) kit from Toyobo Co. Ltd (Osaka, Japan), Phenol-free RPMI-1640 medium, DMEM, and F12K medium, fetal bovine serum (FBS), phosphate-buffered saline (PBS), penicillin/streptomycin, and 0.25% trypsin from Gibco Chemical. Co. (MA, USA), CCK-8 cell counting kit from TransGen Biotech Co. Ltd (Beijing, China), Gigantol from MCE (NJ, USA), TRIzol reagents from Invitrogen (Thermo Fisher Scientific, New Zealand). The human bladder cancer cell lines, SW780, 5637, and T24, and a normal urothelial cell line SVHUC-1 were acquired from Institute of Cell Biology, Chinese Academy of Science (Shanghai, China). UV spectra was recorded using an ELISA microplate reader (Bio-Rad Laboratories Inc., CA, USA). Images were taken using an inverted fluorescence microscope and camera system (Olympus Co., Tokyo, Japan). The Annexin V-PE/7-AAD apoptosis detection kit was provided by BD PharmingenTM (Shanghai, China). The antibodies against β-Actin, Axin2, Survivin, Slug, and Vimentin proteins were purchased from Affinity Biosciences (Melbourne, Australia). All other reagents used were of analytical grade and acquired from local vendors in China.
Cell Culture and Treatment
Cell lines were cultured as monolayers in phenol-free RPMI-1640 (for 5637), DMEM (for T24 and SW780), or F12K mediums (for SVHUC-1) supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin in a humidified chamber with 5% CO2 at 37°C and passaged at near confluent density with trypsin-EDTA. Gigantol powder was dissolved in DMSO, and stock sample was further diluted in culture medium to the appropriate concentration. The final concentration of DMSO in all assays did not exceed 0.1% (v/v), a concentration that did not affect cell viability. Cells were incubated with gigantol (0, 40, 80 and 160 μM) for the indicated times. Experiments were performed in triplicate. The research protocol using commercially available human cell lines was reviewed and approved by the Institutional Ethics Committee of the First Affiliated Hospital of Shenzhen University and the research was carried out in accordance with the principles of the Declaration of Helsinki.
Cell Viability
Cell viability was examined by Cell Counting Kit-8 (CCK-8) assay as previously described.27 Briefly, cells were seeded onto 96-well flat-bottom plats (1×104 cells/well) during exponential growth and incubated overnight for cell attachment. Subsequently, cells were treated with various concentrations of gigantol (0, 40, 80 and 160 μM) for 0, 24, 48 and 72 h. At the end of incubation for indicated period, 100 μL fresh medium with 10% CCK-8 solution was replaced into each well and incubated in the dark for another 3 h at 37°C. The absorbance was read at 450 nm using a microplate reader.
Cell Migration
Cell migration was determined using the wound healing assay. Cells were seeded onto 6-well plates (1×106 cells/well) and grown to confluence. The monolayer cells were vertically scratched with a sterile micropipette tip (200 µL) to generate a wound space. The cells were then washed thrice with PBS to remove cell debris and replaced with fresh serum-free medium containing various concentrations of gigantol (0, 40, 80 and 160 μM) for 24 h. Migrating cells across the wounded space were recorded under an inverted microscope at the indicated time. The relative cell migration was measured by using Image J software (NIH, MD, USA).
Cell Invasion
Cell invasion was assessed by transwell invasion assay using 24-well matrigel pre-coated culture inserts (BD Biosciences, CA, USA). The upper chamber of each insert was plated with 1×105 cells suspended in FBS-free medium, while the lower chamber was filled with 500 µL FBS-free medium to allow for cell attachment overnight. The next day, medium in the upper and the lower chambers was refreshed with medium containing various concentrations of gigantol (0, 40, 80 and 160 μM). Twenty percent FBS was added specifically to the lower chamber. After 24 h of incubation, the leftover cells were removed from upper side of membrane with a cotton swab, whereas invaded cells on the bottom layer surface were rinsed in PBS, fixed with methanol for 20 min, stained with 0.1% crystal violet dye for 25 min, followed by thrice PBS washing. The invaded cells were photographed and analyzed under an inverted microscope with a digital camera.
Quantitative Real-Time PCR Analyses (qRT-PCR)
5637 cells were treated with gigantol (0 and 80 μM) for 48 h. Cells were collected and homogenized for total RNA isolation using TRIzol reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. Total RNA (1 μg) was reverse-transcribed into cDNA using the Revertra Ace qPCR RT Kit with gDNA Eraser (Toyobo Co. Ltd, Japan). qRT-PCR analysis was performed with SYBR Green Premix Kit (Toyobo Co. Ltd, Japan) in the ABI PRISM 7500 Fluorescent Quantitative PCR System (Thermo Fisher Scientific). GAPDH was used as the internal control; other genes and the primers are listed in Table 1.
 |
Table 1 Primers for qRT-PCR Analysis |
Cell Apoptosis Assay
Bladder cancer cell lines SW780 and T24 were seeded in 6-well plates (1x106 cells/well) and treated with different concentrations of gigantol (0, 40, 80, and 160 μM) for 24 h. Cell apoptosis was determined by using flow cytometry. Cells were digested with trypsin (non-EDTA), followed by thrice PBS washing, and then collected and re-suspended in 500 μL binding buffer. The cells were co-mixed with 5 μL of Annexin V-PE and 5 μL of 7-AAD and incubated for 15 min at room temperature in darkness. The proportion of apoptotic cells was immediately analyzed by using flow cytometry (EPICS XL-4, Beckman Counter, USA).
Western Blot Analysis
Bladder cancer 5637 cells were seeded in 6-well plates (1x106 cells/well) and left to grow overnight. Cells were treated with medium containing varied concentrations of gigantol (0, 40, 80, and 160 μM). After 24 h incubation, cells were collected, washed with cold PBS, and lysed with cold RIPA buffer containing the protease inhibitor cocktail. Protein concentration in the cell lysates was measured using the BCA protein assay kit (Thermo Fisher Scientific). Equal amounts of total cell extracts (30 μg protein/lane) were separated by molecular weight on 10% SDS-PAGE gels and electrotransferred onto PVDF membranes. The membranes were then blocked with TBST buffer (Tris-buffered saline and 0.05% Tween-20) containing 5% skimmed milk for 1 h, and incubated with the specific primary antibodies at 4°C overnight. After thrice TBST washing for 10 min each, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG for 1 h at room temperature. Subsequently, the membranes were washed with TBST three times and visualized using the ECL System (GE Healthcare, Italy) according to the protocol.
Statistical Analysis
Present data were collected from at least triplicate samples and expressed as means ± SD. Data processing and statistical analyses were performed using GraphPad Prism 8 software package (La Jolla, CA, USA) and the statistically significant differences were considered at *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, and the lowercase letters indicate the significance (P<0.05) according to one-way ANOVA tests.
Results
Gigantol Suppressed Cell Growth and Migration of Bladder Cancer Cells
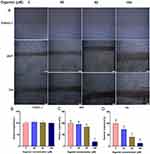
The effect of gigantol on the viability of bladder cancer cells was measured by CCK-8 cell counting assay. Human bladder cancer cell lines (SW780: G1 grade; 5637: G2 grade; T24: G3 grade) generated from different original tumor grades (G1/G2: low grade; G3: high grade)28 and a normal bladder cell line (SVHUC-1) were treated with varied concentrations of gigantol (0, 40, 80 and 160 μM) for indicated times (0, 24, 48 and 72 h). Results showed that gigantol had no significant effect on cell growth of SVHUC-1 cells at all concentrations indicated (Figure 1A), while an obvious reduction of cell growth could be first detected in response to gigantol at a concentration of 40 μM in both SW780 and 5637 cell lines (Figure 1B and C). Gigantol at concentrations of more than 40 μM caused a significant decrease in viability, with approximately 38% and 28% of SW780 cells remaining viable in response to 80 μM and 160 μM of gigantol treatment for 48 h, and with about 61% and 83% growth reduction of 5637 cells under 80 μM and 160 μM treatment of gigantol for 48 h, respectively. Gigantol reduced the viability of bladder cancer cells in a dose- and time-dependent manner with IC50 values (48 h) of 109.7 μM in SW780 cells and 186.5 μM in 5637 cells. Gigantol exhibited less toxicity to higher grade T24 cells as compared with lower grade bladder cancer cells with an observable growth reduction only at 160 μM for 48 h of gigantol treatment (Figure 1D). These results indicated that gigantol has selective cytotoxicity in lower grade bladder cancer cells.
Cancer cell migration is one of the several first key steps during metastasis, allowing for cell detachment from primary site to another where secondary tumors were established. To further evaluate the effect of gigantol on bladder cancer motility, wound-healing assay was performed. Bladder cancer cells 5637 and T24 representing low and high tumor grade, respectively, were treated with various concentrations of gigantol (0, 40, 80, and 160 μM) for 24 h. As shown in Figure 2A, gigantol suppressed the migration of 5637 and T24 cells to the scratched area compared to untreated control cells in a dose-dependent manner. However, the migration of gigantol treated normal SVHUC-1 cells was not significantly affected (Figure 2B). Approximately 16.5% and 80.5% reductions in motile activity were found in the 5637 cells treated with 80 μM and 160 μM of gigantol for 24 h (Figure 2C), and about 26%, 59% and 86% inhibition of cancer motility were recorded in T24 cells incubated with 40 μM, 80 μM and 160 μM of gigantol for 24 h, respectively (Figure 2D). These results clearly demonstrated that gigantol significantly suppresses the migratory behavior of bladder cancer cells.
Gigantol Inhibited Cell Invasion and Wnt/EMT Signaling
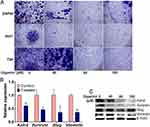
Cancer cell migration and invasion are integral steps for cancer metastasis, which prompted us to further verify the possible anti-invasiveness function of gigantol on bladder cancer cells using matrigel pre-coated transwell assays. Cancer cells representing low-grade tumor (SW780 and 5637) and high-grade tumor (T24) were seeded onto the pre-coated transwell inserts and treated with various concentrations of gigantol (0, 40, 80, and 160 μM) for 48 h. Results showed that gigantol significantly reduced the number of invaded cancer cells at all stages checked in a dose-dependent manner when compared with nontreated control cells (Figure 3A). A sharp decrease of cell invasiveness was first detected in SW780 and 5637 cells at 40 μM of gigantol incubation, while T24 cells exhibited less sensitivity to gigantol (Figure 3A). These results suggested that gigantol has strong inhibitory effects on bladder cancer cell invasion.
To explore the possible molecular mechanisms of gigantol antitumor activity, we analyzed the effect of gigantol on the expression of two well-known Wnt target genes (Axin2 and Survivin) and two EMT marker genes (Slug and Vimentin). Attached 5637 cells were treated with gigantol (0 and 80 μM) for 48 h. The results of qRT-PCR showed that the mRNA expression of Axin2, Survivin, Slug, and Vimentin was significantly decreased after gigantol treatment in bladder cancer 5637 cells (Figure 3B). These results suggested that gigantol might inhibit Wnt signaling and EMT process in bladder cancer cells.
To re-confirm the inhibitory effects of gigantol on Wnt/EMT signaling in bladder cancer cells, we investigated the effect of gigantol on the expression of the two Wnt targets (Axin2 and Survivin) and the two EMT marker proteins (Slug and Vimentin) by Western blot analysis. Attached 5637 cells were treated with indicated concentrations of gigantol (0, 40, 80, and 160 μM) for 48 h. The results showed that gigantol significantly reduced the expression levels of Axin2, Survivin, Slug and vimentin in a dose-dependent manner (Figure 3C), which is in good agreement with their expression at RNA level. Together, these results suggested that gigantol has an ability to suppress Wnt/EMT signaling in bladder cancer cells.
Gigantol Enhanced Cancer Cell Apoptosis
We further evaluated cell apoptosis using the Annexin V-PE and 7-AAD double-staining assay by flow cytometry. SW780 and T24 cells were incubated with gigantol (0, 40, 80, and 160 μM) for 24 h. Our results showed an increased ratio of apoptotic cells with increased concentrations of gigantol in both SW780 and T24 cells (Figure 4), indicating the positive role of gigantol in stimulating cancer cell apoptosis.
Discussion
Metastasis of cancer cell from one primary site to a distant secondary site for tumor initiation is a fundamental cause of death in bladder cancer patients, with increasing rates of incidences and mortality worldwide.29 Therefore, the development of effective anti-cancer therapy focusing on reduction of the metastasis rate is highly needed. It has been reported that several naturally derived compounds have been identified for inhibition of cancer metastasis.23–25 Our previous study also demonstrated that gigantol, a stilbenoid derivative originally isolated from medicinal Dendrobium, suppressed the viability and migration of breast cancer cells via inhibition of Wnt/β-catenin signaling.26 Following an ongoing effort to isolate and characterize bioactive compounds from medicinal plants for treating cancer, the present study has provided further evidence supporting the potential of gigantol as biological agent for bladder cancer therapy. Our results demonstrated that gigantol significantly inhibited cell viability, migratory behavior, and attenuated cell invasion, but strongly induced cell apoptosis in bladder cancer cells.
Charoenrungruang et al23 reported that gigantol inhibited viability of non-small cell lung cancer H460 cells with IC50 value of 247.55 μM. In comparison with their finding, we previously found that gigantol suppressed the viability of breast cancer cells with IC50 values of 115.2 μM in MDA-MB-231 cells and 103.6 μM in MDA-MB-468 cells,26 which were comparable with the present study in bladder cancer cells with IC50 values of 109.7 μM in SW780 cells and 186.5 μM in 5637 cells when incubated with gigantol (Figure 1), suggesting the sensitivities of lower grade bladder cancer cell towards this compound. In contrast, the growth of non-tumorigenic bladder cell SVHUC-1 was not affected at the indicated concentrations (Figure 1), suggesting that gigantol had selective cytotoxicity against cancer cells. We also detected a timely and sensitive response in the decrease of cell viability in lower grade cancer cells at 40 μM for 24 h (Figure 1).
Our previous results and others have shown that gigantol had cytotoxic activity against breast, liver, and lung cancer cells23,25,26 and that gigantol could attenuate certain aggressive phenotypes of cancer cells including proliferation, migration, and invasion, etc.24,30 In consistent with these reports, the present study demonstrated that gigantol suppressed bladder cancer cell proliferation, migration, and invasiveness. Interestingly, our results revealed that gigantol was more cytotoxic to lower grade bladder cancer cells (SW780 and 5637, G1/2 grade) than higher grade cell line T24 (G3 grade) (Figure 1), which was in good agreement with the invasive behavior of cells to gigantol treatment, with SW780 and 5637 cells more sensitive than T24 cells (Figure 3A).
It has been demonstrated that activated Wnt signaling and epithelial–mesenchymal transition (EMT) played vital roles in tumor establishment and that gigantol inhibited cell viability and migration by decreasing the functions of Wnt signaling and EMT process.26,30 The Wnt/β-catenin signaling cascade can be activated when secreted Wnt proteins bind to its coreceptor LRP5/6 and subsequent phosphorylation of Dishevelled (DVL), which stabilizes β-catenin to activate transcription of Wnt target genes including Axin2 and Survivin.19,31 If aberrantly activated, it can contribute to the initiation, progression and metastasis of various human cancers.32 We previously identified gigantol as a novel inhibitor of Wnt/β-catenin signaling in breast cancer cells through inhibition of LRP6 phosphorylation and cytosolic β-catenin accumulation.26 The present study further expanded the mechanism of action of this small molecule to bladder cancer and found that gigantol significantly inhibited the expression of two Wnt target genes, Axin2 and Survivin.
Gigantol could decrease β-catenin expression and “stemness” in lung cancer cells.24,30 It was worth noting that the levels of proteins belonging to the Wnt pathway, which is also related to cancer stem cells (CSCs) phenotypes, were altered under gigantol treatment,33 and that the expression of CSC markers was significantly reduced after 48 h of gigantol incubation.24 Recent study has suggested that CSCs, a group of specialized cells within the tumor, use their ability of self-renewal and differentiation for tumor initiation, progression, metastasis, and cancer recurrence,34 and that CSCs phenotypes are also related to EMT.35 As EMT is a conversion of the terminally differentiated epithelial cells back to their mesenchymal states with an increase in mesenchymal-related proteins, namely, Slug and Vimentin,36 it is very similar to the initiation step of the “stemness” phenotype of CSCs. A sharp expression decrease of Slug and/or Vimentin has been observed according to the gigantol treatment.30,37 Although it is still debatable that EMT is one of the CSC behaviors and a required step driving cancer cell towards CSC, our results showing decreased expression of Wnt targets (Axin2 and Survivin) and EMT markers (Slug and Vimentin) have provided further molecular evidences to connect them together (Figure 3B and C). It is therefore possible that gigantol is able to suppress “stemness” of bladder cancer cells through inhibition of Wnt/β-catenin signaling and attenuation of the EMT process. However, the underlining mechanisms are still to be investigated.
Recent studies showed that gigantol inhibited proliferation and induced apoptosis in liver cancer and lung cancer cells,23,25 which is consistent with its apoptosis stimulatory role as evidenced in the present study (Figure 4). Gigantol has also exhibited protective effects against streptozotocin-induced cataracts in rats, possibly via up-regulated expression and activity of aldose reductase (AR) and inducible nitric oxide synthase (iNOS).18 Its inhibitory activity against lung cancer has also been demonstrated in vivo by subcutaneous inoculation of gigantol-pretreated tumor cells into xenograft mice model, causing dramatic decreases in the cell proliferation and relative tumor weight.33 We could assume from the present study that gigantol treatment attenuated the metastatic properties of bladder cancer cells and enhanced cancer cell apoptosis. Although the results helped us scope the direct action of gigantol on bladder cancer cells, further investigation including in vivo verification of the anticancer activities is still needed for gaining more insights.
Conclusion
The present study demonstrated that gigantol as a representative of small molecules originally isolated from medicinal Dendrobium exerted invasiveness and growth inhibition against human bladder cancer cells through Wnt/EMT signaling. The findings of this study provide confirmation of the anticancer effects of gigantol in human bladder cancer, which could be beneficial to the future development of this compound as an antimetastasis treatment for cancer therapy.
Funding
This work was financially supported by the Medical Science and Technology Research Fund Project of Guangdong Province (A2019093), the Research Grants for Postdocs Settle in Shenzhen from Shenzhen Government to Dr. ZC Wang, the Forestry Science and Technology Innovation Project of Guangdong (2017KJCX062) to Dr. MN Wang; and the National Natural Science Foundation of China (81802537) to Dr. Z Gao. The authors are grateful to Dr. WR Huang at Shenzhen Second People’s Hospital for providing some of the regular reagents and research facilities used in this study.
Disclosure
The authors declare that they have no competing interests. The protocols, analytic methods, and materials used in the present study are available from the authors upon reasonable request.
References
1. Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–461. doi:10.1016/j.eururo.2016.05.041
2. Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145. doi:10.1002/ijc.27711
3. Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–241. doi:10.1016/j.eururo.2012.07.033
4. Malats N, Real FX. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 2015;29(2):177–189. doi:10.1016/j.hoc.2014.10.001
5. Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374(9685):239–249. doi:10.1016/S0140-6736(09)60491-8
6. Cheung G, Sahai A, Billia M, Dasgupta P, Khan MS. Recent advances in the diagnosis and treatment of bladder cancer. BMC Med. 2013;11:13. doi:10.1186/1741-7015-11-13
7. Guidance NI. Bladder cancer: diagnosis and management of bladder cancer. BJU Int. 2017;120(6):755–765. doi:10.1111/bju.14045
8. Svatek RS, Hollenbeck BK, Holmang S, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66(2):253–262. doi:10.1016/j.eururo.2014.01.006
9. Fang Y, Chen Y, Yu L, et al. Inhibition of breast cancer metastases by a novel inhibitor of TGFbeta receptor 1. J Natl Cancer Inst. 2013;105(1):47–58. doi:10.1093/jnci/djs485
10. Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med. 2008;23(6):854–859.
11. Shin SA, Moon SY, Kim WY, Paek SM, Park HH, Lee CS. Structure-based classification and anti-cancer effects of plant metabolites. Int J Mol Sci. 2018;19(9):2651. doi:10.3390/ijms19092651
12. Yang LC, Lu TJ, Hsieh CC, Lin WC. Characterization and immunomodulatory activity of polysaccharides derived from Dendrobium tosaense. Carbohydr Polym. 2014;111:856–863. doi:10.1016/j.carbpol.2014.05.007
13. Tian CC, Zha XQ, Pan LH, Luo JP. Structural characterization and antioxidant activity of a low-molecular polysaccharide from Dendrobium huoshanense. Fitoterapia. 2013;91:247–255. doi:10.1016/j.fitote.2013.09.018
14. Zhou XM, Zhang B, Chen GY, et al. Dendrocoumarin: a new benzocoumarin derivative from the stem of Dendrobium nobile. Nat Prod Res. 2018;32(20):2464–2467. doi:10.1080/14786419.2017.1419241
15. Chen MF, Liou SS, Hong TY, Kao ST, Liu IM. Gigantol has protective effects against high glucose-evoked nephrotoxicity in mouse glomerulus mesangial cells by suppressing ROS/MAPK/NF-kappa B signaling pathways. Molecules. 2018;24(1):80. doi:10.3390/molecules24010080
16. Won JH, Kim JY, Yun KJ, et al. Gigantol isolated from the whole plants of Cymbidium goeringii inhibits the LPS-induced iNOS and COX-2 expression via NF-kappa B inactivation in RAW 264.7 macrophages cells. Planta Med. 2006;72(13):1181–1187. doi:10.1055/s-2006-947201
17. Deciga-Campos M, Palacios-Espinosa JF, Reyes-Ramirez A, Mata R. Antinociceptive and anti-inflammatory effects of compounds isolated from Scaphyglottis livida and Maxillaria densa. J Ethnopharmacol. 2007;114(2):161–168. doi:10.1016/j.jep.2007.07.021
18. Fang H, Hu X, Wang M, et al. Anti-osmotic and antioxidant activities of gigantol from Dendrobium aurantiacum var. denneanum against cataractogenesis in galactosemic rats. J Ethnopharmacol. 2015;172:238–246.
19. Gutierrez RM, Solis RV. Relaxant and antispasmodic effects of extracts of the orchid Encyclia michuacana on isolated guinea pig ileum. J Nat Med. 2009;63(1):65–68. doi:10.1007/s11418-008-0280-x
20. Wu J, Lu C, Li X, et al. Synthesis and biological evaluation of novel gigantol derivatives as potential agents in prevention of diabetic cataract. PLoS One. 2015;10(10):e0141092. doi:10.1371/journal.pone.0141092
21. Fan C, Wang W, Wang Y, Qin G, Zhao W. Chemical constituents from Dendrobium densiflorum. Phytochemistry. 2001;57(8):1255–1258. doi:10.1016/S0031-9422(01)00168-6
22. Miyazawa M, Shimamura H, Nakamura S, Kameoka H. Antimutagenic activity of gigantol from Dendrobium nobile. J Agric Food Chem. 1997;45:2849–2853. doi:10.1021/jf9603902
23. Charoenrungruang S, Chanvorachote P, Sritularak B, Pongrakhananon V. Gigantol, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J Nat Prod. 2014;77(6):1359–1366.
24. Bhummaphan N, Chanvorachote P. Gigantol suppresses cancer stem cell-like phenotypes in lung cancer cells. Evid Based Complement Alternat Med. 2015;2015:836564. doi:10.1155/2015/836564
25. Chen H, Huang Y, Huang J, Lin L, Wei G. Gigantol attenuates the proliferation of human liver cancer HepG2 cells through the PI3K/Akt/NF-kappaB signaling pathway. Oncol Rep. 2017;37(2):865–870. doi:10.3892/or.2016.5299
26. Yu S, Wang Z, Su Z, et al. Gigantol inhibits Wnt/beta-catenin signaling and exhibits anticancer activity in breast cancer cells. BMC Complement Altern Med. 2018;18(1):59.
27. Wang Z, Zhou Q, Li A, Huang W, Cai Z, Chen W. Extracellular matrix protein 1 (ECM1) is associated with carcinogenesis potential of human bladder cancer. OncoTargets Ther. 2019;12:1423–1432. doi:10.2147/OTT.S191321
28. Earl J, Rico D, Carrillo-de-Santa-Pau E, et al. The UBC-40 urothelial bladder cancer cell line index: a genomic resource for functional studies. BMC Genomics. 2015;16:403. doi:10.1186/s12864-015-1450-3
29. Xie R, Chen X, Chen Z, et al. Polypyrimidine tract binding protein 1 promotes lymphatic metastasis and proliferation of bladder cancer via alternative splicing of MEIS2 and PKM. Cancer Lett. 2019;449:31–44.
30. Unahabhokha T, Chanvorachote P, Sritularak B, Kitsongsermthon J, Pongrakhananon V. Gigantol inhibits epithelial to mesenchymal process in human lung cancer cells. Evid Based Complement Alternat Med. 2016;2016:4561674. doi:10.1155/2016/4561674
31. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
32. Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hemat. 2016;99:141–149. doi:10.1016/j.critrevonc.2015.12.005
33. Losuwannarak N, Maiuthed A. Gigantol targets cancer stem cells and destabilizes tumors via the suppression of the PI3K/AKT and JAK/STAT pathways in ectopic lung cancer xenografts. Cancers (Basel). 2019;11(12):2032. doi:10.3390/cancers11122032
34. Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25(1):20. doi:10.1186/s12929-018-0426-4
35. Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22(5–6):396–403.
36. Shi Y, Wu H, Zhang M, Ding L, Meng F, Fan X. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol. 2013;8:89. doi:10.1186/1746-1596-8-89
37. Lee K, Nelson CM. New insights into the regulation of epithelial-mesenchymal transition and tissue fibrosis. Int Rev Cel Mol Bio. 2012;294:171–221.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.