Back to Journals » International Journal of Nanomedicine » Volume 15
GFc7 as a Smart Growth Nanofactor for ex vivo Expansion and Cryoprotection of Humans’ Hematopoietic Stem Cells
Authors Hafizi M , Kalanaky S , Fakharzadeh S, Janzamin E, Arjmandi T, Atashi A , Nazaran MH
Received 2 April 2020
Accepted for publication 28 July 2020
Published 21 August 2020 Volume 2020:15 Pages 6263—6277
DOI https://doi.org/10.2147/IJN.S256104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Maryam Hafizi,1 Somayeh Kalanaky,1 Saideh Fakharzadeh,1 Ehsan Janzamin,2 Tarlan Arjmandi,1 Amir Atashi,3,* Mohammad Hassan Nazaran1,*
1Department of Research and Development, Sodour Ahrar Shargh Company, Tehran, Iran; 2Flowcyt Science-Based Company, Tehran, Iran; 3Stem Cell and Tissue Engineering Research Center, Shahroud University of Medical Sciences, Shahroud, Iran
*These authors contributed equally to this work
Correspondence: Amir Atashi; Mohammad Hassan Nazaran Tel/Fax +98 23 3239 45000
; +98 21 8899 2123
Email [email protected]; [email protected]
Background: Nowadays, smart synthesized nanostructures have attracted wide attention in the field of stem cell nanotechnology due to their effect on different properties of stem cells.
Methods: GFc7 growth nanofactor was synthesized based on nanochelating technology as an iron-containing copper chelator nanocomplex. The effect of this nanocomplex on the expansion and differentiation of hematopoietic stem cells (HSCs) as well as its performance as a cryoprotectant was evaluated in the present study.
Results: The results showed that the absolute count of CD34+ and CD34+CD38− cells on days 4, 7, 10 and 13; the percentage of lactate dehydrogenase enzyme on the same days and CD34+CXCR4 population on day 10 were significantly increased when they were treated with GFc7 growth nanofactor in a fetal bovine serum (FBS)-free medium. This medium also led to delayed differentiation in HSCs. One noticeable result was that CD34+CD38− cells cultured in an FBS medium were immediately differentiated into CD34+CD38+ cells, while CD34+CD38− cells treated with GFc7 growth nanofactor in FBS medium did not show such an immediate significant differentiation. De-freezing GFc7-treated CD34+ cells, which were already frozen according to cord blood bank protocols, showed a higher percentage of cell viability and a larger number of colonies according to colony-forming cell assay as compared to control.
Conclusion: It can be claimed that treating HSCs with GFc7 growth nanofactor leads to quality and quantity improvement of HSCs, both in terms of expansion in vitro and freezing and de-freezing processes.
Keywords: expansion, GFc7, human hematopoietic stem cells, nanocomplex, nanochelating technology
Introduction
Hematopoietic stem cells (HSCs) were first used more than half a century ago and the United States Food and Drug Administration (FDA) have approved HSCs transplant for use against malignancies.1,2 However, the low number of HSCs is the major obstacle to their broader utilization in cell therapy. Despite rapid expansion of HSCs, in vitro studies show that controlling HSCs self-renewal and differentiation has still remained challenging. This problem suggests that additional methods and growth factors for expansion of HSCs are required in vitro studies to tackle this limitation.3,4
It is reported that using frozen HSCs separated from umbilical cord blood (UCB) after 20 years had no negative effect on cell viability and function in HSCs transplant. Although the side effects associated with dimethyl sulfoxide (DMSO) are already recognized, it is still being used as the top standard cryoprotectant in cryoprotection used in UCB banking.5–7
Nanotechnology is the synthesis of functional structures on nanoscale, which results in materials with new chemical, biological and physical characteristics as well as novel conductivity, reactivity, functional conformation and smart structures.8,9 These smart structures provide proper responses, including anti-oxidative, adhesive, migrating and proliferative ones, to physiochemical stimuli in cells in a particular, controlled way.10
Nanotechnology helps stem cells utilize their therapeutic potential for the treatment and repair of damaged tissues. The combination of stem cells and nanotechnology has emerged as a new field of study called “stem cell nanotechnology”. This technology has attracted considerable attention since it greatly contributes to improving regenerative medicine and tissue engineering.11,12
Recently, novel nanostructures have been produced by nanochelating technology with the aim of treating various diseases such as cancer and diabetes.13,14
In the previous study, GFc7 growth nanofactor was synthesized based on nanochelating technology by self-assembly method and its effect on expansion of human mesenchymal stem cells (hMSCs) was analyzed from various aspects. The results showed that GFc7 growth nanofactor improved cell proliferation and maintained the pluripotency properties of hMSCs.15
In the present study, the effect of GFc7 growth nanofactor on the expansion of primitive HSCs (CD34+ and CD34+CD38− cells) as a well‐established marker of human progenitor cells,16–18 their differentiation in ex vivo culture, cell-protection capacity against oxidative stress and quality and quantity after cryoprotection was evaluated.
Materials and Methods
Materials and Instruments
Materials
GFc7 growth nanofactor was synthesized by Sodour Ahrar Shargh Company [Iran]. Nonessential Amino Acids, GlutaMAX, Penicillin G (100 U/mL), Streptomycin (100 mg/mL) and Phosphate-Buffered Solution (PBS) were purchased from Gibco-Life Technologies [USA]. Fetal Bovine Serum (FBS) was taken from PAA Biotech [Austria], hydrogen peroxide (H2O2) from Merck [Germany] and Dimethyl Sulfoxide (DMSO) and Dextran 40 from Cryosure [Germany]. Propidium Iodide (PI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide 99% (MTT), Stemline® II Hematopoietic Stem Cell Expansion Medium, stem cell factor (SCF), Flt-3 ligand (Flt-3L), thrombopoietin (TPO) and Iscove’s Modified Dulbecco’s Media (IMDM) were purchased from Sigma-Aldrich-St Louis [USA]. All antibodies were purchased from Dako [Denmark]. Standard SYBR Green PCR kit was taken from Fermentas [Germany], ALDEFLUOR™ Kit and Methylcellulose from stem cell technologies [Canada] and CD34 and CD38 MicroBead Kit from Milteny Biotec [USA]. Hydroxyl ethyl starch (HES) was purchased from Fresenius Kabi [Germany], Sepax processing kit from Biosafe [Switzerland] and Ficoll-Paque PLUS density gradient from Amersham Biosciences [USA].
Instruments
The list of equipment and instruments is as follows:
Cooling Device [Coolmix-210, Biosafe, Switzerland]. Control Rate Freezer [Planer, UK]. Absorbance Micro Plate Readers [ELx800TM; BioTek, USA]. Flow Cytometry (FACS) [Becton Dickinson, USA]. Rotor Gene 6000 instrument [Corbett, Australia]. Sepax [Biosafe, Switzerland]. Inverted Microscopy (Olympus, USA).
GFc7 Growth Nanofactor
GFc7 growth nanofactor is an iron-containing copper chelator nanocomplex. This new growth factor for the expansion of stem cells was synthesized based on a nanotechnology patented in the United States Patent and Trademark Office (USPTO) with the subject of “Chelate Compounds”.19 The synthesis method is thoroughly explained in the previous study.15
High-resolution transmission electron microscopy (HRTEM) images of GFc7 growth nanofactor were captured by a transmission electron microscope in the University of Tehran Science and Technology Park according to a standard protocol.20
To specify the porosity of GFc7 nanoparticles, BET (Brunauer, Emmett and Teller) Surface Area Analysis was conducted at Tehran University using Quanta Chrome Instruments, version 2.2. In addition, Dynamic Light Scattering for Nanoparticle Size Analysis was conducted using DLS device – Malvern brand, nano ZS (red badge) ZEN 360 model – at KEFA laboratory, Sharif University of Technology.
CD34+ and CD34+CD38− Cells Isolation and Culture
Fresh UCB samples were provided by Iran National Cord Blood Bank (Tehran, Iran).21 Mononuclear Cells (MNCs) of UCB samples were separated by Ficoll-Paque PLUS density gradient according to the manufacturer’s instruction.22 Afterwards, PI solution was used to evaluate cells viability by FACS analysis. In the next stage, CD34+ and CD34+CD38− cells were separated from MNCs.
Isolating CD34+ Cells
According to MicroBeads manufacturer’s instruction, CD34+ cells were isolated from MNCs. Magnetic CD34 MicroBeads were used to label CD34+ cells. Then, they were loaded and retained onto a MACS® Column placed in a MACS Separator, where the unlabeled cells ran through. After removing the MACS® Column from the magnetic field, the CD34+ cells were separated.23,24
Isolating CD34+CD38− Cells
According to MicroBeads manufacturer’s instruction, CD34+CD38− cells were isolated from MNCs of human UCB via two steps. In the first step, CD34+ cells were separated by the procedure explained above. In the second step, magnetic CD38 MicroBeads were used to label the separated CD34+ cells in the first step. Then, they were loaded and retained onto a MACS® Column placed in a MACS Separator. The CD34+CD38+ cells remained on the column, while CD34+CD38− cells ran through.18,25-27
The cells were labelled by PE - anti-human CD34 antibody and FITC- anti-human CD38 antibody in order to specify the purity of separated cells. The groups were labeled according to Table 1 and the design of the study is shown in Figure 1.
 |
Table 1 Design of Dosages and Study Groups |
 |
Figure 1 Design and time line of the study. |
Ex vivo Expansion of CD34+ and CD34+CD38− Cells
Culturing and expansion of both CD34+ and CD34+CD38− cells were performed in two ways:
FBS-Free Medium
2×104 cells per well of both types of cell groups (Groups A & C) were cultured in a stem line medium where supplemented with 100 ng/mL SCF, 100 ng/mL Flt-3L, 100 ng/mL TPO and 1% penicillin G and streptomycin with (Group A & C, Test) and without (Group A & C, Control) 0.1 ng/mL GFc7 growth nanofactor (Table 1).
FBS Medium
2×104 cells per well of both types of cell groups (Groups B & D) were cultured in an IMDM medium where supplemented with 10% FBS, 100 ng/mL SCF, 100 ng/mL Flt-3L, 100 ng/mL TPO and 1% penicillin G and streptomycin with (Groups B & D, Test) and without (Groups B & D, Control) 0.1 ng/mL GFc7 growth nanofactor (Table 1).
Three samples were analyzed in this experiment. The cells were cultured in a humidified incubator (37°C - 5% CO2 - 95% humidity) and were seeded onto 24-well plates in triplicate wells for 13 days.
Absolute Count of CD34+ and CD34+CD38− Cells
The cells number of CD34+ and CD34+CD38− was counted by the hemacytometer, and then the population percentage of CD34+ and CD34+CD38− was determined by FACS analysis. Finally, the absolute counts were obtained using the following formulas:28
“The population percentage of CD34+ × the number of cells”
“The population percentage of CD34+CD38− × the number of cells”
UCB Preparation Process for Cryoprotection
Red blood cells of UCB were depleted by HES (0.6%), and then the bag was attached to Sepax processing kit under sterile conditions. Afterwards, a cell separator (Sepax) was used to separate HSCs from UCB. Later in the process, the buffy coat was extracted and the UCB bag was detached. In the freezing stage, DMSO and Dextran 40 were added to the buffy coat, and then it was transferred to a programmable control rate freezer (Planer) to decrease the sample temperature to −100°C. Finally, the samples were stored in a dry nitrogen shipper first and were then transferred to a liquid nitrogen quarantine tank at −135°C until their use.21
HSCs Recovery After Being Thawed
The samples were thawed by removing them from storage and immediately immersing them with gentle agitation in water at 37°C. After that, their cell viability and colony forming were assessed.21
FACS Analysis
FACS analysis was performed according to the protocol in the previous study to evaluate anti-CD34-PE, anti-CD38-FITC and anti-CXCR4-FITC as well as PI staining. The data were assessed by Win MDI 2.8 software, and the results were illustrated using histograms or dot plots.15
Aldehyde Dehydrogenase Assay
Aldehyde dehydrogenase (ALDH) assay was carried out in CD34+ cells according to the protocol presented in ALDEFLUOR™ Kit. According to this protocol, ALDEFLUOR™ is a reagent used to identify cells which express various levels of ALDH enzyme. The amount of fluorescent reaction was measured by FACS analysis, and a particular inhibitor of ALDH (diethylaminobenzaldehyde) was utilized to control the background fluorescence.29
Colony-Forming Cell Assay
Clonogenic progenitors from thawed CD34+ cells were assayed in MethoCult medium by using 1000 freshly isolated cells. After 14 days of incubation (37°C, 5% CO2), colonies were counted by bright-field inverted microscope.30,31 Different colony-forming units, including granulocyte-monocyte colony-forming unit (CFU-GM), granulocyte-erythrocyte-macrophage-megakaryocyte colony-forming unit (CFU-GEMM) and erythroid burst-forming unit (BFU-E), were counted using a bright-field microscope.
Real-Time PCR Quantification
Evaluating the gene expressions of CD34+ cells was performed 13 days after treatment by real-time PCR analysis, where Trizol was used to extract total RNA, and then the synthesis of cDNA was carried out by M-MuLV Reverse Transcriptase and oligo primers. Gene quantification and real-time PCR quantification (qRT-PCR) analysis was performed using a standard SYBR Green PCR kit by Rotor-Gene 6000 instrument. The details of qRT-PCR analysis are described in the previous study. Rotor-Gene Q software (Corbett) was used for data analysis of the threshold cycle average, and the data were normalized to endogenous controls (GAPDH) and calibrated to untreated cultured cells as control. Finally, the relative mRNA expression levels were measured according to the ΔCT method.15
Measuring Cytoprotective Effect of GFc7 Against Oxidative Stress in CD34+ Cells
MTT assay was performed in H2O2-induced model to measure the cytoprotective effect of GFc7 against oxidative stress in CD34+ cells.32 HSCs were plated at a density of 1×104 cells/well in 96-well plates in 100 μL specific medium. Afterwards, two treatments were conducted to assess the cytoprotective effect of different doses of GFc7 growth nanofactor against oxidative stress in CD34+ cells;
a) GFc7 growth nanofactor treatment for 10 days following H2O2 treatment (5 μM) for 72 hrs.
b) Simultaneous treatment with GFc7 growth nanofactor and H2O2 (5 μM) for 72 hrs.
In control groups, HSCs were treated with H2O2 (control positive) and without any treatments (control negative).
Statistical Analysis
All experiments were conducted in triplicate and the analysis of variance (ANOVA) test was applied to compare multiple groups; the difference was considered significant when the P value was lower than 0.05. All data are shown as mean ± Standard Deviation (SD). Statistical data analyses were performed by the IBM SPSS Statistics (version 25) software.33
Results
Characteristics of GFc7 Growth Nanofactor
The TEM image of GFc7 growth nanofactor showed that the size of the nanoparticles was approximately 80 nm (Figure 2A). Moreover, the BET analysis illustrated the porous structure of this nanocomplex (Figure 2B). Moreover, the results of DLS test showed that there was no significant difference in the size of the molecules compared to the size in TEM image. According to this test, the nanoparticles were approximately 82 nm. Zeta potential was also 147.9 d.nm, proving the molecular stability of GFc7 growth nanofactor (Figure 3).
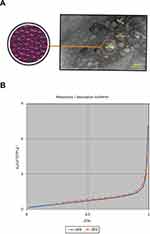 |
Figure 2 (A) HRTEM image of GFc7 growth nanofactor. (B) BET test results of GFc7 growth nanofactor. |
 |
Figure 3 Dynamic light scattering analysis of GFc7 growth nanofactor. |
Purification of CD34+ and CD34+CD38− Cells
The results of surface markers analysis revealed that 98%±1 of HSCs expressed CD34+ and 15%±0.5 of the result cells expressed CD34+CD38−. The results of MNCs analysis showed that the cell viability of HSCs was 98%±1 (Figure S1).
Effect of GFc7 Growth Nanofactor on CD34+ Cells During ex vivo Expansion
FBS-Free Medium
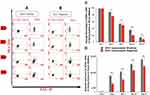
The percentages of CD34+ cells treated with GFc7 (Group A, Test) were 95%±2 (day 4), 72%±1.6 (day 7), 45%±2 (day 10) and 19%±1.5 (day 13), while the percentages in control (Group A, Control) were 88%±2.3, 60%±1.4, 30%±3 and 10%±2 on the same days, respectively. Likewise, the absolute counts of CD34+ cells treated with GFc7 (Group A, Test) were 165±40 ×103 (day 1), 620±95×103 (day 4), 1872±65×103 (day 7), 1550±100×103 (day 10) and 6100±80×103 (day 13), while the absolute counts in control (Group A, Control) were 165±40×103, 600±92×103, 1320±11×103, 1121±120×103 and 4900±8×103 on the same days, respectively (Figure 4).
FBS Medium
The percentages of CD34+ cells treated with GFc7 (Group B, Test) were 88%±2 (day 4), 54%±1.6 (day 7), 36%±1 (day 10) and 14%±8 (day 13), while the percentages in control (Group B, Test) were 76%±2.5, 49%±1.4, 20%±1 and 8%±0.5 on the same days, respectively. Likewise, the absolute counts of CD34+ cells treated with GFc7 (Group B, Test) were 165±40×103 (day 1), 832±20×103 (day 4), 1032±34×103 (day 7), 1420±14×103 (day 10) and 1652±90×103 (day 13), while the absolute counts (Group B, Test) in control were 165±40×103, 420±24×103, 630±11×103, 1092±70×103 and 1303±67×103 on the same days, respectively (Figure 5).
Effect of GFc7 Growth Nanofactor on CD34+CD38− Cells During ex vivo Expansion
FBS-Free Medium
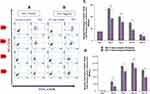
The percentages of CD34+CD38− cells treated with GFc7 (Group C, Test) were 54%±2 (day 4), 40%±1.6 (day 7), 30%±2 (day 10) and 10%±0.6 (day 13), while the percentages in control (Group C, Control) were 37%±1.8, 31%±1.4, 18%±1.2 and 7%±0.5 on the same days, respectively. Likewise, the absolute counts of CD34+CD38− cells treated with GFc7 (Group C, Test) were 17±10×103 (day 1), 413±98×103 (day 4), 1092±65×103 (day 7), 1222±70×103 (day 10) and 915±85×103 (day 13), while the absolute counts in control (Group C, Control) were 17±10×103, 200±91×103, 860±11×103, 100±80×103 and 600±7×103 on the same days, respectively (Figure 6).
FBS Medium
The percentages of CD34+CD38− treated with GFc7 (Group D, Test) were 44%±2 (day 4), 30%±1.4 (day 7), 24%±1.9 (day 10) and 13%±0.5 (day 13), while the percentages in control (Group D, Control) were zero on all four days. Likewise, the absolute counts of CD34+CD38-treated with GFc7 (Group D, Test) were 17±10×103 (day 1), 430±24×103 (day 4), 750±30×103 (day 7), 572±70×103 (day 10) and 1100±67×103 (day 13), while the absolute counts in control (Group D, Control) were zero on all four days (Figure 7).
Effect of GFc7 Growth Nanofactor on CXCR4 Cells During ex vivo Expansion
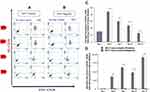
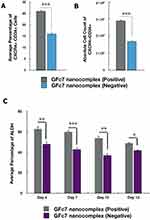
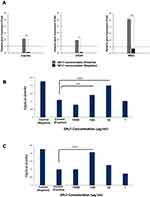
The percentage of CXCR4+CD34+ cells treated with GFc7 was 26%±0.5 on day 10, while the percentage in control was 16%±0.3 on the same day (Figure 8A). Likewise, the absolute count of CXCR4+CD34+ cells treated with GFc7 was 2900±50×103 on day 10, while the absolute count in control was 1700±30×103 on the same day (Figure 8B).
Effect of GFc7 Growth Nanofactor on ALDH During ex vivo Expansion of CD34+ Cells
The percentages of ALDH in CD34+ cells treated with GFc7 were 63%±2.1 (day 4), 60%±1.6 (day 7), 54%±2 (day 10) and 49%±0.6 (day 13), while the percentages in control were 48%±2.3, 43%±1.4, 37%±1.9 and 42%±0.5 on the same days, respectively (Figure 8C).
Effect of GFc7 Growth Nanofactor on Gene Expression
The gene expressions of CD34, CXCR4 and MGCL in CD34+ cells treated with GFc7 increased by 1.9±0.03, 2.1±0.3 and 5.1±0.4, respectively, while the gene expressions in control were 0.1±0.02, 0.1±0.04 and 0.7±0.05 (Figure 9A).
Effect of GFc7 Growth Nanofactor on Protection of CD34+ Cells in the Presence of H2O2
Assessing the cytoprotective effect of GFc7 against oxidative stress in CD34+ cells treated with GFc7 followed by treatment with H2O2: The optical densities with different doses of GFc7 were 0.078±0.005 (1000 μg/mL), 0.140±0.004 (100 μg/mL), 0.199±0.006 (10 μg/mL) and 0.102±0.009 (1 μg/mL), while they were 0.226±0.002 in control negative and 0.109±0.009 in control positive (Figure 9B).
Assessing the cytoprotective effect of GFc7 against oxidative stress in CD34+ cells treated with GFc7 and H2O2 at the same time: The optical densities with different doses of GFc7 were 0.0995±0.005 (1000 μg/mL), 0.207±0.006 (100 μg/mL), 0.126±0.004 (10 μg/mL) and 0.073±0.006 (1μg/mL), while they were 0.226±0.002 in control negative and 0.0995±0.009 in control positive (Figure 9C).
The results showed the cytoprotective effect of GFc7 against oxidative stress in CD34+ cells at 100 μg/mL and 10 μg/mL doses.
Effect of GFc7 Growth Nanofactor on Recovery of CD34+ Cells After Being Thawed
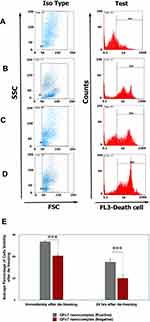
The cell viability of CD34+ cells was assessed in two stages (Figure 10);
Immediately After De-Freezing
The percentage of cell viability in GFc7 group was 80%±3, while it was 61%±2.2 in control (without GFc7).
24 Hrs After De-Freezing
The percentage of cell viability in GFc7 group was 52%±5, while it was 30%±4.5 in control.
Effect of GFc7 Growth Nanofactor on Colony-Forming Cells of CD34+ Cells After Being Thawed
Colony-forming assay was conducted and the number of colonies was counted to evaluate CD34+ cells function. This assay was performed in two stages (Table 2):
 |
Table 2 A Comparison Between the Numbers of the Colonies of CD34+ Cells Treated with and without GFc7 Growth Nanofactor Immediately After De-Freezing (A) and 24 Hrs After (B) |
Immediately After De-Freezing
The numbers of colonies of CFU-GM, CFU-GEMM and BFU-E in GFc7 group were 56±3, 26±1 and 13±1 while they were 60±1, 20±3, 13%±1 in control, respectively.
24 Hrs After De-Freezing
The numbers of colonies of CFU-GM, CFU-GEMM and BFU-E in GFc7 group were 52±2, 22±3 and 15±1, while they were 41±2, 14±2 and 8±2 in control, respectively.
Discussion
HSCs are identified as one of the most well-characterized tissue-specific stem cells with decades of basic research and clinical application, and UCB is the most accessible source to supply this type of stem cells in cell therapy. Adequate number of HSCs is a determining factor in cell therapy. However, the low number of HSCs in UCB unit has restricted the application of these cells to some patients with a specific age and weight range.34,35
The in vitro expansion of HSCs by growth factors before transplant is a priority in this field. T Peled claims that copper chelator tetraethylenepentamine can regulate the balance between self-renewal and differentiation of HSCs when used for expansion.36 Recent studies have shown that the application of nicotinamide as a growth factor expands CD34/CD133− cells in vitro, delays cell differentiation and results in 9-fold increase in cells.37 In another study, it was reported that human and murine expression of PGE2 receptors in HSCs and short-term exposure to PGE2 improved cell viability and expansion of HSCs.38
Recently, various studies have been conducted in the field of nanotechnology to overcome stem cells limitations in cell therapy, such as nanoparticles applied in targeted drug delivery system.39,40 Wang Y experimented on liposome-protamine-DNA complex and stated that cell-specific promoters enabled lipid-based nanoparticles to facilitate genes delivery to retina cells.41 In another study, it was proven that owing to the tumor-homing ability of nano-engineered MSCs, they could be used as vectors to deliver diagnostic and therapeutic nanoparticles into a tumor.42
In the previous study, the positive impact of GFc7 growth nanofactor on hMSCs properties was revealed to be significant in vitro.15 Therefore, the effect of this growth nanofactor on HSCs expansion and their properties after treatment was evaluated in the present study.
CD34 glycosylated transmembrane protein is the defining characteristic of human HSCs. This marker is involved in progenitor cells maintenance in a phenotypically undifferentiated state.43,44 In the current study, the expansion of CD34+ after treatment with GFc7 growth nanofactor was evaluated and compared with control (without GFc7). The results showed that the population percentage of CD34+ was higher than control.
The absolute count of CD34 in one unit of UCB is 2.07±1.31×106, and 170×103 of CD34 cells per kilogram of the bodyweight is required for transplant.21,43 Therefore, it can be claimed that CD34 cells expansion with GFc7 growth nanofactor can provide the proper number of cells required for transplant in an adult weighing over 55 kg.
As CD34 marker alone is not capable of representing actual HSCs, CD34+CD38− stem cell population was measured as well. CD34+CD38− immunophenotype represents a rare and highly primitive population of stem cells in UCB45 with low HLA-Dr expression in all HSCs studies to date. Because CD34+CD38− cells are immediately differentiated into CD34+CD38+ in the presence of FBS, FBS-free mediums are utilized for maintenance and expansion of these stem cells,46 yet these mediums are too costly. As a result, in the second stage of this study, CD34+CD38− cells were expanded in FBS medium in the presence of GFc7 growth nanofactor to investigate their differentiation and expansion so as to find a more economical way than FBS-free medium. The results showed that CD34+CD38− cells lasted to exist even 13 days after expansion, whereas they were differentiated and transformed to CD34+CD38+ just 4 days after expansion in control according to dot plot diagram of FACS analysis.
In this study, after treating HSCs with GFc7 growth nanofactor, the percentage of ALDH, as a marker of HSCs, was computed and then compared with control in various days of expansion.47,48 The results indicated that the treated cells expressed higher percentage of ALDH than control. Therefore, it could be concluded that the treated cells with GFc7 growth nanofactor possessed higher stemness property.
In one experiment, Asfour I evaluated engraftment after transplant in a number of patients and reached the conclusion that SDF-1/CXCR4 in HSCs plays a vital role in engraftment after transplant.49 In another study, it was revealed that the mobilization of CD34+CXCR4+ cells in acute MI has a positive correlation with the improvement of left ventricular ejection fraction.50 As a result, the importance of CD34+CXCR4+ cells after transplant made us assess the expression of these cells after expansion when treated with GFc7 growth nanofactor, and the results showed that expansion in GFc7 group was 10% higher than that of control.
In the next stage of the current study, the gene expressions of CD34, CXCR4 and MGCL were analyzed, showing that they were higher in GFc7 group than control.
Nowadays, private and public UCB banks are established to provide patients with HSCs by freezing these stem cells.1 Higher quality and quantity of these stem cells is essential in the freezing and de-freezing process in order to help the patients with the higher performance of these stem cells after transplant.51
Freezing and de-freezing process naturally reduces stem cells quality due to the presence of free radicals.52,53 In one of the stages of the current study before de-freezing, it was revealed that in the presence of H2O2, HSCs treated with GFc7 growth nanofactor had higher viability.
The freezing process of HSCs was performed by using the standard protocol of UCB banks to evaluate the quality of HSCs treated with GFc7 growth nanofactor after de-freezing. This evaluation was performed in two stages; a) immediately after de-freezing, b) 24 hrs after de-freezing. The results showed that the treated cells with GFc7 growth nanofactor had higher percentage of cell viability than control in all three stages, as it was also confirmed by colony-forming cell assay (this assay was utilized to study hematopoietic progenitors proliferation and differentiation pattern and investigate their ability to form stem cell colonies in a semisolid medium). This higher cell viability could be attributed to the cytoprotective effect of GFc7 against oxidative stress in CD34+ cells.
Conclusion
The results of the present study support the hypothesis that treating HSCs with GFc7 growth nanofactor leads to HSCs quality and quantity improvement in terms of expansion in vitro and freezing and de-freezing process, which consequently improves their efficient performance in the body.
Abbreviations
HSCs, hematopoietic stem cells; SCF, stem cell factor; TPO, thrombopoietin; Flt-3L, Flt-3 ligand; UCB, umbilical cord blood; DMSO, dimethyl sulfoxide; PBS, phosphate buffer solution; H2O2, hydrogen peroxide; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide 99%; hMSCs, human mesenchymal stem cells; FBS, fetal bovine serum; IMDM, Iscove’s Modified Dulbecco’s Media; HES, hydroxyl ethyl starch; FACS, flow cytometry; HRTEM, high-resolution transmission electron microscopy.
Ethics Approval and Consent to Participate
This study received the ethics approval of Shahid Beheshti University of Medical Sciences, Tehran, Iran,7808, and the written consent of the patients to participate in the study.
Acknowledgments
We do appreciate the support of the Department of Research and Development at Sodour Ahrar Shargh Company and Stem Cell Technology Research Center, Tehran, Iran.
Disclosure
Dr Mohammad Hassan Nazaran reports a patent US8288587B2 with royalties paid. The authors declare that they have no competing interests.
References
1. Gluckman E, Ruggeri A, Volt F, et al. Milestones in umbilical cord blood transplantation. Br J Haematol. 2011;154(4):441–447. doi:10.1111/j.1365-2141.2011.08598.x
2. Available from: https://www.medicalnewstoday.com/articles/318091.
3. Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci. 2012;1266(1):138–150. doi:10.1111/j.1749-6632.2012.06549.x
4. Yucel D, Kocabas F. Developments in hematopoietic stem cell expansion and gene editing technologies. Adv Exp Med Biol. 2018;1079:103–125.
5. Shearer WT, Lubin BH, Cairo MS, et al. Cord blood banking for potential future transplantation. Pediatrics. 2017;140(5):e20172695. doi:10.1542/peds.2017-2695
6. Roura S, Pujal J-M, Gálvez-Montón C, et al. The role and potential of umbilical cord blood in an era of new therapies: a review. Stem Cell Res Ther. 2015;6(1):123. doi:10.1186/s13287-015-0113-2
7. Svalgaard JD, Talkhoncheh MS, Haastrup EK, et al. Pentaisomaltose, an alternative to DMSO. Engraftment of cryopreserved human CD34+ cells in immunodeficient NSG mice. Cell Transplant. 2018;27(9):1407–1412. doi:10.1177/0963689718786226
8. Mazzeo A, Santos EJC. Nanotechnology and multipotent adult progenitor cells in reparative medicine: therapeutic perspectives. Einstein (Sao Paulo). 2018;16(4):eRB4587. doi:10.31744/einstein_journal/2018RB4587
9. Raveendran S, Rochani A, Maekawa T, et al. Smart carriers and nanohealers: a nanomedical insight on natural polymers. Materials (Basel). 2017;10(8):929. doi:10.3390/ma10080929
10. Vasilescu I, Vilceanu M, Fitărău A, et al. Serological types and clinical forms in salmonella food toxinfections. Microbiol Parazitol Epidemiol (Bucur). 1971;16(2):159–165.
11. Deb KD, Griffith M, Muinck ED, Rafat M. Nanotechnology in stem cells research: advances and applications. Front Biosci (Landmark Ed). 2012;17(1):1747–1760. doi:10.2741/4016
12. Wang Z, Ruan J, Cui D. Advances and prospect of nanotechnology in stem cells. Nanoscale Res Lett. 2009;4(7):593–605. doi:10.1007/s11671-009-9292-z
13. Fakharzadeh S, Kalanaky S, Hafizi M, et al. DIBc, a nanochelating-based nano metal-organic framework, shows anti-diabetic effects in high-fat diet and streptozotocin-induced diabetic rats. Int J Nanomedicine. 2019;14:2145–2156. doi:10.2147/IJN.S196050
14. Hafizi M, Kalanaky S, moaiery H, et al. A randomized, double-blind, placebo-controlled investigation of BCc1 nanomedicine effect on survival and quality of life in metastatic and non-metastatic gastric cancer patients. J Nanobiotechnology. 2019;17(1):52. doi:10.1186/s12951-019-0484-0
15. Hafizi M, Hajarizadeh A, Atashi A, et al. Nanochelating based nanocomplex, GFc7, improves quality and quantity of human mesenchymal stem cells during in vitro expansion. Stem Cell Res Ther. 2015;6(1):226. doi:10.1186/s13287-015-0216-9
16. Sidney LE, Branch MJ, Dunphy SE, et al. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380–1389. doi:10.1002/stem.1661
17. Wisniewski D, Affer M, Willshire J, et al. Further phenotypic characterization of the primitive lineage- CD34+CD38-CD90+CD45RA- hematopoietic stem cell/progenitor cell sub-population isolated from cord blood, mobilized peripheral blood and patients with chronic myelogenous leukemia. Blood Cancer J. 2011;1(9):e36. doi:10.1038/bcj.2011.35
18. Hao QL, Shah AJ, Thiemann FT, et al. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995;86(10):3745–3753. doi:10.1182/blood.V86.10.3745.bloodjournal86103745
19. Mh N. Chelate compounds. Google Patents US8288587B2. 2012.
20. Meyer JC, Kurasch S, Park HJ, et al. Experimental analysis of charge redistribution due to chemical bonding by high-resolution transmission electron microscopy. Nat Mater. 2011;10(3):209–215. doi:10.1038/nmat2941
21. Jamali M, Atarodi K, Nakhlestani M, et al. Cord blood banking activity in Iran national cord blood bank: a two years experience. Transfus Apher Sci. 2014;50(1):129–135. doi:10.1016/j.transci.2013.09.012
22. Nilsson C, Aboud S, Karlén K, et al. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin Vaccine Immunol. 2008;15(4):585–589. doi:10.1128/CVI.00161-07
23. Rao J, Xu D-R, Zheng F-M, et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med. 2011;9(1):71. doi:10.1186/1479-5876-9-71
24. Hong CS, Muller L, Boyiadzis M, et al. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One. 2014;9(8):e103310. doi:10.1371/journal.pone.0103310
25. Available from: https://www.miltenyibiotec.com/US-en/products/cd34-cd38-cell-isolation-kit-human.html#gref.
26. Albeniz I, TÜRKER-ŞENER L, BAŞ A, et al. Isolation of hematopoietic stem cells and the effect of CD38 expression during the early erythroid progenitor cell development process. Oncol Lett. 2012;3(1):55–60. doi:10.3892/ol.2011.455
27. Rosler E, Brandt J, Chute J, Hoffman R. Cocultivation of umbilical cord blood cells with endothelial cells leads to extensive amplification of competent CD34+CD38- cells. Exp Hematol. 2000;28(7):841–852. doi:10.1016/S0301-472X(00)00177-6
28. Phelan MC, Lawler G. Cell counting. Curr Protoc Cytom. 2001;Appendix 3:Appendix 3A.
29. Available from: https://www.stemcell.com.
30. Wognum B, Yuan N, Lai B, Miller CL. Colony forming cell assays for human hematopoietic progenitor cells. Methods Mol Biol. 2013;946:267–283.
31. Zhang Y, Shen B, Guan X, et al. Safety and efficacy of ex vivo expanded CD34+ stem cells in murine and primate models. Stem Cell Res Ther. 2019;10(1):173. doi:10.1186/s13287-019-1275-0
32. Si C-L, Shen T, Jiang -Y-Y, et al. Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food Chem Toxicol. 2013;59:145–152. doi:10.1016/j.fct.2013.05.051
33. Dea Q. Workload Optimized Systems: Tuning POWER7 for Analytics. Quintero Dea. Workload Optimized Systems: Tuning POWER7 for Analytics.
34. Takizawa H, Schanz U, Manz MG. Ex vivo expansion of hematopoietic stem cells: mission accomplished? Swiss Med Wkly. 2011;141:w13316.
35. Schuster JA, Stupnikov MR, Ma G, et al. Expansion of hematopoietic stem cells for transplantation: current perspectives. Exp Hematol Oncol. 2012;1(1):12. doi:10.1186/2162-3619-1-12
36. Peled T, Mandel J, Goudsmid RN, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6(4):344–355. doi:10.1080/14653240410004916
37. Peled T, Shoham H, Aschengrau D, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40(4):342–55e1. doi:10.1016/j.exphem.2011.12.005
38. Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. doi:10.1182/blood-2009-01-201335
39. Saulite L, Dapkute D, Pleiko K, et al. Nano-engineered skin mesenchymal stem cells: potential vehicles for tumour-targeted quantum-dot delivery. Beilstein J Nanotechnol. 2017;8:1218–1230. doi:10.3762/bjnano.8.123
40. La Francesca S. Nanotechnology and stem cell therapy for cardiovascular diseases: potential applications. Methodist Debakey Cardiovasc J. 2012;8(1):28–35. doi:10.14797/mdcj-8-1-28
41. Wang Y, Rajala A, Cao B, et al. Cell-specific promoters enable lipid-based nanoparticles to deliver genes to specific cells of the retina in vivo. Theranostics. 2016;6(10):1514–1527. doi:10.7150/thno.15230
42. Liu WH, Chang Y-L, Lo W-L, et al. Human induced pluripotent stem cell and nanotechnology-based therapeutics. Cell Transplant. 2015;24(11):2185–2195. doi:10.3727/096368914X685113
43. Pei X. Who is hematopoietic stem cell: CD34+ or CD34-? Int J Hematol. 1999;70(4):213–215.
44. AbuSamra DB, Aleisa FA, Al-Amoodi AS, et al. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017;1(27):2799–2816. doi:10.1182/bloodadvances.2017004317
45. Kadekar D, Kale V, Limaye L. Differential ability of MSCs isolated from placenta and cord as feeders for supporting ex vivo expansion of umbilical cord blood derived CD34+ cells. Stem Cell Res Ther. 2015;6(1):201. doi:10.1186/s13287-015-0194-y
46. Lebkowski JS, Schain LR, Okarma TB. Serum-free culture of hematopoietic stem cells: a review. Stem Cells. 1995;13(6):607–612. doi:10.1002/stem.5530130605
47. Muramoto GG, Russell JL, Safi R, et al. Inhibition of aldehyde dehydrogenase expands hematopoietic stem cells with radioprotective capacity. Stem Cells. 2010;28(3):523–534. doi:10.1002/stem.299
48. Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103(31):11707–11712. doi:10.1073/pnas.0603806103
49. Asfour I, Afify H, Elkourashy S, et al. CXCR4 (CD184) expression on stem cell harvest and CD34 + cells post-transplant. Hematol Oncol Stem Cell Ther. 2017;10(2):63–69. doi:10.1016/j.hemonc.2017.01.002
50. Wyderka R, Wojakowski W, Jadczyk T, et al. Mobilization of CD34+CXCR4+ stem/progenitor cells and the parameters of left ventricular function and remodeling in 1-year follow-up of patients with acute myocardial infarction. Mediators Inflamm. 2012;2012:564027. doi:10.1155/2012/564027
51. Armitage S, Doméné A, Vercouillie J. Cord blood banking standards: autologous versus altruistic. Front Med (Lausanne). 2015;2:94. doi:10.3389/fmed.2015.00061
52. Li P, Xi MD, Du H, et al. Antioxidant supplementation, effect on post-thaw spermatozoan function in three sturgeon species. Reprod Domest Anim. 2018;53(2):287–295. doi:10.1111/rda.13103
53. Hatef B, Taromchi A, Nejatbakhsh R, et al. Supplementation of freezing media with stromal cell-derived factor-1alpha preserves human sperm from cryodamage. Cryobiology. 2017;79:37–42. doi:10.1016/j.cryobiol.2017.09.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.