Back to Journals » International Journal of Nanomedicine » Volume 13
Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice
Authors Zhang L, Xie X, Zhou Y, Yu D, Deng Y, Ouyang J, Yang B, Luo D, Zhang D, Kuang H
Received 23 September 2017
Accepted for publication 11 December 2017
Published 5 February 2018 Volume 2018:13 Pages 777—789
DOI https://doi.org/10.2147/IJN.S152400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Lu Zhang,1,* Xingxing Xie,1,* Yigang Zhou,2,* Dainan Yu,1 Yu Deng,1 Jiexiu Ouyang,3 Bei Yang,1 Dan Luo,1 Dalei Zhang,1 Haibin Kuang1,3
1Department of Physiology, Basic Medical College, Nanchang University, Nanchang, 2Department of Color Ultrasonic Room, No 96716 Hospital of PLA, 3Jiangxi Provincial Key Laboratory of Reproductive Physiology and Pathology, Medical Experimental Teaching Center, Nanchang University, Nanchang, Jiangxi, People’s Republic of China
*These authors contributed equally to this work
Background: Titanium dioxide nanoparticles (TiO2 NPs) have recently found applications in a wide variety of consumer goods. TiO2 NPs exposure significantly increases fetal deformities and mortality. However, the potential toxicity of TiO2 NPs on the growth and development of placenta has been rarely studied during mice pregnancy.
Purpose: The objective of this study was to investigate the effects of maternal exposure of TiO2 NPs on the placentation.
Methods: Mice were administered TiO2 NPs by gavage at 0, 1 and 10 mg/kg/day from gestational day (GD) 1 to GD 13. Uteri and placentas from these mice were collected and counted the numbers of implanted and resorbed embryo and measured the placental weight on GD 13. Placental morphometry was observed by hematoxylin and eosin staining. The levels of Hand1, Esx1, Eomes, Hand2, Ascl2 and Fra1 mRNA were assessed by qRT-PCR. Uterine NK (uNK) cells were detected by using DBA lectin. Laminin immunohistochemical staining was to identify fetal vessels. Western blotting and transmission electron micrograph (TEM) were used to assess the apoptosis of placenta.
Results: No treatment-related difference was observed in the numbers of implanted and resorbed embryos and weight of placenta between the groups. However, 1 mg/kg/day TiO2 NPs treatment significantly reduced the ratio of placenta/body weight on GD 13. The proportion of spongiotrophoblast in the 10 mg/kg/day dose group became higher than that in the control group, yet that of labyrinth was significantly lower in 10 mg/kg/day mice. The expression levels of Hand1, Esx1, Eomes, Hand2, Ascl2 and Fra1 mRNA markedly decreased in TiO2 NP treated placentas. Furthermore, TiO2 NPs treatment impaired the formation of intricate networks of fetal vessels and reduced the number of uNK cells, and inhibited proliferation and induced apoptosis of placenta by nuclear pyknosis, the activation of caspase-3 and upregulation of Bax protein and downregulation of Bcl-2 protein on GD 13.
Conclusion: Gestational exposure to TiO2 NPs significantly impairs the growth and development of placenta in mice, with a mechanism that seems to be involved in the dysregulation of vascularization, proliferation and apoptosis. Therefore, our results suggested the need for great caution while handling of the nanomaterials by workers and specially pregnant consumers.
Keywords: nanoparticles, titanium dioxide, placenta, proliferation, apoptosis, vascularization
Introduction
Nanoparticles (NPs) are materials with at least one dimension ≤100 nm, and this large surface-to-volume ratio results in unique physicochemical characteristics, including high reactivity, lower melting temperature, color changes and greater solar radiation absorption, compared to their corresponding bulk materials.1 Titanium dioxide nanoparticles (TiO2 NPs) have unusual optical properties and therefore are widely used in various applications, such as sunscreens, food production, dietary supplements, food packaging materials, medicine, toothpastes, cosmetics and waste water treatment.2,3 Despite the rapid introduction of nanomaterials to markets and extensive investigations of their toxicological properties, their safety has not yet been well established.
TiO2 was previously considered as innocuous in particle toxicology, while recent studies on TiO2 NPs have been reported to cause toxicity in the livers, kidneys, spleens, brains and hearts of animals.4–8 For example, TiO2 NPs exposure induced hydropic degeneration, cloudy swelling and apoptosis of rat hepatocytes, and infiltration of inflammatory cells in portal vein.9 Intragastric administrations of TiO2 NPs were accumulated in the kidney, resulting in nephric inflammation, cell necrosis and congestion of mesenchyme blood vessels.6 Furthermore, a potentially toxic role of TiO2 NPs on the reproductive system also raises great concern. TiO2 NPs exposure was associated with harmful effects on the reproductive system. Some studies showed that TiO2 NPs could cross the blood–testis barrier to reach the testis and resulted in the decrease of sperm numbers and motility, sperm malformations, alterations in serum sex hormone levels and gene expression in the testes of mice.10,11 Komatsu et al demonstrated that TiO2 NPs were taken up by mouse Leydig TM3 cells in vitro and inhibited the proliferation of Leydig cells and increased the expression of heme oxygenase-1 (a sensitive marker for oxidative stress) and steroidogenic acute regulatory protein.12 TiO2 NPs were also found to accumulate in the ovary and can impair the equilibrium of sex hormones and alter the gene expression of ovary, and decrease the pregnancy rate and numbers of giving birth.13 The treatment of TiO2 NPs to preantral follicle can lead to morphological changes in them and significantly inhibit follicular development and oocyte maturation in rat.14,15 In addition, TiO2 NPs have a potential risk for embryonic development. Maternal exposure to TiO2 NPs could suppress the embryonic development of CD-1 mice, resulting in a statistically significant increase in the resorptions, fetal deformities and dead fetuses.16,17 Similarly, TiO2 NP addition to food led to a significant progeny loss in Drosophila.16 Moreover, it was reported that TiO2 NPs were detected in the placenta by the gamma-ray spectrometer and silver NPs could be transferred to the developing conceptus across placenta.18,19 However, little is known about the effect of TiO2 NPs on the growth and development of placenta. Therefore, our aim of this work was to explore the effect and mechanism of TiO2 NPs on the growth and development of placenta in vivo during mice pregnancy.
Materials and methods
Materials
TiO2 NPs (CAS#: 637254) were purchased from Sigma-Aldrich Co., Ltd (St Louis, MO, USA). The average particle size of anatase TiO2 NPs was <25 nm, while the surface area of sample was 45–55 m2/g. The purity of TiO2 NPs is 99.7% based on trace metals analysis (≤4,000.0 ppm). TiO2 NP powder was dispersed on the surface of 0.5% (w/v) tragacanth solution (Xiya Reagent Co. Ltd., Shanghai, People’s Republic of China), and the solutions containing TiO2 particles were treated ultrasonically for 20–30 min and then mechanically vibrated for 3–5 min. The particle sizes of NPs suspended in 0.5% w/v solution (3 mg/mL) following incubation were determined using a Tecnai-10 transmission electron microscope (TEM) (Philips, Amsterdam, the Netherlands). Mean particle size was determined by measuring more than 100 individual particles, which were randomly sampled. The zeta potential of TiO2 NPs was 9.12 mV.
Animal treatment and tissue collection
Mature mice (Kunming strain) were housed in a consistent environment with a 14 h light: 10 h dark cycle and allowed free access to water and food. The experiment was approved by the Institutional Animal Care and Use Committee of Nanchang University and followed the rules set forth in the Guide for the Care and Use of Laboratory Animals. Adult virgin female mice were mated with fertile males of the same strain to induce pregnancy. The day of finding a vaginal plug was considered as gestational day (GD) 1. Pregnant mice were randomly divided into 3 groups as follows: 1) control group, 2) 1 mg/kg/day TiO2 NPs group, 3) 10 mg/kg/day TiO2 NPs group, with 10 mice in each group. The mice were weighed, a volume of TiO2 NP suspension was calculated for each mouse (0.1 mL/10 g body weight) and the fresh TiO2 NP suspension was administered by gavage from GD 1 to GD 13. The control group was given an equivalent volume of 0.5% w/v tragacanth solution. Treated mice were weighed and euthanized to collect the liver, spleen, kidney, ovary and placenta tissues at 15:00–16:00 on GD 13 by cervical dislocation. Uterus and placenta from these mice were photographed by using a digital camera (Nikon, Tokyo, Japan) and frozen at −80°C for further analysis.
Placental morphometry
After counting the numbers of implanted and resorbed embryo and measuring the placental weight on GD 13, tissues were fixed in Bouin’s solution for 12 h, dehydrated in gradient ethanol, embedded in paraffin blocks, sliced to 6 μm thickness and placed on the glass slides coated with poly-l-lysine. Then sections stained with hematoxylin and eosin (H&E) were examined and photographed using a Nikon DS-Fi1 microscope (Nikon) at a 10× objective magnification for morphological evaluation. The areas of whole placenta, placental spongiotrophoblast and placental labyrinth were measured using sections, with the maximum areas for the layer of whole placenta, using ImageJ software (v. 1.48, National Institutes of Health). The average of the areas was calculated using 5 serial sections from 10 individuals. All placentas were analyzed without treatment knowledge to avoid bias.
Immunohistochemistry
Tissue sections were deparaffinized in xylene, and hydrated in graded ethanol solutions. Endogenous peroxidase activity was blocked by incubating the sections in 3% hydrogen peroxide in PBS for 10 min. Nonspecific binding was blocked in 5% bovine serum albumin (BSA) in PBS for 60 min. Then, the sections were incubated in rabbit anti-laminin (1:1,200, Sigma-Aldrich), rabbit anti-PCNA (1:1,000; Cell Signaling, Danvers, MA, USA) or biotinylated DBA lectin (1:300; Sigma-Aldrich) overnight at 4°C. After washing in PBS 3 times for 5 min each time, the sections were incubated with a secondary antibody for 60 min at 37°C followed by fresh diaminobenzidine (ZSGB-BIO, Beijing, People’s Republic of China) solution, together with counter-staining with Harris’ hematoxylin. Experimental results were observed by light microscopy, and analyses of immunohistochemical staining were taken using an NIS-Elements analysis system (Nikon). Each slide was measured in a randomly selected 10 fields under the same magnification (×400) and light intensity. Mean values of optical density for positive cells were calculated using 5 placenta sections from 10 individuals in each group.
Placental genes expression
Relative expression of placental genes was detected by real-time polymerase chain reaction (PCR) using 2−ΔΔCT method. Total RNA was extracted from placental tissues with RNAiso Plus solution (Takara, Tokyo, Japan) and cDNA was generated using PrimeScript™ II cDNA Synthesis Kit (Takara) according to the manufacturer’s instructions. Gene expression of each sample was examined in a 20 μL reaction volume containing 10 μL of 2× Brilliant SYBR Green Mix (Takara), 2 μL of template cDNA, 0.5 μM primers and 300 nM reference dyes using the ABI thermal cycler 7500 (Applied Biosystems, Waltham, MA, USA). Relative expression of each transcript was normalized to 18S. The primers for placenta-specific genes were described previously.20
Observation of placental ultrastructure by TEM
Placenta tissue blocks of about 1 mm3 in size were placed in 2.5% glutaraldehyde phosphate buffer overnight and fixed in 1% osmium acid for 1 h. Staining was performed for 30 min with 2% uranium acetate solution. The specimens were dehydrated in a graded series of ethanol (50%, 70%, 90% and 100%). Tissues were embedded in Epon 812, sectioned (120 nm thickness), stained with 4% uranyl acetate and lead citrate, and observed with a Tecnai-10 TEM (Philips). Placental apoptosis was analyzed based on the changes in nuclear morphology.
Western blot analysis
Tissues were homogenized and lysed in the ice-cold radioimmunoprecipitation assay buffer supplemented with phenylmethylsulfonyl fluoride solution and a phosphatase inhibitor cocktail (Applygen Technologies, Beijing, People’s Republic of China). Protein content was detected by using the BCA protein assay kit (Applygen Technologies). A total of 20 μg protein extracts were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel for electrophoresis, and then transferred onto the nitrocellulose blotting membrane (Corning Life Sciences, New York, NY, USA). Nonspecific binding was blocked with 5% BSA for 1 h at room temperature and blotting membranes were incubated with the following primary antibodies at 4°C overnight: rabbit anti-Bcl-2, Bax, caspase-3 (Casp-3) and mouse anti-β-actin (Cell Signaling). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies at 1:8,000 dilutions and visualized via enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA, USA). The relative band intensity was acquired by using the Quantity One software. The results were corrected for background, normalized to β-actin expression.
Statistical analysis
Statistical analyses were performed using SPSS 21.0 software (IBM Corporation, Armonk, NY, USA). Values are presented as means ± standard error of mean. The results were analyzed by using Student’s t-test between two groups or using 1-way analysis of variance (ANOVA) followed by least-significant difference’s post-hoc test. Statistical significance for all tests was judged at a probability level of 0.05 (P<0.05).
Results
TiO2 NPs characteristics
X-ray-diffraction (XRD) measurements showed that TiO2 NPs exhibited the anatase structure, and the average particle size calculated from the XRD peak of anatase was <25 nm using Scherrer’s equation (Figure S1). In addition, TEM analyses indicated that the average size of TiO2 powder particles suspended in tragacanth solution was about 10 nm (Figure 1), which was almost consistent with the XRD results.
Effect of TiO2 NPs on the organ weights
Absolute and relative organ weights of pregnant mice exposed to TiO2 NPs are shown in Table 1. At the dose levels tested, TiO2 NPs had no significant effect on the absolute and relative weights of liver, spleen, kidney and ovary on GD 13 (all P>0.05, Table 1).
Effect of TiO2 NPs on embryo implantation and placental weight
Although TiO2 NPs treatment slightly increased the numbers of resorbed embryos, statistical analysis indicated that there was no significant difference between TiO2 NP-treated group and control group (Figure 2A and B). In addition, no detectable changes were noted in the numbers of viable embryos (Figure 2B) and absolute weight of placenta (Figure 3A and B). However, 1 mg/kg/day TiO2 NPs significantly reduced the ratio of placental weight to body weight compared to control group on GD 13 (P<0.05, Figure 3C).
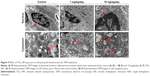
Effect of TiO2 NPs on placental structure
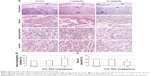
We observed placental histopathologic changes by H&E staining. On GD 13, treatment with 1 mg/kg/day TiO2 NPs had no obvious effect on the area of spongiotrophoblast and labyrinth relative to control group (all P>0.05, Figure 4A–C). Nevertheless, 10 mg/kg/day TiO2 NP treatment significantly increased the area of placental spongiotrophoblast and reduced the area of labyrinth (all P<0.05, Figure 4B and C).
Effect of TiO2 NPs on the expression of placental marker genes
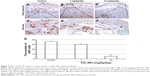
To investigate underlying mechanisms of the dysregulation of spongiotrophoblast and labyrinth development in mice treated with TiO2 NPs, we examined the expression levels of placental marker genes. Compared with the control, quantitative PCR results showed that all doses of TiO2 NPs significantly reduced levels of Hand1, Esx1, Eomes, Hand2, Ascl2 and Fra1 mRNA (all P<0.001, Figure 5A–F).
Effect of TiO2 NPs on the fetal vessels and numbers of uNK cells of decidua
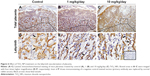
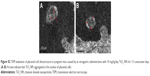
Laminin immunohistochemical staining (a fetal vessels marker) showed that there were numerous intricate fetal vessels in normal control placental labyrinth (Figure 6A and D). However, 1 and 10 mg/kg/day TiO2 NP treatment resulted in the collapse of fetal blood vessels (Figure 6B and C and E and F). DBA lectin staining (a novel uNK-specific marker) indicated that administration of 10 mg/kg/day TiO2 NPs significantly decreased the numbers of uNK cells in the placental decidua compared with control group (P<0.001, Figure 7G), whereas 1 mg/kg/day dose did not significantly affect the numbers of uNK cells.
TiO2 NPs treatment inhibited the proliferation and induced the apoptosis of placenta
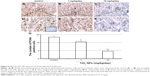
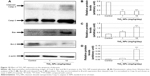
Compared to control group, TiO2 NP administration significantly reduced the numbers of PCNA-positive cells in the placental labyrinth (P<0.05 or P<0.001, Figure 8A and B). Furthermore, TEM analysis showed that TiO2 NPs aggregated in the nucleus of placental cells (Figure S2), and nuclear pyknosis, mitochondrial swelling, expansion of sliding endoplasmic reticulum and abscission of rough endoplasmic reticulum ribosome were observed in the TiO2 NP-treated placental tissues (Figure 9B and C). Western blot analysis showed that intact Casp-3 was cleaved to active fragments after TiO2 NP treatment on GD 13 (Figure 10A) and expression levels of active fragments significantly increased in a dose-dependent manner (P<0.001, Figure 10D). In addition, TiO2 NP treatment also significantly increased the expression level of Bax protein (P<0.05 or P<0.001, Figure 10C and D). However, the expression level of Bcl-2 protein was significantly downregulated on GD 13 (P<0.01 or P<0.001, Figure 10B and D).
Discussion
Recently, information on the toxicity of TiO2 NPs in the liver and kidneys and in male and female reproductive system injury5,6,9,15 is increasing. However, the evidence for placental developmental toxicity in mammalians was limited. In this study, our results demonstrated that 1 mg/kg/day TiO2 NP treatment significantly reduced the ratio of placenta/body weight in mice on GD 13. Histopathologically, compared with control group, the area of placental spongiotrophoblast significantly increased and labyrinth was markedly reduced in the 10 mg/kg/day dose group. The expression levels of Hand1, Esx1, Eomes, Hand2, Ascl2 and Fra1 mRNA were significantly inhibited in TiO2 NP-treated placentas. TiO2 NP administration dramatically disrupted labyrinth vascularization of placentas and reduced the numbers of uNK cells. Moreover, we found that TiO2 NP treatment significantly inhibited the proliferation and induced apoptosis of placenta by the activation of Casp-3, upregulation of Bax and downregulation of Bcl-2 protein.
Under our experimental conditions, statistical analysis showed that treatment with different doses of TiO2 NPs did not affect absolute and relative organ weights of pregnant mice, including liver, spleen, kidney, ovary and body weight. This is almost consistent with Wang et al’s result,21 in which treatment with 5 g/kg TiO2 NPs in adult mice did not induce significant abnormal pathology changes in the spleen, lung, testis, ovary and heart tissues. However, female mice treated with 25 and 80 nm TiO2 NPs for 2 weeks showed high coefficients of liver.22 Upregulation of blood urea nitrogen level and pathology change of kidneys were also observed in the experimental groups. In addition, Hong et al’s study showed that chronic exposure to TiO2 NPs for 9 months led to significant decreases in body weight and increases in kidney organ coefficient compared with those of control group.23 Nevertheless, Ag NP exposure on gestational days 6–19 in rats did not affect maternal body weight, organ weight (brain, liver, spleen, kidney, heart and ovary), and fetal and placental weights.24 These results suggested that effects of TiO2 NPs on the organ weight are probably exposure time dependent, dose dependent, and nanoparticle size and nanomaterial dependent.
In addition, we also found that the numbers of implanted and resorbed embryos and the weight of placenta were not significantly affected by different doses of TiO2 NPs; only 1 mg/kg/day TiO2 NPs significantly reduced placental organ coefficient on GD 13. Consistent with our findings, Warheit et al found that the numbers of implantation sites, early and late resorptions were all comparable to control group values for every dose level tested, which were 0, 100, 300 or 1,000 mg/kg/day TiO2 NPs in Sprague-Dawley rats from GD 6 through GD 20.25 The authors concluded that TiO2 NP exposure produced no evidence of maternal or developmental toxicity at any dose level. However, Zhao et al’s study demonstrated that female mice who were exposed to 2.5, 5 and 10 mg/kg TiO2 NPs by intragastric administration for 90 consecutive days, resulted in significant reduction of relative weight and function of ovary, and pregnancy rate and number of newborns.15 Reduced pregnancy rate, and fertility may be a result of ovarian injury because the fertility of treated females was tested by caging with males of proven fertility after 90 days.13,15
In this study, the significant reduction of developmental related genes Hand1, Esx1, Eomes, Ascl2 and Fra1 expression was observed in TiO2 NP-treated placental tissues. After implantation, Hand1 expression is restricted to placental trophoblast cells and essential for the differentiation of trophoblast.26 Esx1 is required for mouse labyrinthine development and mesoderm formation.27 In addition, Eomes and Fra1 also play a crucial role in the labyrinthine development.28,29 Therefore, downregulation of Hand1, Esx1, Eomes and Fra1 expression would severely impair the development of labyrinth and result in the downregulation of labyrinth area in the 10 mg/kg/day dose group. Ascl2 is essential to spongiotrophoblast maintenance and the development of extraembryonic trophoblast lineage in mice. In Ascl2 mutant placentas, spongiotrophoblast cells and their precursors are absent and chorionic ectoderm is reduced.30 In di-(2-ethylhexyl) phthalate-treated placenta, the area of spongiotrophoblast and expression levels of Ascl2 mRNA dramatically decreased in mice on GD 13.20 However, the area of placental spongiotrophoblast significantly increased in the 10 mg/kg/day TiO2 NP group compared to control group. This suggested that the development of spongiotrophoblast with exposure to TiO2 NPs was not closely related to the downregulation of Ascl2. The confirmation of the underlying mechanism requires further investigation.
Vascularization of placental labyrinth is essential for the growth and development of placenta and establishment of successful pregnancy.31,32 Our results evidenced that TiO2 NP treatment resulted in placental vascular collapse and it impaired the formation of an intricate network of fetal vessels. Similar to our results, Yamashita et al found that spiral artery canals failed to form and blood flow was significantly reduced in the fetal vascular sinuses of 70 nm TiO2 NP-treated mice.19 Stapleton et al’s study showed that TiO2 NP exposure significantly impaired the microvascular reactivity of uterine and coronary circulations of female offspring.32 Furthermore, Stapleton et al reported that thrombosis was observed in the pulmonary vascular system of TiO2 NP-treated mice, which could be induced by the blocking of blood vessels with TiO2 particles.33 In addition, uNK cells are short-lived, terminally differentiated and the most abundant lymphocyte in the uterus, and play a vital role in vascularization, spiral arteriole modification and placentation.34–36 Our results showed that 10 mg/kg/day TiO2 NP treatment significantly decreased the numbers of uNK cells in the placental decidua compared with control group. This suggests that reduction of branched fetal vessels may partly result from the downregulation of uNK cells in the TiO2 NP-treated group. Previous research indicated that Esx1, Hand2 and Fra1 were closely related with vascularization.27,29,37 Therefore, our study showed that downregulation of Esx1, Hand2 and Fra1 mRNA in TiO2 NP-treated placenta possibly also contributed to the decrease of fetal vessels within the labyrinth.
We also observed whether defective growth and development of placentas exposure TiO2 NPs were associated with the proliferation and apoptosis changes of placenta. PCNA staining indicated that TiO2 NP treatment significantly decreased the proliferation of placental trophoblast. TEM analysis showed that nuclear pyknosis, expansion of sliding endoplasmic reticulum and abscission of rough endoplasmic reticulum ribosomes were observed in the TiO2 NP-treated placental tissues. Genes involved in apoptosis cleaved-Casp-3 and Bax proteins were markedly upregulated, while Bcl-2 was significantly downregulated in the mice placentas. In concordance with our report, Li et al found that TiO2 NPs induced apoptosis in mouse spleen by activating Casp-3.38 Overexpression of Bcl-2 prevents cells from undergoing apoptosis in response to a variety of harmful stimuli.39 In our present study, Bcl-2 expression was significantly reduced, suggesting that Bcl-2 may play an important role in TiO2 NP-induced trophoblast apoptosis in the mice placentas. It has been shown that exposure to TiO2 NPs resulted in the upregulation of Casp-3, Nrbp2 and cytochrome c expression, and caused downregulation of SOD, CAT, GST and Bcl-2 expression in the Sertoli cells of mouse testis.22 Consequently, Sertoli cell apoptosis in testicular tissue caused by TiO2 NPs may be involved in oxidative stress. However, underlying mechanisms of TiO2 NP-induced placental apoptosis in mice requires further investigation.
Conclusion
Our results provide evidence that gestational exposure to TiO2 NPs significantly impairs the growth and development of placenta during mice pregnancy with a mechanism that seems to be involved in vascularization, proliferation and apoptosis pathways. The present study provides new insight into the mechanism of TiO2 NP-induced fetal mortality and deformities. Therefore, we believe that the unnecessary supplementation of commercial products with TiO2 NPs should be restrained during pregnancy, until we are sure that it has no detrimental effects on reproductive health.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81671486, 81270668, 31260248 and 81460226) and The 555 project of Jiangxi Province Gan Po Excellence. We thank Min Ren of the Department of Physiology, Basic Medical College, Nanchang University, for providing technical support and Dr Luo Z of the Department of Biochemistry, Boston University School of Medicine and Gong H of the Department of Clinic Medicine, School of Queen Mary, Nanchang University, for proofreading this manuscript.
Author contributions
LZ, XX, YZ and HK conceived and designed the study. LZ, XX and DY, YD and JO carried out all the experiments. BY, DL and DZ performed Western blot analysis. LZ, YZ, and HK drafted the paper. All the authors contributed toward statistical analysis and revising the paper, read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Roduner E. Size matters: why nanomaterials are different. Chem Soc Rev. 2006;35(7):583–592. | ||
Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46(4):2242–2250. | ||
Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. | ||
Li SQ, Zhu RR, Zhu H, et al. Nanotoxicity of TiO(2) nanoparticles to erythrocyte in vitro. Food Chem Toxicol. 2008;46(12):3626–3631. | ||
Cui Y, Gong X, Duan Y, et al. Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J Hazard Mater. 2010;183(1–3):874–880. | ||
Gui S, Zhang Z, Zheng L, et al. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;195:365–370. | ||
Shimizu M, Tainaka H, Oba T, Mizuo K, Umezawa M, Takeda K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part Fibre Toxicol. 2009;6:20. | ||
Sha B, Gao W, Wang S, et al. Nano-titanium dioxide induced cardiac injury in rat under oxidative stress. Food Chem Toxicol. 2013;58:280–288. | ||
Alarifi S, Ali D, Al-Doaiss AA, Ali BA, Ahmed M, Al-Khedhairy AA. Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int J Nanomedicine. 2013;8:3937–3943. | ||
Hong F, Zhao X, Si W, et al. Decreased spermatogenesis led to alterations of testis-specific gene expression in male mice following nano-TiO2 exposure. J Hazard Mater. 2015;300:718–728. | ||
Morgan AM, Ibrahim MA, Noshy PA. Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of Tiron in adult male rats. Biochem Biophys Res Commun. 2017;486(2):595–600. | ||
Komatsu T, Tabata M, Kubo-Irie M, et al. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol In Vitro. 2008;22(8):1825–1831. | ||
Gao G, Ze Y, Li B, et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater. 2012;243:19–27. | ||
Tassinari R, Cubadda F, Moracci G, et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology. 2014;8(6):654–662. | ||
Zhao X, Ze Y, Gao G, et al. Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS One. 2013;8(4):e59378. | ||
Philbrook NA, Winn LM, Afrooz AR, Saleh NB, Walker VK. The effect of TiO(2) and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 mice. Toxicol Appl Pharmacol. 2011;257(3):429–436. | ||
Hong F, Zhou Y, Zhao X, Sheng L, Wang L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int J Nanomedicine. 2017;12:6197–6204. | ||
Melnik EA, Buzulukov YP, Demin VF, et al. Transfer of silver nanoparticles through the placenta and breast milk during in vivo experiments on rats. Acta Naturae. 2013;5(3):107–115. | ||
Yamashita K, Yoshioka Y, Higashisaka K, et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol. 2011;6(5):321–328. | ||
Zong T, Lai L, Hu J, et al. Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice. J Hazard Mater. 2015;297:25–33. | ||
Wang J, Zhou G, Chen C, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168(2):176–185. | ||
Hong F, Zhao X, Chen M, et al. TiO2 nanoparticles-induced apoptosis of primary cultured Sertoli cells of mice. J Biomed Mater Res A. 2016;104(1):124–135. | ||
Hong F, Hong J, Wang L, et al. Chronic exposure to nanoparticulate TiO2 causes renal fibrosis involving activation of the Wnt pathway in mouse kidney. J Agric Food Chem. 2015;63(5):1639–1647. | ||
Yu WJ, Son JM, Lee J, et al. Effects of silver nanoparticles on pregnant dams and embryo-fetal development in rats. Nanotoxicology. 2014;8(Suppl 1):85–91. | ||
Warheit DB, Boatman R, Brown SC. Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment-different grade & 3 nanoscale) demonstrate an absence of effects in orally-exposed rats. Regul Toxicol Pharmacol. 2015;73(3):887–896. | ||
Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18(3):271–275. | ||
Li Y, Behringer RR. Esx1 is an X-chromosome-imprinted regulator of placental development and fetal growth. Nat Genet. 1998;20(3):309–311. | ||
Russ AP, Wattler S, Colledge WH, et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404(6773):95–99. | ||
Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF. Placental vascularisation requires the AP-1 component fra1. Development. 2000;127(22):4937–4948. | ||
Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371(6495):333–336. | ||
Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitoneal injection. J Appl Toxicol. 2009;29(4):330–337. | ||
Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21(1):15–25. | ||
Stapleton PA, Nichols CE, Yi J, et al. Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology. 2015;9(8):941–951. | ||
Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev. 2006;214:161–185. | ||
Chaouat G. Current knowledge on natural killer cells, pregnancy and pre-eclampsia. Introduction. Reprod Biomed Online. 2008;16(2):170–172. | ||
Gong H, Chen Y, Xu J, et al. The regulation of ovary and conceptus on the uterine natural killer cells during early pregnancy. Reprod Biol Endocrinol. 2017;15(1):73. | ||
Tsuchihashi T, Maeda J, Shin CH, et al. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol. 2011;351(1):62–69. | ||
Li N, Duan Y, Hong M, et al. Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticles. Toxicol Lett. 2010;195(2–3):161–168. | ||
Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26(7):877–897. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.