Back to Journals » Clinical Epidemiology » Volume 12
Geographical and sociodemographic differences in discontinuation of medication for Chronic Obstructive Pulmonary Disease – A Cross-Classified Multilevel Analysis of Individual Heterogeneity and Discriminatory Accuracy (MAIHDA)
Authors Khalaf K , Axelsson Fisk S , Ekberg-Jansson A, Leckie G , Perez-Vicente R, Merlo J
Received 27 January 2020
Accepted for publication 11 May 2020
Published 20 July 2020 Volume 2020:12 Pages 783—796
DOI https://doi.org/10.2147/CLEP.S247368
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Vera Ehrenstein
Kani Khalaf,1,* Sten Axelsson Fisk,1,* Ann Ekberg-Jansson,2,3 George Leckie,1,4 Raquel Perez-Vicente,1 Juan Merlo1,5
1Unit for Social Epidemiology, Faculty of Medicine, Lund University, Malmö, Sweden; 2Department of Research and Development, Region Halland, Halmstad, Sweden; 3Department of Internal Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; 4Centre for Multilevel Modelling, University of Bristol, Bristol, UK; 5Center for Primary Health Care Research, Region Skåne, Malmö, Sweden
*These authors contributed equally to this work
Correspondence: Kani Khalaf Email [email protected]
Background: While discontinuation of COPD maintenance medication is a known problem, the proportion of patients with discontinuation and its geographical and sociodemographic distribution are so far unknown in Sweden. Therefore, we analyse this question by applying an innovative approach called multilevel analysis of individual heterogeneity and discriminatory accuracy (MAIHDA).
Patients and Methods: We analysed 49,019 patients categorized into 18 sociodemographic contexts and 21 counties of residence. All patients had a hospital COPD diagnosis and had been on inhaled maintenance medication during the 5 years before the study baseline in 2010. We defined “discontinuation” as the absolute lack of retrieval from a pharmacy of any inhaled maintenance medication during 2011. We performed a cross-classified MAIHDA and obtained the average proportion of discontinuation, as well as county and sociodemographic absolute risks, and compared them with a proposed benchmark value of 10%. We calculated the variance partition coefficient (VPC) and the area under the receiver operating characteristics curve (AUC) to quantify county and sociodemographic differences. To summarize the results, we used a framework with 15 scenarios defined by the size of the differences and the level of achievement in relation to the benchmark value.
Results: Around 18% of COPD patients in Sweden discontinued maintenance medication, so the benchmark value was not achieved. There were very small county differences (VPC=0.35%, AUC=0.54). The sociodemographic differences were small (VPC=4.98%, AUC=0.57).
Conclusion: Continuity of maintenance medication among COPD patients in Sweden could be improved by reducing the unjustifiably high prevalence of discontinuation. The very small county and small sociodemographic differences should motivate universal interventions across all counties and sociodemographic groups. Geographical analyses should be combined with sociodemographic analyses, and the cross-classified MAIHDA is an appropriate tool to assess health-care quality.
Keywords: COPD, socioeconomic inequity, multilevel analysis, equity in health care, health care quality, compliance, discriminatory accuracy
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive and irreversible disorder that impairs quality of life,1 increases the risk of premature mortality and conveys considerable costs for both the individual and society.2 While smoking cessation reduces mortality among COPD patients,3 life-long inhaled maintenance medication of COPD reduces symptoms and exacerbations, increases activity tolerance and improves health-related quality of life.4–6 Life-long inhaled maintenance medication is recommended in both international5 and the national Swedish guidelines for COPD management, except for the mildest stage.7 Because COPD is a chronic condition, once a patient has initiated inhaled maintenance medication, it should not be discontinued, unless the initial diagnosis was incorrect or the patient suffers intolerable side effects from the medication, which is not a frequent problem.5,8
The evidence is divergent regarding the influence of socioeconomic factors on adherence with inhaled maintenance medication among COPD patients. Low socioeconomic position was associated with more moderate adherence in the USA,9 while in Denmark one study found an association with lower,10 and another with higher adherence.11 In a Swedish study, adherence was equal across age and gender categories but socioeconomic factors were not analysed.12 In Sweden, health-care management is a county council responsibility and geographical differences between counties in health-care quality are regularly monitored by the Swedish authorities.13 However, it is still unknown whether there are geographical and sociodemographic differences in discontinuation of inhaled maintenance medication (henceforth “discontinuation”). Therefore, the aim of our study was to evaluate such possible differences. We analysed 49,019 patients from 18 different sociodemographic contexts and residing in the 21 Swedish counties in 2010.
For the purpose of our investigation, we apply an innovative methodological approach called multilevel analysis of individual heterogeneity and discriminatory accuracy (MAIHDA).14–17 MAIHDA is not a new methodology per se, but it may be viewed as a reorganization of existing multilevel modelling concepts. The MAIHDA approach proposed here stresses the relevance of performing a systematic analysis that simultaneously considers county and sociodemographic differences in the average risk of discontinuation and the extent of individual variation around such averages. This methodology allows the disentangling of geographical from sociodemographic inequalities. It also maps and quantifies the sizes of such inequalities and provides information on the discriminatory accuracy of the sociodemographic and geographical information when predicting discontinuation in COPD patients. Compared with traditional analysis based on differences between group averages, the MAIHDA methodology provides an improved tool for auditing geographical and sociodemographic inequalities in quality of health care.
Patients and Methods
Databases and Study Population
We analysed a database constructed by record linkage between several Swedish registers with national coverage: the Swedish Population Register19 and the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA), administrated at Statistics Sweden; as well as data from the National Patient Register (NPR),20 the Swedish Prescribed Drug Register (SPDR)21 and the Cause of Death Register,22 administrated by the National Board of Health and Welfare. We linked the registers by means of the anonymized personal identification number provided by the Swedish authorities.
Initially, we selected all 4,994,992 individuals aged 35–80 years who resided in Sweden on 31st December 2010. We then restricted this to 69,391 patients with a COPD diagnosis defined according to the International Classification of Diseases, 10th edition (codes at any position) as emphysema (J43) or other chronic obstructive pulmonary disease (J44). The NPR includes information from all Swedish hospitals on both outpatient external visits and inpatient discharges. However, it does not cover information on diagnoses in primary health care. Next, we excluded 16,402 patients without previous inhaled maintenance pharmacotherapy (see Assessment of Variables, below) between 1st January 2006 and 31st December 2010. For this purpose, we used the SPDR, which records all medications dispensed by the Swedish pharmacies, excluding storage in hospitals and nursing homes. Finally, we excluded 3640 patients who died during 2011 and 330 patients who had resided in Sweden for less than 5 years at baseline.
In summary, the study population consisted of 49,019 patients with a hospital COPD diagnosis. The patients were 35–80 years old and had resided in Sweden for at least 6 years by 31st December 2011. All patients had complete information on demographic and socioeconomic variables and were using inhaled maintenance pharmacotherapy before 31st December 2010 (Figure 1).
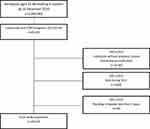 |
Figure 1 Flowchart indicating the selection of patients in the study sample. |
Ethical Statement
The Regional Ethics Review Board in southern Sweden (no. 2012/637), as well as the data safety committees from the National Board of Health and Welfare and from Statistics Sweden, approved the construction of the database.
Data Accessibility
The original databases are available from the Swedish National Board of Health and Welfare, and Statistics Sweden. In Sweden, register data are protected by strict rules of confidentiality23 but can be made available for research after a special review that includes approval of the research project by both an Ethics Committee and the authorities’ own data safety committees. The Swedish authorities under the Ministry of Health and Social Affairs do not provide individual-level data to researchers abroad. Instead, they normally advise researchers in other countries to cooperate with Swedish colleagues and analyse data in collaboration according to standard legal provisions and procedures.
Assessment of Variables
Discontinuation of Inhaled Maintenance Medication (the Outcome Variable)
We first retrieved information from the SPDR. Thereafter, we defined inhaled maintenance medication as any dispensation of the following substances: long-acting β2-agonists (LABA), including salmeterol, formoterol and indacaterol; long-acting muscarinic antagonists (LAMA), including tiotropium bromide; and combinations of LABA and inhaled corticosteroids (LABA-ICS), including formoterol and budesonide, salmeterol and fluticasone, and formoterol and beclometasone. We specify the Anatomical Therapeutic Chemical Classification system (ATC) codes of these substances in the supplementary material. The Stata do-file can easily be adapted by readers for use on their own data.
Based on the Swedish guidelines at the time of the study,7 we assumed that the patients in our sample fulfilled the criteria for inhaled maintenance medication since they all had a COPD diagnosis in the NPR as well as previous inhaled maintenance medication in the SPDR. We defined “discontinuation” as the absolute lack of retrieval from a pharmacy of inhaled maintenance medication between 1st January and 31st December 2011.
Sociodemographic Variables
We defined three age categories: 35–49, 50–64 and 65–80 years. These cut-off values were chosen to create three groups with a similar age-span and to separate individuals aged 65 and older, as 65 years is the official age of retirement. Gender was defined in a binary manner according to legal sex as male or female. We used information on individualized disposable family income for the years 2000, 2005 and 2010 to compute a cumulative measurement that is more stable to temporary fluctuations in income than single measurements.24 We used information on absolute income, which takes into account the size of the household and the consumption weight of the individuals. In each of the three years, income was categorized into 25 groups (coded 1–25) by quantiles using the complete Swedish population. The groups from the three years were then summed up, so a patient could have a value between 3 (always in the lowest income group) and 75 (always in the highest income group). Thereafter, we categorized the cumulative income in three groups by tertiles. Individuals with missing values for income during 2000 or 2005 (N=381) were assigned the tertile values of the year 2010. No individuals in our study population had missing income data for 2010.
Finally, we created a multicategorical sociodemographic variable composed of 18 sociodemographic contexts consisting of all possible combinations of categories of gender, age and income-level variables (2×3×3).
Geographical Information
At the time of our study, Sweden was divided into 21 counties, and each patient was assigned to the county where the individual resided on 31st December 2010.
Multicategorical Geographical and Sociodemographic Matrix
For the purpose of the cross-classified multilevel analyses (see the description in the following subsection), we created a multicategorical matrix with 372 strata defined by the unique combinations of the 18 sociodemographic contexts and the 21 counties (ie, 18×21 minus 6 empty strata).
Multilevel Analysis of Individual Heterogeneity and Discriminatory Accuracy (MAIHDA)
Two-Way Cross-Classified Multilevel Model
We analysed the risk of discontinuation of the patients using cross-classified multilevel logistic regression models with COPD patients simultaneously nested within 18 sociodemographic contexts and within 21 counties. Underneath these two higher levels of analysis, there were the 372 strata.
To avoid giving a higher weight to patient categories with a large number of individuals, as in the case of a traditional single-level analysis, we calculated the average proportion of discontinuation across the geographical and sociodemographic categories. We also considered the reliability and precision of the strata information by using multilevel models, as they are based on reliability-weighted strata residuals (ie, shrunken residuals) and average proportions.25
In addition, crude geographical (eg, county) differences in discontinuation may be confounded by the different composition of the counties in relation to the demographic and socioeconomic characteristics of the patients. Analogously, sociodemographic categories may be confounded by the different health-care management policies of the counties where the patients reside. Ideally, to investigate county and sociodemographic differences, they should be disentangled from one another. Therefore, we performed a two-way cross-classified multilevel model that decomposes the higher level variance into county and sociodemographic components. Let 1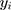 denote the number of patients who discontinue in stratum
denote the number of patients who discontinue in stratum  (
( ). The model is written as
). The model is written as
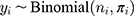
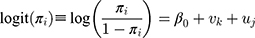
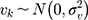
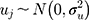
where 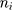 denotes the total number of patients in that stratum,
denotes the total number of patients in that stratum, 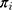 denotes the probability of discontinuation,
denotes the probability of discontinuation, 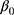 denotes the intercept,
denotes the intercept, 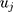 denotes the random effect for sociodemographic context
denotes the random effect for sociodemographic context 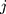 (
(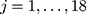 ) and
) and 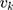 denotes the random effect for county of residence
denotes the random effect for county of residence 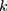 (
(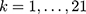 ). The random effects are assumed to be normally distributed with mean 0 and variances
). The random effects are assumed to be normally distributed with mean 0 and variances 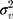 (between counties),
(between counties),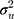 (between sociodemographic contexts). The intercept,
(between sociodemographic contexts). The intercept, 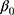 , is the average proportion (on the log-odds scale) of discontinuation (ie, grand mean) across all counties and sociodemographic categories, defined as the 372 strata.
, is the average proportion (on the log-odds scale) of discontinuation (ie, grand mean) across all counties and sociodemographic categories, defined as the 372 strata.
This model has three purposes:
1. Mapping county and sociodemographic differences in discontinuation risk
The first purpose was to obtain an improved mapping of how the individual risk of discontinuation is distributed across counties and sociodemographic strata. We use the predicted random effects (ie, shrunken residuals) from the multilevel regression to calculate the absolute risk (AR) of discontinuation and its 95% credible interval (CI) in each sociodemographic context and county. To do so, we transformed the predicted logit of discontinuation into predicted proportions.
For the county-level prediction, we used the following formula, and calculated the absolute risk (ARC):
2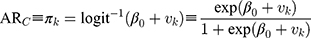
For the sociodemographic context prediction, we used the following formula:
3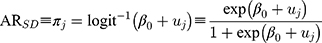
Observe that in Formulas 2 and 3, the predictions isolate the county and sociodemographic differences while holding the other source of differences constant and, in this way, the values are adjusted for each other.
An advantage of multilevel modelling is that in the presence of higher level units with a small number of patients, the shrunken residuals enable one to obtain precision-weighted AR predictions and also to overcome the limitation of model convergence in the presence of small groups.25,26
The graphical or tabulated representation of the ARs facilitates the evaluation of how the individual risk of discontinuation is distributed across counties of residence and sociodemographic contexts. However, this information is based on differences between average ARs, and it does not inform us about individual patient heterogeneity around such averages.14 Therefore, for a complete evaluation, the mapping of risk needs to be accompanied by measures of county, sociodemographic context and individual patient components of variance and/or discriminatory accuracy.
2. Evaluating the components of variance: the variance partition coefficient (VPC)
The second purpose, therefore, was to take into account the individual heterogeneity around the averages and quantify the share of the total individual differences in the latent propensity of discontinuation that existed at the different levels of the analysis. Consequently, we calculated a VPC based on the latent response formulation of the model, as it is an approach widely adopted in applied work.27–29
The VPC for the county level (VPCC) informs on the share of the total individual differences in the underlying propensity for discontinuation that existed at the county level. The 5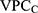 expresses what has been called the general contextual effect;14 that is, the potential ceiling influence of the geo-administrative boundaries of the counties on the individual outcome without any other specific county-level information. The higher the
expresses what has been called the general contextual effect;14 that is, the potential ceiling influence of the geo-administrative boundaries of the counties on the individual outcome without any other specific county-level information. The higher the 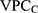 , the higher the county general contextual effect; in other words, the more relevant the county context for understanding individual variation in the latent risk for discontinuation. We computed the
, the higher the county general contextual effect; in other words, the more relevant the county context for understanding individual variation in the latent risk for discontinuation. We computed the 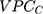 as
as
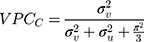
where  denotes the mathematical constant 3.1416, and
denotes the mathematical constant 3.1416, and 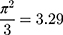 is the variance of the standard logistic distribution. We then multiplied the
is the variance of the standard logistic distribution. We then multiplied the 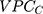 by 100 and interpreted it as a percentage.
by 100 and interpreted it as a percentage.
Analogously, the VPC for the sociodemographic level ( 6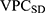 ) can be calculated as
) can be calculated as
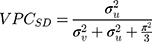
The 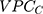 and the
and the 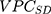 can be directly compared with each other in order to evaluate the relative relevance of geographical versus sociodemographic factors when it comes to understanding patient differences in the latent propensity of discontinuation.
can be directly compared with each other in order to evaluate the relative relevance of geographical versus sociodemographic factors when it comes to understanding patient differences in the latent propensity of discontinuation.
3. Evaluating the discriminatory accuracy (DA) of the information on county of residence and sociodemographic context
A well-known measure of DA is the area under the receiver operating characteristics curve (AUC).14,30 The AUC measures the accuracy of geographical and/or sociodemographic information for discriminating patients according their treatment status (discontinuing or not).
The 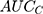 computed for the county level obtained from Formula 2 and the
computed for the county level obtained from Formula 2 and the 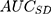 computed for the sociodemographic level from Formula 3 provide complementary information to the
computed for the sociodemographic level from Formula 3 provide complementary information to the 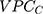 and
and 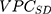 .14,31 One advantage of the use of the AUC is that this measure is already an established concept in clinical epidemiology.
.14,31 One advantage of the use of the AUC is that this measure is already an established concept in clinical epidemiology.
Software and Estimation Methods
All models were run in MLwiN 3.02,32 called from Stata 14.1 using the runmlwin command.33 We note that MLwiN can equally be called from within R using the R2MLwiN package,34 and so our analysis can also be replicated by readers in that statistical package.
We performed all estimations via Markov chain Monte Carlo (MCMC) methods with diffuse (vague, flat or minimally informative) prior distributions for all parameters. We used quasi-likelihood methods to provide starting values for all parameters. For each model, the burn-in length and monitoring chains were set to 5000 and 10,000 iterations. We analysed the parameter chains and standard MCMC convergence diagnostics to evaluate whether the model was adequate.
An advantage of the MCMC is that the resulting parameter chains can be used to construct 95% credible intervals (CI) for all model predictions to communicate statistical uncertainty. MCMC is easy to apply using available software.32,35,36
An advantage of our approach is that the multilevel analyses can be performed using a simple table or matrix with the 372 strata. The only information necessary for the analysis is the number of patients and the number of cases with discontinuation in each stratum. This aggregated approach maintains the joint distribution of the socioeconomic strata and the counties and provides exactly the same model results (parameter estimates, predictions and standard errors) as when analysing the underlying individual-level data. The aggregated approach allows a large number of patients to be analysed in just a few hundred strata, which leads to computationally efficient (fast) estimation. In addition, working with tabulated data reduces ethical problems of confidentiality (statistical disclosure).
The Stata do-file used for our analysis is available as supplementary material.
Auditing Sociodemographic and Geographical Differences in Discontinuation of Inhaled Maintenance Medication
In traditional analysis, geographical (ie, county) differences are evaluated by means of figures (eg, league tables) and sociodemographic differences are appraised by measures of association such as odds ratios or relative risks (ie, socioeconomic gradients). In both cases, the information is only based on differences between group averages. However, as explained in a previous publication,17 in order to perform an improved epidemiological evaluation of sociodemographic and geographical differences in discontinuation, we need at least two types of information.
First, we need a predetermined benchmark or target value informing on the highest percentage of patients with discontinuation that is considered as acceptable. Ideally, this target value should be zero, since there are no formal reasons for discontinuation once maintenance with inhaled therapy is indicated. However, based on standards of ≥90% treatment proposed among Danish COPD patients with documented dyspnoea37 and findings of a prevalence of non-adherence of 5% among patients attending pulmonary outpatient clinics in Denmark,11 we propose a benchmark of 10%, which could be acceptable considering that in some cases medication can be discontinued because of side effects or because the COPD diagnosis was incorrect. Therefore, we propose that a percentage under 10% should be considered as a full achievement, between 10% and 15% as a close achievement and >15% as an insufficient achievement. However, further studies are needed to establish an appropriate benchmark level and the level of achievement. In our study, rather than the country proportion of discontinuation, we used the average proportion (ie, grand mean) across the 372 strata defined in the multicategorical geographical and sociodemographic matrix.
The main questions that we asked in the evaluation were: Has the benchmark value been insufficiently, closely or fully reached? What is the size of the inequities between the counties and between sociodemographic groups? To answer these questions, we created a framework (Table 1) with 15 scenarios combining benchmark value achievement and the size of the county/sociodemographic difference measures according to the VPC and the AUC. First, we located the overall achievement in relation to the predefined benchmark value of acceptable prevalence of discontinuation. Second, we quantified the size of the county and sociodemographic differences expressed as VPC and AUC. Those scenarios can be used to orient the interpretation of an analysis.
 |
Table 1 Framework for Evaluating Continuity of Maintenance Medication Among COPD Patients |
Results
Table 2 presents the characteristics of the 49,019 COPD patients and absolute risk of discontinuation by county of residence, and Table 3 by sociodemographic category. There was a slight overrepresentation of women, and the mean age was around 68 years. In the whole country as well as in all counties except Stockholm, low income was overrepresented in the COPD patient population.
 |
Table 2 Characteristics by County |
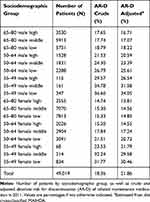 |
Table 3 Number of Patients and Absolute Risk of Discontinuation by Sociodemographic Category |
Overall in Sweden, 8998 patients discontinued inhaled maintenance medication during 2011, giving a national prevalence close to 18%. However, the crude county averages ranged between 14% in Värmland and 21% in Stockholm. The national average percentage of discontinuation across the geographical and sociodemographic strata and accounting for the reliability of the information was 21.9% (95% CI 19.1–25.0%). Table 4 illustrates the results from the cross-classified multilevel model of discontinuation.
According to the framework presented in Table 1, the county differences were very small since the 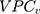 was only 0.4% and the
was only 0.4% and the 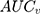 was 0.54. The differences between the sociodemographic categories were higher than the geographical differences but still those differences were small, as the
was 0.54. The differences between the sociodemographic categories were higher than the geographical differences but still those differences were small, as the 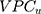 was 5.0% and the
was 5.0% and the 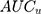 value was 0.57.
value was 0.57.
The cross-classified multilevel model provided information on the predicted average risk of discontinuation for the 21 counties and the 18 sociodemographic contexts simultaneously adjusted for each other. The adjusted county average risk of discontinuation ranged between 19% in the county of Värmland and 26% in the county of Stockholm (Figure 2). The sociodemographic differences were more pronounced than the geographical ones. They varied between 14% in 65–80-year-old women with high income and 34% in 35–49-year-old men with low income (Figure 3). Discontinuation decreased with age, but we did not find clear income gradients. The pattern of discontinuation across sociodemographic categories was similar in men and women, with men having a marginally higher proportion of discontinuation. This difference was not obvious in young patients. However, it was conclusive for patients aged 50–64 and 65–80 years across all three income categories.
An appropriate interpretation of the observed county and sociodemographic differences in Figures 2 and 3 needs to be made in the light of the information provided by the VPC and the AUC (Tables 1 and 4 and Figure 4). Figure 4 shows the AUCs for the county and the sociodemographic information, and it illustrates very clearly their low discriminatory accuracy.
In summary, at the time of our study, there were very small county differences and the sociodemographic inequalities were small, but the proportion of discontinuation was unjustifiably high overall in Sweden. Geographical differences in discontinuation of inhaled maintenance medication can be placed in scenario C and the sociodemographic differences in scenario F (see Table 1) in the framework that we propose.
Discussion
We aimed to evaluate geographical and sociodemographic differences in discontinuation of maintenance with inhaled medication therapy. As far as we know, our study is original in this area and it demonstrates a high prevalence of discontinuation in Sweden. The discontinuation rate across all geographical and sociodemographic categories was 21.86% and, overall, around 18% of the COPD patients who should be on maintenance therapy were not dispensed any such medication during a whole year. While we found statistically significant average differences between both county and sociodemographic strata, those differences only explained very small (geographical case) and small (sociodemographic case) proportions of the individuals’ propensities for discontinuation. Both the VPC and the AUC indicated that discontinuation presented a homogeneous distribution across counties in Sweden. Sociodemographic categorizations appeared to have a higher relevance than counties as determinants of discontinuation.
Our results indicate that measures to reduce the discontinuation of inhaled maintenance medication could be improved among COPD patients in Sweden. Using our proposed benchmark of 10%, the prevalence of discontinuation was double the desired level. However, neither counties nor sociodemographic factors seem relevant to understanding patient discontinuation. Other geographical and sociodemographic contexts may play a more relevant role for understanding patients’ adherence to inhaled medication. For example, the clinics where patients are treated on a regular basis and even physician-prescribing behaviour have been shown to be relevant for adherence to other medications, such as statins.38,39 In addition, in countries with different health-care systems, counties and sociodemographic factors may have a larger influence on adherence.
The prevalence of discontinuation in our study was similar to that observed in previous publications,40–42 in spite of different definitions of medication adherence/discontinuation being used. Haupt et al41 saw that among patients who had received any inhaled medication, 24% received it only once during a 5-year period. However, short-acting pharmacological agents that may be prescribed for non-chronic conditions were included in that study. In another study, Sundh et al40 found that 22% of COPD patients treated at hospitals lacked prescribed maintenance medication. Those results concerning discontinuation, low-dosage coverage or no maintenance treatment on discharge from hospital are in line with our findings.
The high prevalence of discontinuation may have several explanations. Compliance with COPD medication is influenced by many different factors. One possible reason for the high prevalence of discontinuation is offered by publications suggesting a considerable prevalence of COPD overdiagnosis.43,44 If COPD was erroneously diagnosed, the patient would not benefit from maintenance medication and discontinuation would be an adequate response to an incorrect diagnosis. In this scenario, discontinuation of therapy could be a relevant process indicator of COPD health-care quality.
The negative association between age and therapy discontinuation could be explained by the findings by Ingebrigtsen et al11 that adherence and use of maintenance therapy increase with the increased severity of COPD, since COPD is often more severe among older patients. Tottenborg et al presented similar results regarding the relationship between young age and non-use of maintenance therapy, in a Danish cohort study of COPD patients.10
As indicated in Figure 3, and while not statistically significant in all age categories, we found men to have a higher absolute risk of therapy discontinuation than women, which is in line with previous research.40,45 Possible explanations include findings that the lung function of female smokers deteriorates more rapidly than among male smokers, causing more severe COPD46,47 and, thereby, increased adherence with maintenance therapy. However, we need more research on gender disparities in COPD maintenance treatment.
Finally, we did not find obvious income gradients in discontinuation, except among middle-aged women. This observation is in line with findings of small differences in adherence across income groups in Denmark.10 The absence of effect of income on propensity of discontinuation could be explained by the Swedish reimbursement scheme for prescription medication, which is available for all individuals residing in Sweden, and has a co-payment ceiling that by 2011 was at SEK 1800 (~EUR 180) in a given 12-month period. It is also possible that higher disease severity among patients with low income increases adherence and counterbalances a possible income gradient. However, we did not have access to information on COPD severity. In any case, because of the limited success in reducing socioeconomic disparities achieved by behavioural interventions,48 socioeconomic determinants of health higher up in the causal pathway should be addressed in order to reduce inequalities.
It is possible that using only three categories of age and income may result in an underestimation of the variance attributed to the sociodemographic level and wider credible interval. Therefore, we performed a sensitivity analysis using, besides the two categories of sex, nine categories of age and 25 categories of income. In this analysis, the VPC=2.7% (CI: 2.0–3.5%) was lower than in the primary analysis. In absolute terms, our conclusion on the low/moderate relevance of the sociodemographic context remains in both analyses. Using the AUC rather than the VPC values indicates that a finer categorization (ie, AUC=0.59) does not improve the discriminatory accuracy of the original sociodemographic categorization (ie, AUC= 0.57). However, a more detailed categorization would result in more empty cells.
As a supplementary analysis, we also investigated potential interaction effects between county of residence and sociodemographic strata by constructing a third interaction model (see elsewhere for details and an empirical example49). However, we did not find any obvious interaction, suggesting that the degree of sociodemographic inequalities varies across different counties.
Strengths and Limitations
The major limitation of this observational register study is the lack of information on the disease stage and COPD severity of the patients. Since both overdiagnosis and underdiagnosis of COPD are common problems, it is likely that we have both missed COPD patients who ideally should be included and included some patients with erroneous COPD diagnoses. According to the guidelines in Sweden at 2010, all individuals with COPD stages 2, 3 and 4 should be prescribed a bronchodilator as maintenance therapy.7 The study population consisted of individuals treated at hospitals, and with previous prescriptions of LAMA, LABA or LAMA/LABA. Therefore, we assumed that all COPD patients included in our study had COPD stage 2 or higher and needed maintenance therapy. While our assumption seems very probable, we need further studies with exact information on COPD stage since patients with more severe disease have better adherence compared to those with milder cases.50 We did not have information on the type of inhaler or the frequency of dosing, which also influence adherence.51 Another limitation is that patients who are treated only in primary health care are not covered by the NPR.
Overall, the data used in this study are of high quality since all socioeconomic parameters are based on national registers. A total of 3636 individuals died during 2011 and it cannot be ruled out that this fact introduces a selection bias. Since follow-up was only one year and the proportion of patients who died during the follow-up time accounted for 6.9% of the study population, we do not think that it would alter our conclusions if we ran a survival analysis instead. In a sensitivity analysis where the patients who died during 2011 were not excluded, we found similar results. Our results are based on a large database comprising all patients with COPD diagnosed at hospital wards or specialist outpatient clinics. The validity of the ICD diagnoses of COPD has been judged to be suitable for epidemiological research.52
Another strength of this study is the application of measurements of discriminatory accuracy for investigating socioeconomic and geographical inequities in both public health16,53 and health-care epidemiology.14,54 For instance, in order to assess whether it would be preferable to target certain groups (eg, counties or sociodemographic strata) or to perform a universal intervention, we need measures of general contextual effects. If the general contextual effect is low, targeting only those counties or strata with a high average risk may lead to inefficient interventions, and also raises ethical issues related to risk communication and the perils of stigmatization of individuals from specific strata.55
Multilevel models have a number of advantages compared to traditional single-level models and we refer to previous publications for extended explanations.28,29,56–61 However, the present study emphasizes the advantage of using average proportions based on the reliability-weighted strata rather than on the population of individuals, especially when the interest focuses on measuring the proportion of patients with a specific quality indicator (eg, discontinuation of maintenance medication in our case) in relation to geographical and sociodemographic categories.25 The crude proportion of patients with discontinuation may provide information on the burden of discontinuation in, for instance, the country, but it is less representative of the county and sociodemographic contexts of interest in this study.
Implications and Conclusions
The MAIHDA methodology used in this study converges with the current movement of precision (ie, individualized, personalized, stratified) medicine, and its efforts towards understanding not only differences between group averages but also individual heterogeneity around such averages. Nevertheless, a fundamental conceptual distinction exists between the MAIHDA and individualized medicine: rather than considering only individual characteristics, MAIHDA tries to identify the components of individual heterogeneity in health that are at different contextual levels of analysis. The fundamental statement is that individual and population health are not dislocated study objects. Rather, we need to consider the existence of a continuous distribution of individual outcome heterogeneity that can be articulated at different levels of analysis.15,18
One key question for policy makers is to what degree public health interventions should be universal (ie, similarly directed towards the whole population) or targeted to specific groups. The framework outlined in this study provides a tool to guide such decisions. If the insufficient overall achievement had been accompanied by large disparities, as in scenario O in Table 1, targeted health interventions would be justified for categories above the benchmark value. For the case of discontinuation of COPD maintenance medication (scenarios C and F), our results support the public health concept of proportionate universalism.62,63 Since the overlap between both county and sociodemographic strata is substantial, interventions to improve adherence need to be universal and not exclusively target those groups with increased risk of discontinuation. However, the existence of small sociodemographic disparities and even smaller county-level disparities means that interventions should be proportionately more intense among sociodemographic strata with higher average risk of discontinuation, and to a lesser extent in counties with increased risk of discontinuation. One example of an efficient universal intervention is presented by Tottenborg et al,64 who showed how a systematic quality improvement initiative managed to eliminate socioeconomic inequalities in COPD health care. Such universal incentives should be initiated in sociodemographic strata and counties with higher risk of discontinuation. Our study demonstrates the use of MAIHDA to assess differences between geographical areas (ie, counties) and between sociodemographic contexts. Evaluations of geographical differences in health-care performance should always consider sociodemographic factors, and MAIHDA is an appropriate tool to perform such analyses.
Abbreviations
AUC, area under the receiver operating characteristics curve; COPD, chronic obstructive pulmonary disease; CI, confidence interval; DA, discriminatory accuracy; NPR, National Patient Register; SPDR, Swedish Prescribed Drug Register; GCE, general contextual effect; MAIHDA, multilevel analysis of individual heterogeneity and discriminatory accuracy; VPC, variance partition coefficient.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Carrasco Garrido P, de Miguel Diez J, Rejas Gutierrez J, et al. Negative impact of chronic obstructive pulmonary disease on the health-related quality of life of patients. Results of the EPIDEPOC study. Health Qual Life Outcomes. 2006;4(1):31. doi:10.1186/1477-7525-4-31
2. Loddenkemper R, Gibson GJ, Sibille Y. The burden of lung disease in Europe: why a European white book on lung disease? Eur Respir J. 2003;22(6):869. (). doi:10.1183/09031936.03.00107803
3. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi:10.7326/0003-4819-142-4-200502150-00005
4. Ferguson GT. Maintenance pharmacotherapy of mild and moderate COPD: what is the evidence? Respir Med. 2011;105(9):1268–1274. doi:10.1016/j.rmed.2011.02.005
5. GOLD. From the global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD) 2017; 2017. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/2017. Official homepage for Global Strategy for the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD).
6. Stuart BC, Simoni-Wastila L, Zuckerman IH, et al. Impact of maintenance therapy on hospitalization and expenditures for Medicare beneficiaries with chronic obstructive pulmonary disease. Am J Geriatr Pharmacother. 2010;8(5):441–453. doi:10.1016/j.amjopharm.2010.10.002
7. [Läkemedelsverket]. SMPA. Chronic obstructive pulmonary disease - treatment recommendations [Swedish: kroniskt Obstruktiv Lungsjukdom - behandlingsrekommendationer]; 2015. Available from: https://lakemedelsverket.se/upload/halso-och-sjukvard/behandlingsrekommendationer/Kroniskt_obstruktiv_lungsjukdom_KOL_behandlingsrekommendation.pdf2015.
8. Melani AS. Long-acting muscarinic antagonists. Expert Rev Clin Pharmacol. 2015;8(4):479–501. doi:10.1586/17512433.2015.1058154
9. Oates GR, Hamby BW, Stepanikova I, et al. Social determinants of adherence to pulmonary rehabilitation for chronic obstructive pulmonary disease. Copd. 2017;14(6):610–617. doi:10.1080/15412555.2017.1379070
10. Tottenborg SS, Lange P, Johnsen SP, Nielsen H, Ingebrigtsen TS, Thomsen RW. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir Med. 2016;119:160–167. doi:10.1016/j.rmed.2016.09.007
11. Ingebrigtsen TS, Marott JL, Nordestgaard BG, et al. Low use and adherence to maintenance medication in chronic obstructive pulmonary disease in the general population. J Gen Intern Med. 2015;30(1):51–59. doi:10.1007/s11606-014-3029-0
12. Henoch I, Strang S, Löfdahl C-G, Ekberg-Jansson A. Management of COPD, equal treatment across age, gender, and social situation? A register study. Int J Chron Obstruct Pulmon Dis. 2016;11:2681–2690. doi:10.2147/COPD.S115238
13. Landsting S Kvalitetsindikatorer i hälso- och sjukvården; 2012. Available from: www.skl.se/publikationer.
14. Merlo J, Wagner P, Ghith N, Leckie G. An original stepwise multilevel logistic regression analysis of discriminatory accuracy: the case of neighbourhoods and health. PLoS One. 2016;11(4):e0153778. doi:10.1371/journal.pone.0153778.
15. Merlo J. Invited commentary: multilevel analysis of individual heterogeneity-a fundamental critique of the current probabilistic risk factor epidemiology. Am J Epidemiol. 2014;180(2):208–212. doi:10.1093/aje/kwu108
16. Merlo J. Multilevel analysis of individual heterogeneity and discriminatory accuracy (MAIHDA) within an intersectional framework. Social Sci Med. 2018;203:74–80. doi:10.1016/j.socscimed.2017.12.026
17. Merlo J, Wagner P, Leckie G. A simple multilevel approach for analysing geographical inequalities in public health reports: the case of municipality differences in obesity. Health Place. 2019;58:102145. doi:10.1016/j.healthplace.2019.102145
18. Merlo J. Multilevel analysis of individual heterogeneity and discriminatory accuracy (MAIHDA) within an intersectional framework. Soc Sci Med. 2017;203: 74–80.
19. Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136.
20. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi:10.1186/1471-2458-11-450
21. Wettermark B, Hammar N, Fored CM, et al. The new swedish prescribed drug register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. doi:10.1002/pds.1294
22. Brooke HL, Talback M, Hornblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773. doi:10.1007/s10654-017-0316-1
23. Public Access to Information and Secrecy Act: Government Offices of Sweden; 2009. Available from: http://www.government.se/information-material/2009/09/public-access-to-information-and-secrecy-act/.
24. Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007;81–82(1):21–37. doi:10.1093/bmb/ldm001
25. Jones K, Johnston R, Manley D. Uncovering interactions in multivariate contingency tables: a multi-level modelling exploratory approach. Methodol Innovations. 2016;9:1–17. doi:10.1177/2059799116672874
26. Evans CR, Williams DR, Onnela J-P, Subramanian S. A multilevel approach to modeling health inequalities at the intersection of multiple social identities. Soc Sci Med. 2017;203:64–73.
27. Goldstein H, Browne WJ, Rashbash J. Partitioning variation in multilevel models. Understanding Stat. 2002;1(4):223–231. doi:10.1207/S15328031US0104_02
28. Merlo J, Yang M, Chaix B, Lynch J, Rastam L. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health. 2005;59(9):729–736. doi:10.1136/jech.2004.023929
29. Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–297. doi:10.1136/jech.2004.029454
30. Wagner P, Merlo J. Measures of discriminatory accuracy in multilevel analysis. Eur J Epidemiol. 2013;28(1, Supplement):135.
31. Wagner P, Merlo J. Discriminatory accuracy of a random effect in multilevel logistic regression. Int J Epidemiol. 2014;44(suppl_1):i49–i50. doi:10.1093/ije/dyv097.190
32. Charlton C, Rasbash J, Browne WJ, Healy M, Cameron B. MLwiN Version 3.00. Centre for Multilevel Modelling, University of Bristol.; 2017.
33. Leckie G, Charlton C. runmlwin - A program to run the MLwiN multilevel modelling software from within stata. J Stat Softw. 2013;52(11):1–40.
34. Zhang Z, Parker R, Charlton C, Leckie G, Browne WJ. R2MLwiN - A program to run the MLwiN multilevel modelling software from within R. J Stat Softw. 2016;72(10):1–43. doi:10.18637/jss.v072.i10
35. Leckie G, Charlton C. runmlwin - A program to run the MLwiN multilevel modelling software from within stata. J Stat Softw. 2013;52(11):1–40. doi:10.18637/jss.v052.i12
36. Browne WJ MCMC estimation in MLwiN. Version 2.31. Centre for multilevel modelling, university of Bristol. 2014;ISBN: 978-0-903024-99-0Available from: http://www.bristol.ac.uk/cmm/media/migrated/2-31/mcmc-print.pdf.
37. Lange P, Tottenborg SS, Sorknaes AD, et al. Danish Register of chronic obstructive pulmonary disease. Clin Epidemiol. 2016;8:673–678. doi:10.2147/clep.s99489;.
38. Hjerpe P, Ohlsson H, Lindblad U, Bostrom KB, Merlo J. Understanding adherence to therapeutic guidelines: a multilevel analysis of statin prescription in the Skaraborg Primary Care Database. Eur J Clin Pharmacol. 2010;67:415–423.
39. Ohlsson H, Lindblad U, Lithman T, et al. Understanding adherence to official guidelines on statin prescribing in primary health care - a multi-level methodological approach. Eur J Clin Pharmacol. 2005;61(9):657–665. doi:10.1007/s00228-005-0975-9
40. Sundh J, Aberg J, Hasselgren M, et al. Factors influencing pharmacological treatment in COPD: a comparison of 2005 and 2014. Eur Clin Respir j. 2017;4(1):1409060. doi:10.1080/20018525.2017.1409060
41. Haupt D, Krigsman K, Nilsson JL. Medication persistence among patients with asthma/COPD drugs. Pharm World Sci. 2008;30(5):509–514. doi:10.1007/s11096-008-9197-4
42. Luftvägsregistret; 2017. Available from: https://lvr.registercentrum.se/om-registret/om-luftvagsregistret/p/BJGNnV3Zl2017.
43. Sator L, Horner A, Studnicka M, et al. Overdiagnosis of COPD in subjects with unobstructed spirometry: A BOLD Analysis. Chest. 2019;156(2):277–288. doi:10.1016/j.chest.2019.01.015
44. Gershon AS, Thiruchelvam D, Chapman KR, et al. Health services burden of undiagnosed and overdiagnosed COPD. Chest. 2018;153(6):1336–1346. doi:10.1016/j.chest.2018.01.038
45. Ingebrigtsen TS, Marott JL, Vestbo J, et al. Characteristics of undertreatment in COPD in the general population. Chest. 2013;144(6):1811–1818. doi:10.1378/chest.13-0453
46. Gan WQ, Man SF, Postma DS, Camp P, Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7(1):52. doi:10.1186/1465-9921-7-52
47. Sorheim IC, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–485. doi:10.1136/thx.2009.122002
48. Vilhelmsson A, Ostergren PO. Reducing health inequalities with interventions targeting behavioral factors among individuals with low levels of education - A rapid review. PLoS One. 2018;13(4):e0195774. doi:10.1371/journal.pone.0195774.
49. Axelsson Fisk S, Mulinari S, Wemrell M, et al. Chronic obstructive pulmonary disease in sweden: an intersectional multilevel analysis of individual heterogeneity and discriminatory accuracy. SSM Popul Health. 2018;4:334–346. doi:10.1016/j.ssmph.2018.03.005
50. Humenberger M, Horner A, Labek A, et al. Adherence to inhaled therapy and its impact on chronic obstructive pulmonary disease (COPD). BMC Pulm Med;2018:18(1); 163. doi:10.1186/s12890-018-0724-3
51. Koehorst-ter Huurne K, Movig K, van der Valk P, et al. The influence of type of inhalation device on adherence of COPD patients to inhaled medication. Expert Opin Drug Deliv. 2016;13(4):469–475. doi:10.1517/17425247.2016.1130695
52. Inghammar M, Engstrom G, Lofdahl CG, Egesten A. Validation of a COPD diagnosis from the Swedish Inpatient Registry. Scand J Public Health. 2012;40(8):773–776. doi:10.1177/1403494812463172.
53. Merlo J. Multilevel analytical approaches in social epidemiology: measures of health variation compared with traditional measures of association. J Epidemiol Community Health. 2003;57(8):550–552. (). doi:10.1136/jech.57.8.550
54. Ghith N, Frolich A, Merlo J. The role of the clinical departments for understanding patient heterogeneity in one-year mortality after a diagnosis of heart failure: A multilevel analysis of individual heterogeneity for profiling provider outcomes. PLoS One. 2017;12(12):e0189050. doi:10.1371/journal.pone.0189050
55. Merlo J, Mulinari S, Wemrell M, Subramanian SV, Hedblad B. The tyranny of the averages and the indiscriminate use of risk factors in public health: the case of coronary heart disease. SSM Popul Health. 2017;3:684–698. doi:10.1016/j.ssmph.2017.08.005
56. Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling.
57. Hox JJ. Multilevel Analysis: Techniques and Applications.
58. Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods.
59. Goldstein H, Goldstein H. Multilevel Statistical Models.
60. Merlo J, Chaix B, Yang M, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: linking the statistical concept of clustering to the idea of contextual phenomenon. J Epidemiol Community Health. 2005;59(6):443–449. doi:10.1136/jech.2004.023473
61. Merlo J, Chaix B, Yang M, et al. A brief conceptual tutorial on multilevel analysis in social epidemiology: interpreting neighbourhood differences and the effect of neighbourhood characteristics on individual health. J Epidemiol Community Health. 2005;59(12):1022–1028. doi:10.1136/jech.2004.028035
62. Marmot M, Bell R. Fair society, healthy lives. Public Health. 2012;126 Suppl 1:S4–s10. doi:10.1016/j.puhe.2012.05.014
63. Carey G, Crammond B, De Leeuw E. Towards health equity: a framework for the application of proportionate universalism. Int J Equity Health. 2015;14(1):81. (). doi:10.1186/s12939-015-0207-6
64. Tottenborg SS, Lange P, Thomsen RW, Nielsen H, Johnsen SP. Reducing socioeconomic inequalities in COPD care in the hospital outpatient setting - A nationwide initiative. Respir Med. 2017;125:19–23. doi:10.1016/j.rmed.2017.02.016
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




