Back to Journals » Infection and Drug Resistance » Volume 12
Gentamicin susceptibility of Neisseria gonorrhoeae isolates from 7 provinces in China
Authors Liu JW , Xu WQ , Zhu XY, Dai XQ, Chen SC , Han Y, Liu J , Chen XS , Yin YP
Received 7 May 2019
Accepted for publication 12 July 2019
Published 9 August 2019 Volume 2019:12 Pages 2471—2476
DOI https://doi.org/10.2147/IDR.S214059
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Jing-Wei Liu,1,2 Wen-Qi Xu,1,2 Xiao-Yu Zhu,1,2 Xiu-Qin Dai,1,2 Shao-Chun Chen,1,2 Yan Han,1,2 Jun Liu,3 Xiang-Sheng Chen,1,2 Yue-Ping Yin1,2
1Institute of Dermatology and Hospital for Skin Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, People’s Republic of China; 2National Center for Sexually Transmitted Diseases Control, Chinese Center for Disease Control and Prevention, Nanjing, People’s Republic of China; 3SYNAPSE Center, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Purpose: Gentamicin is a promising antimicrobial for the treatment of gonorrhea. The study aimed to evaluate gentamicin minimum inhibitory concentrations (MICs) of Neisseria gonorrhoeae isolates in China.
Methods: In this study, the agar dilution method was used to determine the MICs of 470 isolates collected in 2016 to four effective antimicrobials (gentamicin, azithromycin, ceftriaxone, and spectinomycin).
Results: Gentamicin MICs ranged from 1 to 8 mg/L. No isolate was resistant to gentamicin. Of seven isolates simultaneously resistant to azithromycin and ceftriaxone, 6 isolates demonstrated MICs of 4 mg/L or less to gentamicin. No cross relationships were found between MICs of gentamicinand susceptibility profiles of azithromycin, ceftriaxone, and spectinomycin.
Conclusion: The in vitro results suggest that gentamicin can be a promising treatment option for gonococcal infections in China. Clinical trials to evaluate the therapeutic efficacy of gentamicin are required.
Keywords: Neisseria gonorrhoeae, agar dilution method, gentamicin, antimicrobial surveillance
Introduction
Neisseria gonorrhoeae (N. gonorrhoeae) is a sexually transmitted pathogen causing gonorrhea, which is the fourth most commonly reported notifiable communicable disease in China, with 133,156 reported cases in 2018.1 If left untreated, N. gonorrhoeae can cause adverse health outcomes. It also facilitates the transmission of HIV, causing immense morbidity and socioeconomic consequences.2 Without an effective vaccine against N. gonorrhoeae, antimicrobials are the only medical approaches to gonococcal treatment and control. However, N. gonorrhoeae has developed resistance to multiple classes of antibiotics over the past 60 years, and previously effective therapeutics such as penicillin, tetracycline, and fluoroquinolones are no longer recommended.3
Ceftriaxone is currently recommended as the first-line treatment option for gonococcal infection in American, Australian, Canadian, Chinese, European, and World Health Organization (WHO) guidelines for the treatment of sexually transmitted diseases.4,5 Azithromycin has also been prescribed as a part of dual treatment regimen. Besides, spectinomycin remains susceptible to a large amount of N. gonorrhoeae isolates and is used as an alternative treatment option.6,7 Moreover, the emergence of N. gonorrhoeae isolates with a multidrug resistant or extremely-drug resistant profile highlights the importance of considering alternatives for future therapeutic options. Notably, in China, the rates of isolates resistant to azithromycin or with decreased susceptibility to ceftriaxone were high from 2013 to 2016, with 18.6% to azithromycin and 10.8% to ceftriaxone.8 Although isolates resistant to spectinomycin were sporadic in China, its inferior efficacy for treating oropharyngeal infections and its high potential to develop resistance limits its application.6,9
Gentamicin has been recommended as an alternate therapy to ceftriaxone in American, European, and WHO guidelines.7,10,11 In addition, it is an aminoglycoside antimicrobial that has been used in many developing countries to treat gonorrhea, with the advantage of having both high therapeutic efficacy and low cost.12 However, there is no published data on gentamicin use in China. If gentamicin can be considered as part of an alternative strategy, the knowledge of the susceptibility of Chinese N. gonorrhoeae isolates to gentamicin is essential. This study evaluated gonococcal susceptibility to gentamicin in China for the first time, in conjunction with susceptibility testing for azithromycin, ceftriaxone, and spectinomycin. This study design allowed for the analysis of statistical relations between these four antimicrobials.
Materials and methods
Ethics approval
The ethics approval for the study was obtained from the Medical Ethics Committee at the Institute of Dermatology, the Chinese Academy of Medical Sciences & Peking Union Medical College, and the National Center for Sexually Transmitted Disease Control at Nanjing (2014-LS-026) for the use of anonymized samples collected from patients attending local dermatology and/or Sexually Transmitted Disease clinics.
Gonococcal isolates
Clinical isolates were consecutively collected from different clinics in 7 provinces (Beijing, Hainan, Liaoning, Sichuan, Tianjin, Xinjiang, and Zhejiang) within the China Gonococcal Resistance Surveillance Programme (China-GRSP) in from 1st January to 31st December 2016 as previously described.8 At every clinic, routine medical treatments were provided as regular. Patients with inflammatory secretions or positive risk factors for gonococcal infection were sampled to conduct isolates cultures. Then samples were inoculated into selective Thayer-Martin (TM) medium and cultured at 36–36.5 °C in a moist 5% CO2-enrichment atmosphere for 24–48 hrs. With the culture-positive isolates, presumptive N. gonorrhoeae isolate was identified based on colonial morphology (growth of typical appearing colonies), Gram stain (Gram-negative) and oxidase test (oxidase-positive diplococci in stained smears). If the identification of isolates were still ambiguous, sugar fermentation tests were further conducted. Subsequently, isolates were sub-cultured from the selective TM medium to a non-inhibitory medium. If the sub-cultured isolates were not pure, serial sub-cultures of individual colonies must be performed until a pure culture was obtained. After 18–20 hrs incubation, colonies of confirmed gonococcal isolates from the pure culture were suspended heavily in skim milk and frozen to −70 °C until transported to our reference laboratory at the National Center for Sexually Transmitted Disease Control, Chinese Center for Disease Control and Prevention (Nanjing, China). Then the frozen isolates collected in 2016 were randomly selected for further antimicrobial susceptibility tests. Of note, strains in Hainan, Sichuan, Tianjin, Xinjiang, and Zhejiang have been determined for susceptibility profiles to ceftriaxone and azithromycin in our previous study.8
Antimicrobial susceptibility tests by agar dilution
Antimicrobial susceptibility tests of all of the isolates to gentamicin, azithromycin, ceftriaxone, and spectinomycin were determined by the agar dilution procedure. Specifically, the isolates were cultured from frozen skimmed milk onto selective TM medium and sub-cultured on GC agar base medium (Oxide, Hampshire, England) supplemented with 10% defibrinated sheep blood (Bianzhen Biotechnology, Nanjing, China) and 1% Iso VitaleX Enrichment (BD Diagnostics, New Jersey, USA) at 36 °C in a moist 5% CO2-enrichment atmosphere for 18–20 hrs. We used morphology and oxidase tests for repetitive gonococcal confirmation before antimicrobial susceptibility tests. Then colonies were scraped to the sterile saline solution (Pengyao Pharmacy, Wuxi, China), and the bacterial suspensions of 107 CFU were prepared. The suspension was inoculated to antibiotic-containing medium (GC agar base medium supplemented with 1% Iso VitaleX Enrichment) on a 9-cm diameter plate by using a multipoint inoculator (104 CFU per point). The plates were cultured at 36 °C in a moist 5% CO2-enrichment atmosphere for 18–24 hrs, and the growth of N. gonorrhoeae in different concentrations of antibiotic was observed and recorded.13 For the four antibiotics, their concentrations were 1, 2, 4, 8, 16, and 32 mg/L for gentamicin; ≤0.125, 0.25, 0.5, 1, 2, and ≥4 mg/L for azithromycin; ≤0.008, 0.015, 0.03, 0.06, 0.125, 0.25, and ≥0.5 mg/L for ceftriaxone; and ≤8, 16, 32, 64, and >64 mg/L for spectinomycin. The minimum inhibitory concentration (MIC) of isolates was defined as the lowest concentration of the antibiotic that inhibited growth. Interpretive criteria were in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST, Version 8.1) guidelines to categorize profiles of susceptibility or resistance. For azithromycin, we categorized MIC as susceptibility (MIC ≤0.5 mg/L) or resistance (MIC ≥1.0 mg/L). For ceftriaxone, we defined MIC as susceptibility (MIC ≤0.125 mg/L) or resistance (MIC ≥0.25 mg/L). For spectinomycin, we categorized MIC as susceptibility (MIC ≤64 mg/L) or resistance (MIC >64 mg/L).14 There are no standardized criteria for gentamicin in EUCAST and Clinical and Laboratory Standards Institute (CLSI) methodologies thus far. Previous published interpretative criteria were used: MIC ≤4 mg/L, susceptible; MIC 8–16 mg/L, intermediately susceptible; MIC ≥32 mg/L, resistant.15 For quality assurance, four WHO N. gonorrhoeae reference strains (J, K, L, and P) were used as controls.16
Data analysis
In this study, we described the distribution of the randomly selected and successfully cultured N. gonorrhoeae isolates and their MICs to gentamicin, azithromycin, ceftriaxone, and spectinomycin. Also, the rates were calculated to show the susceptibility profiles for the four antibiotics. Bivariate analyses of their susceptibility profiles were conducted by chi-square statistics between every two antimicrobials. The relationships between gentamicin MIC values and susceptibility profiles of azithromycin, ceftriaxone, and spectinomycin were analyzed by Wilcoxon Signed-Rank Test. A p-value <0.01 was considered statistically significant. Statistical analyses were carried out using Excel (Microsoft, Washington, USA) and SPSS software (IBM, New York, USA).
Results
Distribution of N. gonorrhoeae isolates
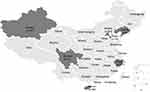
A total of 470 clinical N. gonorrhoeae isolates were randomly selected to test for antimicrobial susceptibility in 2016 from 7 provinces (Beijing, Hainan, Liaoning, Sichuan, Tianjin, Xinjiang, and Zhejiang). The geographic locations of these provinces and specific amounts were shown in Figure 1. The particular numbers were 64, 67, 60, 57, 74, 87, and 61, respectively. Among them, 339 isolates from Hainan, Sichuan, Tianjin, Xinjiang, and Zhejiang were previously reported for their susceptibility to ceftriaxone and azithromycin.8
Antimicrobial susceptibility
MIC values obtained from the WHO N. gonorrhoeae reference strains were identical or within 1 MIC dilution of those previously reported in every batch of tests, and they were excluded from the data analysis.16 All of the 470 clinical isolates were determined for their MIC values to four antimicrobials. Gentamicin MICs ranged from 1 to 8 mg/L, and MIC50 and MIC90 were 4 mg/L and 8 mg/L (Table 1). Of 470 isolates, 404 (86.0%) demonstrated full susceptibility to gentamicin, 66 (14.0%) strains showing intermediate susceptibility, and none demonstrated resistance. Three hundred and twenty-five (69.1%) isolates were located at the breakpoint for susceptibility interpretation (MIC=4 mg/L), with 66 (14.0%) demonstrating one dilution greater than the breakpoint. Seventy-three (15.5%) isolates were resistant to azithromycin; 7 of these isolates also demonstrated intermediate susceptibility to gentamicin (chi-square=1.4, p=0.23). Of the 19 (4.0%) isolates demonstrating resistance to ceftriaxone, 6 were intermediately susceptible to gentamicin (chi-square=3.6, p=0.06). One of the two isolates resistant to spectinomycin demonstrated intermediate susceptibility to gentamicin. Seven (1.5%) isolates were resistant to azithromycin and simultaneously resistant to ceftriaxone (chi-square=5.3, p=0.02); 6 of these isolates demonstrated full susceptibility to gentamicin. The p-values of cross relationships between gentamicin MICs and susceptibility profiles of other antibiotics were 0.94 for azithromycin, 0.29 for ceftriaxone, and 0.18 for spectinomycin (Table 1).
 |
Table 1 Antimicrobial susceptibility of Neisseria gonorrhoeae isolates in China |
Discussion
Increased resistance of N. gonorrhoeae over the past several years has limited the options for effective treatment and added considerable public health concern worldwide. Since N. gonorrhoeae is sporadically resistant to the current first-line antimicrobial, ceftriaxone, and this drug can cause allergic reactions, new antimicrobial need to be considered as an alternate. Other substitute recommendations shown decreased susceptibility worldwide for cefixime and high potential to develop resistance for spectinomycin.7,10,11 Gentamicin was an effective and inexpensive antimicrobial that has been used in many developing countries to treat gonorrhea for over 20 years, without reports about its trend of declining susceptibility.17 Furthermore, gentamicin has been listed as a treatment option when treatment failure occurs after a dual therapy of ceftriaxone and azithromycin in WHO guidelines or resistance to extended-spectrum cephalosporin is identified in European guidelines.10,11 In addition, gentamicin was recommended by the American Centers for Disease Control and Prevention (CDC) as a regimen to substitute for ceftriaxone in case of allergy, intolerance, and adverse reactions.7 However, clinical trials for gentamicin (combining with azithromycin) are scarce, one showing full microbiological cure rate in 213 patients,18 and the other indicating inferior potency to ceftriaxone particularly in pharyngeal and rectal infections.19 In China, gentamicin has been prescribed clinically for gram-negative bacillary infections but not for gonorrhea due to the absence of its susceptibility data.
Evaluation of susceptibility in vitro is crucial to provide a rational basis for formulating more effective therapies for gonorrhea in the future. To our knowledge, the present study is the first surveillance of susceptibility of N. gonorrhoeae to gentamicin in China. Previous studies have assessed the gentamicin susceptibility by agar dilution method or E-test method in other countries. The Gonococcal Isolate Surveillance Project (GISP) in America reported the data of gentamicin susceptibility on 10,403 gonococcal isolates collected in 2015 and 2016. Among these isolates, 73% demonstrated MICs from 8 to 16 mg/L, and 27% were 4 mg/L.20 The 2009 survey of European gonococcal population’s susceptibility to gentamicin found that 95% isolates were within a narrow MIC range (4–8 mg/L), with 79% showing a MIC of 8 mg/L.21 The antimicrobial susceptibility surveillance conducted in Malawi found that after 14 years of use, all the isolates collected in 2007 were fully susceptible to gentamicin (MIC ≤4 mg/L).17 Our findings also demonstrated the full susceptibility to gentamicin but differed from previous researches in showing lower MICs. With 85.9% isolates fully susceptible to gentamicin, MICs detected by agar dilution method in China were approximately one dilution lower than that in Europe and America, but consistent with their results from E-test method,15,21 indicating that gentamicin may have treatment potency in China.
Our study showed that 15.3% gonococcal isolates were resistant to azithromycin, and 12.8% demonstrated decreased-susceptibility to ceftriaxone (according to the criteria in the previous study8). These results are consistent with our surveillance data from 2013 to 2016 in China,8 highlighting the importance of antimicrobial susceptibility surveillance and implications for future gonococcal treatment options. None of the isolates resistant to azithromycin or ceftriaxone were resistant to gentamicin, and we found no relationships between gentamicin MICs and susceptibility profiles of other antimicrobials, which is in tune with a previous study in India.22 Relevant mutations in 23S rRNA, penicillin-binding protein 2 or efflux pump genes6 causing the resistance of azithromycin or ceftriaxone may play no role in forming resistance in gentamicin. The in vitro results suggested that gentamicin might be an effective treatment option for the gonococcal patients who fail the dual therapy.
The present study is the first nationwide research reporting the N. gonorrhoeae susceptibility to domestically-produced gentamicin in China. Moreover, our results led to the preliminary foundation for the future implement of gentamicin for gonococcal infections in China. There are a few limitations to our study. Primarily, the antimicrobial susceptibility evaluation was conducted in vitro. The in vitro tests cannot analyze differences of the drug penetration in genital, rectal, or oropharyngeal sites. Also, a clinical trial of 720 participants indicated that gentamicin was inferior to ceftriaxone, particularly for pharyngeal and rectal infections. Furthermore, standardized resistance breakpoints for gentamicin have not been established by EUCAST and CLSI. The interpretative criteria used in our study was according to a previous study, which documented that MICs from treatment failure strains ranged from 4 to ≥16 mg/L.15 Lastly, this study was a national survey of gentamicin susceptibility with isolates collected from 7 provinces in China, the strains account for only 0.4% (470/115,024) of reported cases of gonorrhea in 2016. Furthermore, the provinces located in central China have not been included in our study (Figure 1). All of these potential biases may limit the generalizability of the current study to all the patients infected with gonorrhea in China.
Conclusion
In conclusion, the current study was the first in vitro study on the susceptibility of N. gonorrhoeae to gentamicin with implications for a future treatment option for gonorrhea. Isolates with resistance to gentamicin were not observed in China as yet. The high incidence of gonorrhea coupled with increasing antimicrobial resistance to azithromycin and ceftriaxone warrants the consideration of alternative therapies. Gentamicin is an effective, inexpensive, and relatively safe antimicrobial that has been prescribed in Malawi,17 whereas more clinical trials are needed for in vivo therapeutic efficacy. Our study for gentamicin assessment was included in the ROADMAP23 (Resistance surveillance, Outcomes due to antimicrobial resistance, Antibiotic stewardship and application, Diagnostic tools, Mechanisms of antimicrobial resistance, Antimicrobial assessment, and Population pharmacokinetics and pharmacodynamics) plan. Moreover, it provided references to the future revision of the guidelines for the treatment of N. gonorrhoeae in China and gave hints of adding gentamicin as a routine surveillance antimicrobial in the China-GRSP. Furthermore, this project contributes to sibling countries for their gonococcal treatment, surveillance and control. Continued surveillance of gentamicin susceptibility and further investigation of gentamicin for clinical use are urgently needed.
Acknowledgments
This work was supported by the Chinese Academy Medical Sciences Initiative Medicine (2016-I2M-3-021), the National Science and Technology Major Project (2018ZX10101001-004-003), the Jiangsu Province Youth Fund Project (BK20180156), and the Fundamental Research Funds for the Central Universities (3332018122). The sponsors of the study had no role in study design, data collection, data analysis, data interpretation or writing of the article. The authors thank the patients who provided the specimens and acknowledge LF Yuan, N Zhong, ZG Zhang, G Yong, ZJ Zheng, Q Zhi, LH Hu and their participants for collecting the isolates.
Disclosure
The authors report no conflicts of interest in this work.
References
1. National Health Commission of the PRC [homepage on the Internet]. Overview of the national epidemic of infectious diseases in 2018; 2018. Available from: http://www.nhc.gov.cn/jkj/s3578/201904/050427ff32704a5db64f4ae1f6d57c6c.shtml.
2. Cohen MS. Classical sexually transmitted diseases drive the spread of HIV-1: back to the future. J Infect Dis. 2012;206(1):1–2. doi:10.1093/infdis/jis303
3. Workowski KA, Berman SM, Douglas JM. Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann Intern Med. 2008;148(8):606–613. doi:10.7326/0003-4819-148-8-200804150-00005
4. Wang F, Liu J, Liu H, et al. Evaluation of the accuracy of molecular assays targeting the mutation A2059G for detecting high-level azithromycin resistance in Neisseria gonorrhoeae: a systematic review and meta-analysis. Infect Drug Resist. 2019;12:95–104. doi:10.2147/IDR.S183754
5. Han Y, Yin YP, Zhou Y, et al. Nonadherence to national guidelines for antibiotic treatment of uncomplicated gonorrhea in China: results from a nationwide survey. Sex Transm Dis. 2018;45(9):600–606. doi:10.1097/OLQ.0000000000000819
6. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587–613. doi:10.1128/CMR.00010-14
7. Workowski KA, Bolan GA; for Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR–03):1–137.
8. Yin YP, Han Y, Dai XQ, et al. Susceptibility of Neisseria gonorrhoeae to azithromycin and ceftriaxone in China: a retrospective study of national surveillance data from 2013 to 2016. PLoS Med. 2018;15(2):e1002499. doi:10.1371/journal.pmed.1002593
9. Boslego JW, Tramont EC, Takafuji ET, et al. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and of penicillinase-producing Neisseria gonorrhoeae. N Engl J Med. 1987;317(5):272–278. doi:10.1056/NEJM198707303170504
10. Bignell C, Unemo M. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24(2):85–92. doi:10.1177/0956462412472837
11. World Health Organization [homepage on the Internet]. WHO guidelines for the treatment of Neisseria gonorrhoeae; 2016. Available from: http://apps.who.int/iris/bitstream/10665/246114/1/9789241549691-eng.pdf?ua=1.
12. Ross JD, Lewis DA. Cephalosporin resistant Neisseria gonorrhoeae: time to consider gentamicin? Sex Transm Infect. 2012;88(1):6–8. doi:10.1136/sextrans-2011-050362
13. Ye S, Su X, Wang Q, Yin Y, Dai X, Sun H. Surveillance of antibiotic resistance of Neisseria gonorrhoeae isolates in China, 1993–1998. Sex Transm Dis. 2002;29(4):242–245.
14. The European Committee on Antimicrobial Susceptibility Testing [homepage on the Internet]. Breakpoint tables for interpretation of MICs and zone diameters, version 8.1; 2019. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf.
15. Daly CC, Hoffman I, Hobbs M, et al. Development of an antimicrobial susceptibility surveillance system for Neisseria gonorrhoeae in Malawi: comparison of methods. J Clin Microbiol. 1997;35(11):2985–2988.
16. Unemo M, Golparian D, Sanchez-Buso L, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother. 2016;71(11):3096–3108. doi:10.1093/jac/dkw288
17. Brown LB, Krysiak R, Kamanga G, et al. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis. 2010;37(3):169–172. doi:10.1097/OLQ.0b013e3181bf575c
18. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59(8):1083–1091. doi:10.1093/cid/ciu521
19. Ross JD, Brittain C, Cole M, et al. Gentamicin compared with ceftriaxone for the treatment of gonorrhoea (G-ToG): a randomised non-inferiority trial. Lancet. 2019;393(10190):2511–2520. doi:10.1016/S0140-6736(18)32817-4
20. Mann LM, Kirkcaldy RD, Papp JR, Torrone EA. Susceptibility of Neisseria gonorrhoeae to gentamicin-gonococcal isolate surveillance project, 2015–2016. Sex Transm Dis. 2018;45(2):96–98. doi:10.1097/OLQ.0000000000000693
21. Chisholm SA, Quaye N, Cole MJ, et al. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J Antimicrob Chemother. 2011;66(3):592–595. doi:10.1093/jac/dkq476
22. Bala M, Singh V, Bhargava A, Kakran M, Joshi NC, Bhatnagar R. Gentamicin susceptibility among a sample of multidrug-resistant Neisseria gonorrhoeae isolates in India. Antimicrob Agents Chemother. 2016;60(12):7518–7521. doi:10.1128/AAC.01907-16
23. Chen XS, Yin YP, Li XY. A ROADMAP plan to address research needs for gonococcal antimicrobial resistance in China. Clin Infect Dis. 2018;68(3):505–510. doi:10.1093/cid/ciy566
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.