Back to Journals » Cancer Management and Research » Volume 13
Genotype Distribution and Prevalence of Human Papillomavirus Infection in Women in Northern Jiangsu Province of China
Authors Wang L, Chen G, Jiang J
Received 9 August 2021
Accepted for publication 11 September 2021
Published 22 September 2021 Volume 2021:13 Pages 7365—7372
DOI https://doi.org/10.2147/CMAR.S332769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Lixin Wang, Gang Chen, Jingui Jiang
Department of Pathology, Jinhu County People’s Hospital, Huaian City, People’s Republic of China
Correspondence: Jingui Jiang
Department of Pathology, Jinhu County People’s Hospital, Huaian City, Jiangsu Province, People’s Republic of China
Tel +86 18952332618
Fax +86 51786808703
Email [email protected]
Objective: Human papillomavirus (HPV) infection is the risk factor for cervical cancer. Consequently, HPV DNA testing is an essential method for cervical cancer screening. Yet, data on the prevalence and genotype distribution of HPV and cervical cytological among women in northern Jiangsu Province of China are very limited.
Materials and Methods: A total of 36,500 women were enrolled at the Department of Pathology of Jinhu County People’s Hospital between January 1, 2016 and December 31, 2019. HPV genotypes was performed using YanengBio® Human Papillomavirus Genotyping Kit. Thin liquid cytology tests (TCT) were conducted, based on cytology conditions set by Bethesda in 2001.
Results: The overall prevalence of HPV infection was 28.95%; it was age-dependent; the number of infections was highest in the 40– 49 age group. Six thousand three hundred eighty-two women (16.87%) were found to have a pure high-risk HPV infection, while 918 women (2.43%) were found to have a pure low-risk HPV infection. The 6 most predominant genotypes were HPV 52, 58, 16, 53, 56, and 33. Moreover, 8923 women (45.09%) were affected by ASCUS, 4531 (22.90%) by LSIL, 3726 (18.83%) by ASC-H, and 2610 (13.19%) by HSIL.
Conclusion: This study revealed a high burden of HPV infection among women in northern Jiangsu Province of China and identified the distribution of the prevalent top 6 HPV genotypes in this area, which can be used as a useful reference for future work.
Keywords: human papillomavirus, prevalence and genotype distribution, infection, cervical cancer, northern Jiangsu Province
Corrigendum for this paper has been published.
Introduction
Cervical cancer (CC) is the second most deadly disease among women in the world and is the leading cause of death from cancer.1 In 2018, there were 570,000 new diseases and 311,000 deaths worldwide.2 Viral infections contribute to 15–20% of all cancers, and several viruses play an important role in the multifaceted development of malignant cancers. Over the past two decades, several viruses have been shown to play an important role in the development of human cancer. Oncogenic viruses can facilitate different stages of carcinogenesis and are human papillomavirus (HPV), a virus that contributes to cancer statistics.3 HPV is a sexually transmitted virus that contains high-risk HPV DNA in 99.7% of uterine cancers.4 Within 12–25 months of infection, 90% of HPV infections are eliminated or inactive. However, high-risk strains of HPV infection continue to increase the risk of developing uterine cancer.5
Human papillomavirus is the most common sexually transmitted pathogen affecting men and women. New epidemiological evidence has revealed that the virus is closely related to cervical cancer, penile cancer, vulvar cancer, and anal canal cancer.6–8 HPV is a small-scale DNA virus that has a dual-strand DNA genome of about 8 KB in circumference and is grouped according to the DNA sequence of their genes. HPVs have less than 90% resemblance to other nucleotide levels.9,10 More than 150 genotypes of HPV have been identified by DNA sequencing and were stratified according to oncogenic risk. Seventeen genotypes are clearly linked to the development of adenocarcinoma and squamous cell carcinoma of the cervix and therefore were ranked as high-risk HPV: −16, −18, −31, −33, −35, −39, −45, −51, −52, −53,56, −58, −59, −66,-68, −73, and −82. Following six genotypes are considered as low risk HPV: −6, −11, −42, −43, −81, −83.11 In China, the HPV infection rate varied. It was 28.4% in northeast China, 20.16% in south China and 14.2% in northwest China.12–14 But there is currently no relevant research in Jiangsu. So we conduct the following research.
In the current study, on January 1, 2016, and December 31, 2019, we identified and genetically determined the HPV infection in women attending the pathology department of Jinhu Prefecture Hospital. The aim of this study was to assess the prevalence and genetic spread of HPV and uterine cytology among women in Jiangsu Province in northern China.
Materials and Methods
Study Design and Patient Recruitment
Over recent years, the Jiangsu Provincial Government has conducted various cervical cancer screening projects for women living in the province. Samples were taken from all women at the gynecology department of Jinhu County People’s Hospital, after which the specimens were sent to the pathology department for processing. Participants who met the following criteria were included: permanent residents in northern Jiangsu Province; aged 15–89; not pregnant; did not receive vaccination against HPV; did not undergo hysterectomy; without a history of cervical surgery, and did not undergo pelvic radiation therapy. The time between January 1, 2016 and December 31, 2019. All participants included in the study gave their written informed consent. This study was approved by the Jinhu County People’s Hospital ethics committee.
Cervical Specimen Collection and HPV DNA Extraction
Samples of the uterine test-based uterus were collected from the collected cells. Cell samples from the uterus were taken as part of a routine examination by a gynecologist. Two extracellular samples were collected independently for a fluid-based cytological diagnosis and HPV DNA gene processing assay, respectively. The solvent slides were prepared by liquid-based cytology. Disease-level cytological classifications were performed according to the Bethesda 2001 criteria (TBS2001)15 H), internal epithelial lesion of low-grade scoliosis (L-SIL), and high-grade scoliosis internal epithelial damage (H-SIL). Cells isolated for HPV DNA extraction are stored in the vehicle. Samples were placed in a protective solution of the cell and stored at 2–8 C C until HPV DNA extraction and gene processing. The HPV genetic test of the specimens was completed within a week.
Detection and Genotyping of HPV
The HPV genes were determined using the YanengBio® Human Papillomavirus Genotyping Kit (Shenzhen: YanengBio, Inc., China) on the instructions of the HPV gene manufacturer. Among the identified HPV species, there are 17 high-risk species (HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-53, HPV-56, HPV-58, HPV-59, HPV-66, HPV-68, HPV-73, and HPV-82) and six low-risk species (HPV-6, HPV-11, HPV-42, HPV) −43, HPV-81, HPV-83). Based on data provided by the YanengBio® Human Papillomavirus Genotyping Kit (Shenzhen: YanengBio, Inc., China), the sensitivity and accuracy of this test was at least 1.0 × 103/mL and 98% higher than the HPV load for each sample. Sincerely.
Statistical Analysis
SPSS 22.0 statistical software was used to process the data, and the measurement data is shown as a standard ± standard deviation. Calculation data were expressed as a percentage (%). Herpes simplex HPV types were scientifically stabilized, and HPV type world trends were calculated by Student’s t-test. P<0.05 is considered statistically significant.
Results
Participants and HPV Positivity and Age
A total of 37,826 women who accepted HPV DNA testing at the Department of Pathology of Jinhu County People’s Hospital between January 1, 2016 and December 31, 2019 were enrolled in the study. One thousand three hundred twenty-six women were excluded due to the lack of age-related information. Finally, a total of 36,500 participants were included in the analysis Figure 1. Here, we have determined the age distribution of the HPV virus in Jiangsu Province in northeastern China. As shown in Table 1 and Figure 2, HPV infections often occur between the ages of 40–49, 50–59, and 30–39 years, accounting for more than 70% of all positive cases. In addition, with a prevalence of 9.66%, women between the ages of 40–49 emerged as the highest risk population for HPV. However, the effectiveness of the HPV strain varied at different age levels. Combined HPV infection has increased in women aged 40–49 and decreased in women 50 and older. Comparing HPV status among different age groups revealed a statistically significant difference (P < 0.05).
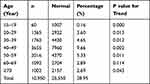 |
Table 1 Distribution of HPV Infection in Different Age Groups |
 |
Figure 1 The flow-chart of exclusion criteria. |
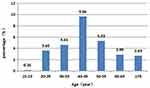 |
Figure 2 HPV infection rates by age. |
Prevalence of HPV Infection
During this period, 10,950 women (28.95%) contracted HPV. 8633 (22.8%) women, 1258 (3.33%) women LR, 1059 (2.80%) women HR and LR HPV were infected with HR. Meanwhile, 7.37% and 2.28% of women had double and multiple HPV infections, respectively (Table 2).
 |
Table 2 Prevalence of HPV Infection in Study Participants from Northeastern Jiangsu Province of China |
HPV Genotype Distribution
In the present study, we identified twenty-three different HPV subtypes. HPV 52, 58, 16, 53, 56 and 33 were the six most predominant subtypes, with proportions of 20.93%, 14.43%, 12.63%, 9.67%, 7.91% and 6.08%, respectively. Other genotypes that ranked top ten were HPV 18, 81, 53, 33, 31, 51 and 39, with corresponding proportions of 6.01%, 5.45%, 5.10%, 4.94%, 3.80%, 3.69% and 3.28%, respectively (Table 3 and Figure 3). To be noted, HPV81 was the only LR subtype with a relatively high infection rate among women. Double or multiple HPV infections were common, especially among the HR subtypes like HPV 52, 16, and 56.
 |
Table 3 Genotypes of the HPV Infection in Study Participants from Northeastern Jiangsu Province of China |
 |
Figure 3 HR-HPV infection rates by genotypes. |
Prevalence of HPV Types Stratified by the Cervical Cytological Result
In this study, 45.09%, 18.83%, 22.90% and 13.19% of HPV infected women had cytological results of ASCUS, ASCH, L-SIL and H-SIL, respectively (Table 4 and Figure 4). HPV 16 and 52 were responsible for 6885 cytological defects in the uterus. In particular, HPV16 had the highest frequency among all classes, with ASCUS, ASC-H, LSIL, and HSIL having the highest frequencies of 20.93%, 12.63%, 6.99%, and 2.62%, respectively, while HPV52 was the second most common. HPV16 had the highest frequency among high-risk HPVs, followed by ASCUS, ASC-H, LSIL, and HSIL at 3.35%, 1.92%, 0.54%, and 0.06%, respectively.
 |
Table 4 HPV Genotype Distribution in Different Cytological Result |
 |
Figure 4 HR-HPV genotype distribution in different cytological result. |
Discussion
To the best of our knowledge, this is the first large-scale study on prevalence and genotype distribution of HPV among women in northeastern Jiangsu Province of China. As HPV is the most common sexually transmitted infection with very high prevalence, it has become a major health concern. There are lots of programs regarding HPV vaccination for HPV prophylaxis in a different part of the world with different types of strains. The study on HPV prevalence and its subtype distribution may provide relevant information needed for routine vaccination and the types of HPV strains to be used in vaccination. The prevalence of crude oil and refined HPV among women with average cytological findings worldwide ranges from 11.70% to 7.20%. The highest prevalence was in sub-Saharan Africa (24.00%), Latin America and the Caribbean (16.10%), Eastern Europe (14.20%) and Southeast Asia (14.00%).16,17 HPV was found to be positive. Our study found that 28.95% of women who underwent cervical cancer screening in Jinhu province in northern China had an HPV diagnosis in the uterus. Previous studies found that 29.10% of Shanghai’s population18 had Beijing,19 53.70% and three cities in Xi’an (Suzhou, Nanjing and Suzhou) (26.92%).20 Our results showed lower results compared to the three cities in Jiangsu Province (Suzhou, Nanjing and Suzhou), compared to Shanghai and Beijing, and showed differences in the region. HPV infection can occur at any age, and the infection is related to age. In the present study, the positive rate among patients aged 20–69 years showed a rising trend. The positive rate of high-risk HPV infection in patients aged 40–49 years was as high as 9.66%, and the positive rate among patients aged ≥70 years significantly decreased. This may be due to the low autoimmunity and hormone levels in the 60–69 years age group, which leads to the poor ability of the cervix to resist HPV infection. Cervical epithelium in patients≥70 years of age tends to atrophy, and sexual life is significantly reduced, which may explain the significant decrease in the HPV positive rate in this age group.
Analysis of different subtypes of high-risk HPV infection revealed that HPV types 16, 18, 58, 33, 52, 31, 45, and 59 are the eight major high-risk HPV subtypes of cervical cancer in China.21 Among them, HPV 16 is the most important subtype of infection with the strongest carcinogenicity. In the second study conducted in Western Kazakhstan, the prevalence of HPV was found to be 25%, and the most common HPV genotypes were HPV-16 (26.40%), HPV- 31 (10.10%), HPV-51 (9.40%), HPV-52 (9%), and HPV-6 (7.90%).22 The major subtypes resulting in high-risk HPV infection types in Jinhu area were 52, 58, 16, 53, 56, and 33, which was not consistent with previous reports on different subtype infection rates across most regions of China. HPV16, together with HPV 18, is known to be responsible for approximately 70% of cervical cancer cases worldwide.23 The prevalence of HPV18, ranging between 1.80% and 16.30%, has been reported in other studies.24 Furthermore, HPV-52 was the highest type reported in Thai women, which is commonly found in Asians.25 Our results revealed that the first type was 52, which was similar to the Thai study and Zhoupu District, Shanghai City, China.26 But inconsistent with other studies. The majority of women in this study were middle-aged and elderly, which may explain the observed differences.
In the present study, a single infection was most commonly found among women (19.30%), while the multiple infections were the rarest (2.28%). Although multiple infections are less frequent than single infections, multiple HPV infections can double the risk of cervical lesions. Previous studies have shown that high-risk HPV multiple infections can increase the incidence of cervical cancer by 31.8 times and single infections by 19.9 times.27 Accordingly, the diagnosis and treatment of patients with multiple infections and high-risk HPV are particularly crucial for the early detection and early treatment of cervical cancer. A previous study reported an increasing trend in the overall prevalence of HPV infection in parallel with an increase in the degree of cervical cytological abnormalities. Guan et al28 reported an overall HPV prevalence of 84% and 85% in H-SIL cases in Europe and globally, respectively. Our results were lower compared to these, which may be explained with a more liberal view on sex, a higher number of sexual partners, or by geographical, environmental, and climate conditions. At the same time, our research also found that HPV16 and HPV52 have the highest curative effects in ASCUS, ASC-H, LSIL, and HSIL, which should be paid attention to. If the cytology test is negative and there are HPV16 and HPV52 infections, relevant treatment must be carried out. Various vaccines are currently in use, such as 2‐V vaccine, 4‐V vaccine, and 9‐V vaccine. Developed countries generally inject 9‐V vaccine due to economic reasons, while developing countries generally rarely inject vaccines. Through our research, We suggest that at least 2-V vaccine injection should be promoted nationwide to reduce or prevent the occurrence of cervical cancer.
Conclusion
This was an early study on cervical cancer and precancerous lesions in the Jinhu area of northern Jiangsu, which may provide useful references for disease identification and early treatment. Understanding the different subtypes of HPV infection and the characteristics of infection across different age groups in the Jinhu area, as well as clarifying the regional differences of HPV infection, are particularly important for the health of women in the Jinhu area of northern Jiangsu. This article also has some limitations. This article is a single-center study, which cannot reflect the HPV prevalence in the entire Jiangsu area, and the sample size is small.
Ethical Approval
All studies were done in compliance with the regulations and guidelines of Jinhu County People’s Hospital institutional and conducted according to the Helsinki and the IACUC guidelines. Informed consent was obtained from all individual participants included in the study. Patients under the age of 18 have signed an informed consent form with themselves and their parents, and have been informed of the relevant circumstances. They are all voluntary.
Acknowledgments
The National Natural Science Foundation of China, Grant 81560088, supported this work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochim Biophys Acta Mol Basis Dis. 2008;1782(3):127–150.
4. Chan CK, Aimagambetova G, Ukybassova T, Kongrtay K, Azizan A. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination-review of current perspectives. J Oncol. 2019. doi:10.1155/2019/3257939
5. Asiaf A, Ahmad ST, Mohammad SO, Zargar MA. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23(3):206–224. doi:10.1097/CEJ.0b013e328364f273
6. Creed S, Walsh E, Foley T. A qualitative study of parental views of HPV vaccination in Ireland. Eur J Gen Pract. 2021;27(1):1–9. doi:10.1080/13814788.2020.1851677
7. Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–265. doi:10.1136/jcp.55.4.244
8. Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi:10.1056/NEJMoa021641
9. de Villiers E-M. Cross-roads in the classification of papillomaviruses. Virology. 2013;445(1–2):2–10. doi:10.1016/j.virol.2013.04.023
10. Hoffmann M, Quabius ES. Relevance of human papillomaviruses in head and neck cancer-what remains in 2021 from a clinician’s point of view? Viruses. 2021;13(6):1173. doi:10.3390/v13061173
11. Yanyun L, Xu C. Human papillomavirus-related cancers. Adv Exp Med Biol. 2017;1018:23–34.
12. Luo G, Sun X, Li M, et al. Cervical human papillomavirus among women in Guangdong, China 2008–2017: implication for screening and vaccination. J Med Virol. 2019;91(10):1856–1865. doi:10.1002/jmv.25520
13. Jiang L, Tian X, Peng D, et al. HPV prevalence and genotype distribution among women in Shandong Province, China: analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PLoS One. 2019;14(1):e0210311. doi:10.1371/journal.pone.0210311
14. Wang J, Tang D, Wang K, et al. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Womens Health. 2019;19:90. doi:10.1186/s12905-019-0785-3
15. Yüksel S, Şimşek E, Yetkinel S, et al. Clinicopathologic importance of atypical glandular cells in cervico-vaginal cytology. J Turk Ger Gynecol Assoc. 2020;21(2):102–106. doi:10.4274/jtgga.galenos.2019.2019.0059
16. Bruni L, Diaz M, Castellsague X, et al. Cervical human papillomavirus prevalence in 5 continents: metaanalysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799. doi:10.1086/657321
17. Bekmukhambetov YZ, Balmagambetova SK, Jarkenov TA, Nurtayeva SM, Mukashev TZ, Koyshybaev AK. Distribution of high risk human papillomavirus types in Western Kazakhstan — retrospective analysis of PCR data. Asian Pac J Cancer Prev. 2016;17(5):2667–2672.
18. Zhang WY, Xue YZ, Chen M, et al. Prevalence of high-risk human papillomavirus infection in different cervical lesion among organized health-examination women in Shanghai, China. Chin Med J. 2008;121:1578–1582. doi:10.1097/00029330-200808020-00015
19. Ding X, Liu Z, Su J, et al. Human papillomavirus type-specific prevalence in women referred for colposcopic examination in Beijing. J Med Virol. 2014;86(11):1937–1943. doi:10.1002/jmv.24044
20. Zhang C, Cheng W, Liu Q, et al. Distribution of human papillomavirus infection: a population-based study of cervical samples from Jiangsu Province. Virol J. 2019;16(1):67. doi:10.1186/s12985-019-1175-z
21. Bao YP, Li N, Wang H, Qiao YL. Study on the distribution of human papillomavirus types in cervix among Chinese women: a meta-analysis. Chin J Epidemiol. 2007;28(10):941–946. Article in Chinese.
22. Balmagambetova S, Gabutti G, Koyshybaev A, et al. Cervical screening in Western Kazakhstan: liquid-based cytology ‘cell scan’ versus azur-eosin staining. J Med Screen. 2020;27:90–95. doi:10.1177/0969141319885409
23. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a Retrospective Cross-Sectional Worldwide Study. Lancet Oncol. 2010;11:1048–1056. doi:10.1016/S1470-2045(10)70230-8
24. Meloni A, Pilia R, Campagna M, et al. Prevalence and molecular epidemiology of human papillomavirus infection in Italian women with cervical cytological abnormalities. J Public Health Res. 2014;3:157. doi:10.4081/jphr.2014.157
25. Kantathavorn N, Mahidol C, Sritana N, et al. Genotypic distribution of human papillomavirus (HPV) and cervical cytology findings in 5906 Thai women undergoing cervical cancer screening programs. Infect Agent Cancer. 2015;10(1):1–9. article 7. doi:10.1186/s13027-015-0001-5
26. Li H, Li P, Huang L, et al. Prevalence characteristics of cervical human papillomavirus (HPV) infection in the Zhoupu District, Shanghai City, China. Virol J. 2020;17(1):84. doi:10.1186/s12985-020-01352-8
27. Li N, Zhang J. Analysis of the distribution of human papillomavirus in the female genital tract of different ages. J Chin Pract Diagn Ther. 2012;26(9):909–911. Article in Chinese.
28. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–2359. doi:10.1002/ijc.27485
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
