Back to Journals » Clinical Epidemiology » Volume 13
Genetically Predicted Cigarette Smoking in Relation to Risk of Polycystic Ovary Syndrome
Authors Tao Y, Liu B, Chen Y, Hu Y, Zhu R, Ye D , Mao Y , Sun X
Received 19 March 2021
Accepted for publication 11 June 2021
Published 2 July 2021 Volume 2021:13 Pages 527—532
DOI https://doi.org/10.2147/CLEP.S311785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Vera Ehrenstein
Yingli Tao, 1 Bin Liu, 2 Ying Chen, 2 Yiduoduo Hu, 2 Rui Zhu, 3 Ding Ye, 2 Yingying Mao, 2 Xiaohui Sun 2
1Department of Reproductive Immunology, Tongde Hospital, Hangzhou, 310012, People’s Republic of China; 2Department of Epidemiology and Biostatistics, School of Public Health, Zhejiang Chinese Medical University, Hangzhou, 310053, People’s Republic of China; 3Institute of Pharmacology, College of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, 310053, People’s Republic of China
Correspondence: Xiaohui Sun; Yingying Mao
Department of Epidemiology and Biostatistics, School of Public Health, Zhejiang Chinese Medical University, 548 Binwen Road, Hangzhou, 310053, People’s Republic of China
Tel +86-571-8663-3143; +86-571-8661-3603
Fax +86-571-8663-3143; +86-571-8661-3693
Email [email protected]; [email protected]
Background: Evidence from observational studies has suggested a link between cigarette smoking and the risk of polycystic ovary syndrome (PCOS). However, it remains uncertain whether the observed relationship is causal or due to biases inherent in observational studies. Therefore, we adopted two-sample Mendelian randomization (MR) design to assess the potential causal association between smoking and the risk of PCOS.
Methods: Summary level data of PCOS was obtained from a genome-wide association study (GWAS) meta-analysis including 4,138 cases and 20,129 controls of European ancestry. Single-nucleotide polymorphisms (SNPs) associated with smoking initiation (n=360) were selected and used as genetic instrumental variables (IVs). MR analysis was performed using inverse-variance weighted (IVW) method, supplemented with the likelihood-based method, weighted median method, MR pleiotropy residual sum and outlier (MR-PRESSO) test, and MR-Egger regression.
Results: Genetically predicted smoking initiation was associated with an increased risk of PCOS in the primary analysis (odds ratio (OR) =1.38, 95% confidence interval (CI) =1.12– 1.69). MR-Egger regression did not detect the horizontal pleiotropy. Sensitivity analyses using alternative MR methods and restricted IVs produced similar results.
Conclusion: Our study provided evidence to support a potential causal association between smoking initiation and an increased risk of PCOS, providing a better understanding of the etiology and prevention of PCOS. Further studies are warranted to clarify the underlying biological mechanisms of smoking in the development of PCOS.
Keywords: causal inference, cigarette smoking, Mendelian randomization, polycystic ovary syndrome, single-nucleotide polymorphism
Corrigendum for this paper has been published
Introduction
Polycystic ovary syndrome (PCOS) is a hormonal and metabolic disorder with a global prevalence among women of reproductive age.1–3 It is recognized that genetic and epigenetic aspects and lifestyle changes could contribute to the development of PCOS.4–6 Nevertheless, its etiology and underlying biological mechanisms remain unclear. A better understanding of the disease risk factors, particularly modification of adverse lifestyle factors, is critical in the prevention and management of PCOS.7
Smoking, an important modifiable risk factor, has been uncovered to play a role in reproductive disorders.8 The complex mixture of chemical substances contained in cigarette smoke can exert composite effects on different targets, such as the ovary, oviduct, and uterus.9–11 Recently, the adverse effects of smoking on the risk of PCOS has caused much research interest, although most of the evidence is gathered from observational studies.11,12 For instance, Legro et al revealed that nearly 40% of PCOS patients (n=626) reported a current or past smoking history.13 Another retrospective study demonstrated that smoking exerted an adverse effect on both PCOS patients with anovulation (odds ratio [OR] = 3.69, 95% confidence interval [CI]: 2.47–5.52) and those with menses (OR=2.39, 95% CI: 1.47–3.89), compared with the normal women.14 Although this evidence provided a suggestive link between smoking and the risk of PCOS, residual confounding and reverse causation are difficult to eliminate in observational studies.
Mendelian randomization (MR) is a genetic epidemiological approach in which genetic variants, such as single-nucleotide polymorphisms (SNPs) are used as instrumental variables (IVs) to assess the potential causality of a risk factor of interest upon an outcome.12 Since these analyses rely on the natural random assortment of genetic variants during meiosis, the results of the MR study are less susceptible to confounding and reverse causality bias. Several studies have implemented such approaches to identify the potential casual risk factors for PCOS, such as obesity.15,16 However, no MR reports are assessing the potential causal relationship between smoking and PCOS to date. Therefore, in the present study, we applied a two-sample MR design to examine whether genetically predicted smoking was associated with the risk of PCOS, which can provide a better understanding of the etiology and prevention of PCOS.
Methods
Study Design and Data Sources
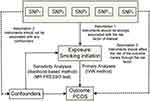
Two-sample MR design was implemented in this study (Figure 1). There are three key assumptions for an MR approach. These include 1) the genetic variants selected as IVs should be strongly associated with the exposure; 2) the IVs should not be associated with confounders; 3) the IVs should affect the risk of the outcome only through the risk factor, not via alternative pathways.17,18 In the present study, the SNPs which achieved genome-wide significance for smoking initiation were used as IVs in the MR analysis. Summary-level data for the genetic associations between SNPs with PCOS risk was obtained from a GWAS meta-analysis published in 2018 by Day et al, which consisted of 24,357 individuals (4,138 cases and 20,129 controls) of European ancestry. Briefly, cases were diagnosed with PCOS based on criteria of the National Institute of Health (NIH) or Rotterdam Criteria.19 Detailed information regarding the GWAS genetic dataset employed in the present study is shown in Supplementary Table 1. Since the present study was based on publicly available summary-level data of genome-wide association studies (GWAS), no additional ethical approval was required.
Genetic Instrumental Variables Selection
The genetic instruments for smoking initiation were obtained from a GWAS meta-analysis, including data from up to 1,200,000 individuals of European ancestry.20 In total, 378 conditionally independent SNPs associated with smoking initiation (ever being a regular smoker vs never being a regular smoker) achieving the genome-wide significance threshold were identified. There were 14 SNPs excluded due to high linkage disequilibrium (LD) (r2>0.1). Besides, four SNPs associated with smoking initiation was not available in the PCOS summary statistics. Finally, a total of 360 SNPs associated with smoking initiation were used as genetic instruments in the subsequent MR analysis. Detailed information on the included SNPs is listed in Supplementary Table 2.
Statistical Analysis
The strength of the IVs was evaluated by F-statistics.21 It was defined as the ratio of the square of the gene-exposure association to the square of the corresponding standard error (SE).22 The R2 statistic was the proportion of variance in the smoking-related phenotypes by the SNP. It was approximately equal to 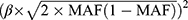 , where β is the effect size estimate of the SNP in association with smoking initiation and MAF is the minimum allele frequency.23
, where β is the effect size estimate of the SNP in association with smoking initiation and MAF is the minimum allele frequency.23
The primary MR analysis was conducted using the inverse-variance weighted (IVW) method based. The estimate obtained from the IVW method is the inverse variance weighted mean of Wald ratio estimates from all genetic instruments.24 We also used Cochran’s Q test to evaluate the heterogeneity. In cases Cochran’s Q statistic in the MR-IVW fixed effects analyses was significant, the MR-IVW random-effects model was adopted. Besides, the likelihood-based method and MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test were used as sensitivity analyses. In the likelihood-based method, the IVs associated with smoking initiation and with PCOS for each SNP are modeled directly by a bivariate normal distribution.25 The MR-PRESSO test was used to detect and correct for directional pleiotropic outliers via outlier removal in multi-instrument summary-level MR testing.26 To test potential directional pleiotropy, we also performed MR-Egger regression. In the MR-Egger method, the smoking-PCOS association was estimated by weighted linear regression of SNP-PCOS against SNP-smoking effect estimates. It can produce an intercept term indicative for directional pleiotropy.21 Moreover, we scanned the SNPs used as genetic instruments for their potential secondary phenotypes using the GWAS Catalog (http://www.ebi.ac.uk/gwas, accessed on November 20, 2020), and further performed sensitivity analyses excluding the potentially pleiotropic SNPs.
Estimates of the association between smoking initiation and PCOS are presented as ORs with 95% CIs. All statistical analysis was performed using R software version 3.6.0 (https://www.r-project.org/). The packages used for MR analyses were “MendelianRandomization” and “MR-PRESSO”.27
Results
For smoking initiation, all F-statistics were above 10, ranging from 24.9 to 73.4, suggesting less possibility of weak instrument bias. The IVs for smoking initiation can account for approximately 2.1% of the variance.
The association estimates between smoking initiation and risk of PCOS are displayed in Figure 2. Genetically predicted smoking initiation was positively correlated with an increased risk of PCOS (OR=1.38, 95% CI=1.12–1.69). The results were consistent in sensitivity analyses using the likelihood-based method (OR =1.39, 95% CI=1.12–1.71). The weighted median method produced a directionally similar estimate (OR=1.28, 95% CI: 0.93–1.71). MR-Egger regression did not indicate a potential pleiotropic bias for the IVs used for smoking initiation. By using the MR-PRESSO test, one outlier SNP (rs4759229) was identified. After correcting for the possible outlier, the effect estimate of the association between genetically predicted smoking initiation and risk of PCOS did not change markedly (OR = 1.40, 95% CI = 1.15–1.72).
In addition, we used the GWAS Catalog to scan the SNPs used as IVs for their potential-associated secondary phenotypes. A total of 69 SNPs were found to be accompanied by other traits (Supplementary Table 3). After excluding these potential pleiotropic SNPs, we found the association between genetically predicted smoking initiation and risk of PCOS remained consistently in both direction and magnitude (OR = 1.32, 95% CI = 1.06–1.64 by IVW) (Supplementary Table 4).
Discussion
To the best of our knowledge, this is the first MR study to examine whether smoking is causally associated with the risk of PCOS. Our study indicated that genetically predicted smoking initiation was associated with an increased risk of PCOS. Compared with never smokers, genetically predicted smokers had a 38% increased risk of developing PCOS.
Previous observational studies have uncovered the potential association between smoking and PCOS. Legro et al found that nearly 40% of PCOS patients (n=626) in the Pregnancy in Polycystic Ovary Syndrome Study (PPCOS I) reported a current or past smoking history.13 Similarly, a study of 346 women with PCOS from Germany revealed that 28% of the study participants were current smokers, and another study from Denmark (n=650) showed that nearly 40% of the patients with PCOS were smokers.28,29 Moreover, the aforementioned case–control study among 2,217 PCOS women and 279 controls demonstrated a positive association between smoking and risk of PCOS after adjusting age and BMI.14 Consistent with these prior observational studies, our data supported the hypothesis that smoking may be associated with an increased risk of PCOS.
Although the biological role of smoking in the development of PCOS is still unclear, several studies have provided plausible explanations for this. One possible interpretation may be the adverse effect of smoking on the increase of vascular stiffness, resistance and endothelial injury of ovarian tissue, which may play a role in the development of PCOS.30 Secondly, the positive correlation between smoking and androgen metabolism was reported.31 For example, some chemical substances contained in cigarette smoke could cause changes in luteal steroidogenesis, affecting progesterone and estradiol production, as well as suppression of the oocyte-cumulus complex expansion.32 Another influence of smoking could be its effect on insulin. It was reported that PCOS cases who were smoking showed increased free testosterone and risking insulin resistance.28 Although these biologically theoretical explanations are plausible, further studies are warranted to understand the underlying mechanisms of smoking in the development of PCOS.
The main novelty of this study is the adoption of the MR approach, which has been widely used to investigate the causal nature of the association between lifestyle-related factors and the risk of PCOS. The SNPs used in this study are confirmedly associated with smoking initiation at genome-wide significance threshold, thereby reducing possible violation of the first assumption of MR. Moreover, the F-statistics for the IVs were all satisfying the threshold of F-statistics >10, indicating that the analysis was unlikely to be affected by weak instrument bias.33 The MR-Egger regression was performed to analyze the data and did not manifest the presence of directional pleiotropy.21 In addition, we manually scanned each of the SNPs used as IVs for potential secondary phenotypes in the GWAS Catalog, and MR analyses excluding these SNPs produced similar results. These consistent results suggested the robustness of our findings.
There several limitations of the present study. First, since our analysis was restricted to participants of European ancestry, the conclusions may not necessarily apply to other populations. However, this fact also greatly reduced the potential impacts of population stratification bias. Second, although the MR approach could provide an unbiased result due to diminishing the confounding factors, the gene–environment and gene–gene interactions may affect the development of PCOS unavoidably. Third, we were unable to use sex-specific genetic estimates for smoking initiation due to lack of data, and further study were still warranted by using sex-adjusted genetic estimates. Finally, the MR analysis of PCOS were based on summary statistics with relatively small sample sizes, and the potential adverse effect of smoking on the risk of PCOS should be explored further in larger samples.
Conclusion
In summary, our study provided evidence to support a potential causal association between smoking initiation and an increased risk of PCOS among participants of European ancestry. Lowering smoking rates should be considered when constructing the prevention strategies for PCOS. However, the exact role and its underlying biological processes of smoking in the development of PCOS warrant further investigation.
Acknowledgment
We thank all the participants and researchers involved in the relevant studies. Data on smoking initiation have been contributed by Liu et al (2019). Data on polycystic ovary syndrome have been contributed by Day et al (2018) and were downloaded from https://doi.org/10.17863/CAM.36024.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81602917), the Natural Science Foundation of Zhejiang Province (LQ20H260008, LQ21H260001), the Talent Project of Zhejiang Association for Science and Technology (2018YCGC003), the Foundation of Zhejiang Chinese Medical University (KC201905, 2020ZR09, 2020ZG01, 2020ZG06), and the Medical Health Science and Technology Project of Health Commission of Zhejiang Province (2019RC134, 2020KY195).
Disclosure
The authors have declared no conflicts of interest.
References
1. Li S, Qi J, Tao Y, Zhu Q, Sun Y. Elevated levels of arachidonic acid metabolites in follicular fluid of PCOS patients. Reproduction. 2019;159(2).
2. Lin J, Huang J, Wang N, Kuang Y, Cai R. Effects of pre-pregnancy body mass index on pregnancy and perinatal outcomes in women with PCOS undergoing frozen embryo transfer. BMC Pregnancy Childbirth. 2019;19(1):487. doi:10.1186/s12884-019-2611-1
3. Bruni V, Capozzi A, Lello S. The role of genetics, epigenetics and lifestyle in polycystic ovary syndrome development: the state of the art. Reprod Sci. 2021. doi:10.1007/s43032-021-00515-4
4. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. doi:10.1038/nrendo.2018.24
5. Hiam D, Moreno-Asso A, Teede HJ, et al. The genetics of polycystic ovary syndrome: an overview of candidate gene systematic reviews and genome-wide association studies. J Clin Med. 2019;8(10):1606. doi:10.3390/jcm8101606
6. Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. doi:10.1016/j.jsbmb.2018.04.008
7. Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13(6):251–257. doi:10.1016/S1043-2760(02)00612-4
8. Budani MC, Tiboni GM. Ovotoxicity of cigarette smoke: a systematic review of the literature. Reprod Toxicol. 2017;72:164–181. doi:10.1016/j.reprotox.2017.06.184
9. Talbot P, Riveles K. Smoking and reproduction: the oviduct as a target of cigarette smoke. Reprod Biol Endocrinol. 2005;3:52. doi:10.1186/1477-7827-3-52
10. Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15(1):31–44. doi:10.1093/humupd/dmn046
11. de Angelis C, Nardone A, Garifalos F, et al. Smoke, alcohol and drug addiction and female fertility. Reprod Biol Endocrinol. 2020;18(1):21.
12. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926. doi:10.1001/jama.2017.17219
13. Legro RS, Chen G, Kunselman AR, et al. Smoking in infertile women with polycystic ovary syndrome: baseline validation of self-report and effects on phenotype. Hum Reprod. 2014;29(12):2680–2686. doi:10.1093/humrep/deu239
14. Zhang B, Zhou W, Shi Y, Zhang J, Cui L, Chen ZJ. Lifestyle and environmental contributions to ovulatory dysfunction in women of polycystic ovary syndrome. BMC Endocr Disord. 2020;20(1):19. doi:10.1186/s12902-020-0497-6
15. Harris HR, Cushing-Haugen KL, Webb PM, et al. Association between genetically predicted polycystic ovary syndrome and ovarian cancer: a Mendelian randomization study. Int J Epidemiol. 2019;48(3):822–830. doi:10.1093/ije/dyz113
16. Zhao Y, Xu Y, Wang X, et al. Body mass index and polycystic ovary syndrome: a 2-sample bidirectional Mendelian randomization study. J Clin Endocrinol Metab. 2020;105(6):1778–1784. doi:10.1210/clinem/dgaa125
17. Lawlor DA. Commentary: two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol. 2016;45(3):908–915. doi:10.1093/ije/dyw127
18. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;30(7).
19. Group REA-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi:10.1016/j.fertnstert.2003.10.004
20. Liu M, Jiang Y, Wedow R. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nature Genetics. 2019;51(2):237–244. doi:10.1038/s41588-018-0307-5
21. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi:10.1093/ije/dyv080
22. Bowden J, Del Greco MF, Minelli C, Smith GD, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I-2 statistic. Int J Epidemiol. 2016;45(6):1961–1974. doi:10.1093/ije/dyw220
23. Park JH, Wacholder S, Gail MH, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42(7):570–575. doi:10.1038/ng.610
24. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. doi:10.1002/gepi.21758
25. Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–1906. doi:10.1002/sim.6835
26. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases (vol 50, 693, 2018). Nat Genet. 2018;50(8):1196. doi:10.1038/s41588-018-0164-2
27. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics. 2018;50(5):693–698. doi:10.1038/s41588-018-0099-7
28. Cupisti S, Häberle L, Dittrich R, et al. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil Steril. 2010;94(2):673–677. doi:10.1016/j.fertnstert.2009.03.062
29. Glintborg D, Mumm H, Hougaard DM, Ravn P, Andersen M. Smoking is associated with increased adrenal responsiveness, decreased prolactin levels and a more adverse lipid profile in 650 white patients with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28(3):170–174. doi:10.3109/09513590.2011.589926
30. Emekci Ozay O, Ozay AC. Smoking reduces ovarian stromal blood flow in polycystic ovary syndrome patients. Ginekol Pol. 2020;91(4):201–206. doi:10.5603/GP.2020.0041
31. Konstantinidou F, Stuppia L, Gatta V. Looking inside the world of granulosa cells: the noxious effects of cigarette smoke. Biomedicines. 2020;8(9):309. doi:10.3390/biomedicines8090309
32. Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152(4):491–499. doi:10.1530/eje.1.01867
33. Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223–224. doi:10.1177/0962280210394459
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.