Back to Journals » Drug Design, Development and Therapy » Volume 17
Genetic Diversity and Characteristics of Drug Resistance Among Treatment-Naive People Living with HIV in Xi’an, China
Authors Xia H, Jin J, Ba H, Zhang Y, Li J, Guo R, Li Y, Ma P, Zhang Y
Received 29 January 2023
Accepted for publication 29 April 2023
Published 17 May 2023 Volume 2023:17 Pages 1485—1494
DOI https://doi.org/10.2147/DDDT.S406255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Huan Xia,1,2,* Juan Jin,3,* Huanhuan Ba,3 Yuan Zhang,3 Jiajia Li,3 Rui Guo,3 Ying Li,3 Ping Ma,1,2 Yan Zhang3
1Department of Infectious Diseases, Tianjin Second People’s Hospital, Tianjin, 300192, People’s Republic of China; 2Tianjin Association of STD/AIDS Prevention and Control, Tianjin, 300011, People’s Republic of China; 3Department of Infectious Diseases, Xi’an Eighth’s Hospital, Xi’an, Shaanxi, 710061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Zhang, Xi’an Eighth’s Hospital, 2 Zhangba East Road, Yanta District, Xi’an, Shaanxi, 710061, People’s Republic of China, Tel/Fax +86-29-85393973, Email [email protected] Ping Ma, Tianjin Second People’s Hospital, 7 Sudi South Road, Nankai District, Tianjin, Tianjin, 300192, People’s Republic of China, Tel/Fax +86-22-27468129, Email [email protected]
Purpose: The genetic diversity and genetic predisposition for drug resistance mutations are the primary features of human immunodeficiency virus type 1 (HIV-1), which could cause the incidence of failure of antiretroviral therapy (ART). This study investigates the distribution of various HIV-1 genotypes and the incidence of pretreatment drug resistance (PDR) in the antiretroviral-naive HIV-1 infected participants in Xi’an, China.
Patients and Methods: In this study, a cross-sectional analysis was carried out at the Xi’an Eighth Hospital between January 2020 and December 2021 among newly-diagnosed ART-naive HIV-1 infected participants. A nested PCR technique was used for amplifying the target segment of 1.3 kb present in the pol gene that spanned the reverse transcriptase and the protease regions. HIV-1 genotypes and the PDR-associated mutations were identified using the Stanford HIV Drug Resistance Database.
Results: A total of 317 pol gene sequences were retrieved, amplified, and sequenced. The circulating recombinant form (CRF), CRF07_BC (51.7%) was seen to be the most prevalent HIV-1 genotype, followed by other genotypes like CRF01_AE (25.9%), B (14.2%), and CRF55_01B (4.7%). PDR was found in 18.3% of the population. The PDR mutation frequency in the non-nucleoside reverse transcriptase inhibitor (NNRTI) (16.1%) was significantly higher compared to that of the nucleoside reverse transcriptase inhibitor (NRTI) (4.4%) and the protease inhibitor (0.9%). V179D/E (both were 4.4%) was seen to be the most predominant type of NNRTI mutation. K65R and M184V (1.3%) were the most frequent NRTI-associated mutations. About half (48.3%) of the sequenced HIV-1 strains that had mutations could show a potential low-level NNRTI resistance owing to V179D/E. Multivariate regression analysis revealed one PDR mutation associated with subtype CRF01_AE (p=0.002) and CRF55_01B (p< 0.001) as a higher risk mutation.
Conclusion: Diverse and complex HIV-1 genotypes are distributed in Xi’an, China. Considering new evidence, it is necessary to screen for baseline HIV-1 drug resistance among the newly-diagnosed HIV-1 individuals.
Keywords: human immunodeficiency virus, drug resistance, pre-treatment, China
Introduction
The human immunodeficiency virus (HIV) has infected ≈37.7 million individuals across the globe and are living with the virus, thereby making HIV a severe public health hazard.1 By the end of 2021, the number of people living with HIV (PLWH) in China had risen to 1.05 million, presenting a substantial challenge to the country’s HIV control efforts. Access to antiretroviral therapy (ART) has considerably improved since 2003 after China’s National Free Antiretroviral Therapy Program was established, significantly reducing HIV-related morbidity and mortality.2,3 However, the extensive use of ART, the high genetic diversity of HIV, and the selection pressure exerted by ART drugs have resulted in the spread of drug-resistant variants of the virus, thereby reducing the treatment efficiency. The long-term success of ART is jeopardized due to drug resistance. It lowers the viral suppression rates, decreases antiretroviral medication durability and efficiency, increases the chances of new infections, acquired immunodeficiency syndrome (AIDS)-related mortality, and treatment expenses.4,5 The World Health Organization (WHO) devised a novel Global Action Plan on HIV drug resistance. This plan aims to understand the incidence of pretreatment drug resistance (PDR) in the PLWH, who were never exposed previously to antiretroviral drugs and acquired drug resistance in PLWH on ART should be assessed.6 However, the economic conditions do not permit China to perform routine pretreatment HIV drug resistance genotype testing for ART-naive individuals. According to a nationwide study conducted in 2017, China has an overall 6.8% prevalence of PDR.7 The varying prevalence of PDR has been reported in different regions of China, and PDR is 6.1% in Beijing,8 13.5% in Tianjin,9 and 17.4% in Shanghai.10
Xi’an (capital of the Shaanxi Province) is an old city in Northwest China, with a population of over 13 million. In 1992, Xi’an reported its first confirmed HIV type 1 (HIV-1) case, and since then, the total number of documented HIV/AIDS cases had reached 8900 by the end of December 2021, with 900 new cases reported in 2021. Males showed a higher incidence of HIV infection, compared to females, while sexual contact was the primary mode of transmission, amongst which homosexual transmission accounted for more than half of all new diagnosis.11 Chang et al, 2020 reported that the most prevalent subtype strains in Xi’an were circulating recombinant forms (CRF)01_AE and CRF07_BC.11 However, the present pretreatment HIV drug resistance status in Xi’an is yet unknown. Therefore, this study aimed to determine the distribution of the HIV-1 genotype and the prevalence of PDR in the newly-diagnosed ART-naive people in Xi’an, China.
Methods
Study Participants and Sample Collection
A cross-sectional, prospective study was conducted between January 2020 and December 2021 at the Xi’an Eighth Hospital, which is the only hospital that offered ART and HIV testing support in Xi’an, China. Ethical approval was obtained from Xi’an Eighth Hospital’s institutional review board (2019-03), and all the participants were asked to provide their written informed consent before enrollment. The study was conducted in accordance with the Declaration of Helsinki. The inclusion criteria were as follows: 1) HIV/AIDS diagnosis within 3 months of enrollment; 2) ≥18 years of age; 3) ART-naive; 4) provided signed consent for PDR testing. Participants’ demographic information, such as gender, age, self-reported transmission, and marital status, was obtained through a questionnaire upon enrollment. The baseline plasma viral load and CD4 count were quantified at Xi’an Eighth Hospital. The plasma was separated from the collected blood samples within 6 hours, and stored at −80°C for subsequent use.
Extraction, Amplification, and Sequencing of the HIV-1 RNA
HIV-1 RNA was isolated from the plasma samples (200 μL) with the help of the QIAamp Viral RNA Mini kit (QIAGEN, Germany) following the instructions provided on the kit. Thereafter, a nested PCR was conducted for amplifying the 1.3 kb target sequence in the pol gene (which encodes for protease and the initial 300 amino acids of the reverse transcriptase). For the first round of PCR, the following primer sequences were used:
Outer forward primer, MAW26: 5′-TTGGAAATGTGGAAAGGAAGGAAGGAC-3′
Outer reverse primer, RT21: 5′-CTGTATTTCTGCTATTAAGTCTTTTGATGGG-3′
For the second round of PCR, the following sequences were used,
Inner forward primer, PRO-1: 5′-CAGAGCCAACAGCCCCACCA-3′
Inner reverse primer, RT20: 5′-CTGCCAGTTCTAGCTCTGCTTC-3′
The amplification cycle for the first PCR round was as follows: Initial denaturation for 5 mins at 94°C, followed by 30 cycles at 94°C for 30s, at 55°C for 30s, and 72°C for 2.5 mins, and the final extension for 10 mins at 72°C. The second PCR reaction was set using the following conditions: Initial denaturation for 5 mins at 94°C, followed by 35 cycles at 94°C for 30s, at 63°C for 30s, and at 72°C for 2.5 mins, and the final extension for 10 mins at 72°C. Negative controls were used to check for contamination. The direct sequencing primers were designed as per the sequences mentioned elsewhere.12 Amplicons were sequenced by KingMed Diagnostics (Xi’an, China).
Sequence and Mutation Analysis
The REGA HIV subtyping tool (version 3.0) was employed for subtyping the various HIV strains13 and the recombinants were identified and confirmed using the Recombinant Identification Program version 3.0.14 Furthermore, the Stanford HIV Drug Resistance Database, HIVdb (version 9.0) (http://hivdb.stanford.edu/hivdb/by-sequences/), was used for determining the drug resistance mutations. The HIV drug resistance mutation level was classified into five categories based on genotypic susceptibility: sensitive susceptible (score ranging between 0 and 9), potential low-level resistance (scores ranging between 10 and 14), low-level resistance (score ranging between 15 and 29), intermediate resistance (score ranging between 30 and 59), and the high-level resistance (scores ≥60), as per the classification listed in the HIBdb (https://hivdb.stanford.edu/page/release-notes#resistance.summary). In this study, the low, intermediate, and high-level drug resistance sequences were all considered to be resistant.
Statistical Analysis
The data was statistically analyzed using the SPSS software (version 26.0) for Windows (SPSS, Chicago, IL). The Fisher exact test or the χ2 test was employed to compare the categorical variables, while the continuous variables were compared using the Mann–Whitney U-test. The probable associated factors that were related to at least one PDR mutation were identified using the univariate and multivariate logistic regression analyses.
Based on the results, we included the top four HIV-1 subtypes in the multivariable model for post-hoc analysis. The statistically significant variables obtained using univariate logistic regression analysis and possible factors influencing PDR reported previously, such as CD4 count, viral load, age, and gender, were included and analyzed in the multivariable regression model. The strength of the associations was calculated using the odds ratio (OR) and the adjusted OR (aOR) values, having a 95% Confidence Interval (95% CI). Every test that was performed was two-tailed, and a significance level of p<0.05 was set.
Results
Characteristics of the Study Participants
A total of 333 ART-naive participants were assessed in this study. RNA was successfully isolated from 317 plasma samples, and PCR was performed to amplify the pol gene. Table 1 represents the clinical and demographic features of all participants. The participants had a median age of 32 years [Interquartile Range (IQR) 27–42]. Male participants (90.9%, 288/317) were more in number compared to the female participants (9.1%, 29/317). The participants were further divided based on marital status, and the results reveal most of the study participants (58.7%) were single, followed by the married (30.9%) and divorced (10.4%) participants. Sexual contact was the most common mode of transmission of HIV-1 infection. Homosexual transmission (65.0%) was higher than heterosexual transmission (34.4%). 0.6% of the participants reported injection drug use. Further tests revealed that the participants had a median CD4 count of 288 cells/μL (IQR 146–470), while their median HIV viral load was recorded to be 66,000 copies/mL (IQR 24,123–143,500). The participants were divided based on their PDR status, and the results revealed no significant differences in their clinical or demographic characteristics across all groups (p>0.05).
 |
Table 1 Demographic and Clinical Characteristics Based on PDR Status |
Distribution of HIV-1 Genotypes
The genotyping results revealed that CRF07_BC (51.7%, 164/317) was the most prevailing HIV-1 genotype. It was followed by the CRF01_AE (25.9%, 82/317), B (14.2%, 45/317), CRF55_01B (4.7%, 15/317), CRF08_BC (1.3%, 4/317), and C (0.6%, 2/317) genotypes. Besides, we determined and identified 4 additional strains as the other CRFs, CRF67_01B (0.6%, 2/317), CRF59_01B (0.3%, 1/317), and CRF68_01B (0.3%, 1/317). Here, one sequence (0.3%) was identified as a unique recombinant form since it was not clustered with the available reference sequences. Further analysis revealed that CRF67_01B/CRF68_01B was the most likely recombinant form. The distribution of genotypes is shown in Figure 1.
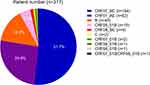 |
Figure 1 HIV-1 genotype distribution amongst the newly-diagnosed HIV/ AIDS patients in Xi’an, China. |
Prevalence of PDR Mutations
The results depicted in Figure 2 showed that the overall prevalence of the PDR mutations in the ART-naive HIV-1-infected participants was 18.3% (58/317). Most of these variants (84.5%, 49/58) were single-class mutations. The prevalence of the non-nucleoside reverse transcriptase inhibitor (NNRTI)-resistant mutation was 16.1% (51/317), NRTI-resistant mutation was 4.4% (14/317), and the prevalence of the protease inhibitor (PI)-resistant mutation was seen to be 0.9% (3/317).
Furthermore, seven variants (2.2%, 7/317) harbored mutations associated with both NRTI and NNRTI, and one variant (0.3%, 1/317) harbored NNRTI and PI-associated mutations. One variant (0.3%, 1/317) harbored mutations associated with all three types of drugs (NRTI, NNRTI, and PI).
In ART-naive HIV-1-infected participants, 22 mutation patterns were associated with NNRTIs, 14 mutation patterns were associated with NRTIs, and six mutation patterns were associated with PI. The most common NNRTI-associated mutation pattern in ART-naive HIV-1-infected participants was V179D and V179E (both were 4.4%, 14/317), followed by K103N (2.2%, 7/317), V106I (0.9%, 3/317) and V106VI (0.9%, 3/317). The most predominant NRTI-associated mutations in ART-naive HIV-1-infected participants were K65R (1.3%, 4/317) and M184V (1.3%, 4/317), followed by A62AV (0.6%, 2/317), L210W (0.6%, 2/317), and Y115F (0.6%, 2/317). Six major PI associated mutation found in three ART-naive HIV-1-infected participants were I50V (0.3%, 1/317), I54V (0.3%, 1/317), L90M (0.3%, 1/317), M46I (0.3%, 1/317), M46L (0.3%, 1/317), and V82A (0.3%, 1/317). Furthermore, most resistant strains (79.3%, 46/58) harbored a single mutation; the remaining resistant strains (1.9%, 6/317) harbored multiple (≥3) mutation patterns, as shown in Figure 2.
Resistance Level to Different ART Drugs
For analyzing the HIV-1 resistance to various ART drugs, we tested a total of 15 commonly utilized ART drugs, namely tenofovir disoproxil fumarate (TDF), didanosine (DDI), stavudine (D4T), abacavir (ABC), zidovudine (AZT), lamivudine (3TC), and emtricitabine (FTC) for NRTI, efavirenz (EFV), rilpivirine (RPV), doravirine (DOR), nevirapine (NVP), and etravirine (ETR) for NNRTI, atazanavir/ ritonavir (ATV/r), darunavir/ ritonavir (DRV/r) and lopinavir/ ritonavir (LPV/r) for PI. Table 2 represents a detailed description of the resistance level according to anchor antiretroviral drugs.
 |
Table 2 Resistance Levels to Individual Antiretroviral Drugs Among ART-Naive Participants |
Twelve participants showed drug resistance to NRTI, 8 (2.5%) participants showed resistance against ABC, 3 (0.9%) participants showed resistance against AZT, 9 (2.8%) participants showed resistance against D4T, 7 (2.2%) participants showed resistance against DDI, 7 (2.2%) participants showed resistance against 3TC, 7 (2.2%) participants showed resistance against FTC, and 6 (1.9%) participants had resistance against TDF.
For NNRTI, NVP displayed the highest resistance frequency (5.7%, 18/317), followed by EFV (5.0%, 16/317), RPV (3.5%, 11/317), DOR (2.8%, 9/317), and ETR (1.9%, 6/317). However, a substantial number of these participants generated potential low-level resistance (11.0%, 35/317) to NNR; hence, this trend cannot be disregarded.
Three participants showed resistance to PI, including 2 (0.6%) participants who were resistant to ATV/r, 2 (0.6%) participants who were resistant to LPV/r, and 1 (0.3%) participant to DRV/r.
Predicting Factors Associated with Pretreatment Drug Resistance Mutations
Some of the factors that were independently related to the increased risk of PDR mutation, included the genotypes such as CRF01_AE (aOR 3.15, 95% CI 1.51–6.57, p=0.002) and CRF55_01B (aOR 32.41, 95% CI 8.02–130.95, p<0.001; Table 3). No differences in age, gender, CD4 count, marital status, viral load, and the self-reported risk factors, were found in patients harboring/not harboring at least one PDR.
 |
Table 3 Factors Associated with Drug Resistance Mutations |
Discussion
This study revealed that the most prevalent HIV-1 genotype in Xi’an was CRF07_BC (51.7%), followed by CRF01_AE (25.9%) and B (14.2%). PDR was prevalent in 18.3% of newly diagnosed HIV/AIDS antiretroviral-naive participants in Xi’an. The prevalence of PDR mutations in NNRTIs (16.1%) was significantly higher than that in NRTIs (4.4%) and PIs (0.9%). Furthermore, 48.3% (28/58) of the mutation HIV-1 strains were attributed to V179D/E as potential low-level NNRTI resistance. Moreover, CRF01_AE and CRF55_01B strains were independently associated with at least one PDR.
Our data indicated that the CRF07_BC and the CRF01_AE genotypes were the most prevalent in Xi’an. These findings were consistent with results from Tianjin, Shanghai, Beijing, Shenzhen, and other Chinese regions.7–10,15,16 The CRF07_BC is a juvenile HIV strain that accounts for 39.7% of all cases and was first seen in injection drug users in China.17 CRF07_BC has distinct traits different from its parent strain (subtypes B and C).18 The reasons for the predominance of recombinant forms over parent subtypes in many regions of China could be attributed to its huge population, higher rate of sexual transmission, and the mass migration from one region to another in the country, all of which may contribute to a higher rate of HIV infection and cross-infection in the Chinese population.
The standard first-line ART regimen for HIV-infected adults in China is TDF/AZT+3TC+EFV/NVP.2 TDF or EFV if there are no contraindications. The second-line treatment suggested by the HIV treatment guidelines substitutes protease inhibitors (LPV/r) for NNRTI. According to our findings, the PDR mutation frequency in NNRTI was significantly higher than that in NRTI and PI among the ART-naive HIV-1-infected participants in Xi’an, thereby highlighting the importance of including PDR testing before treatment. According to the WHO’s updated guidelines related to the public health response to PDR, in countries that show a national PDR mutation frequency for NNRTI >10%, the first-line antiretroviral regimen should be modified from NNRTI to the non-NNRTI drugs, including integrase inhibitors [INSTI]).19 Hence, a PI/INSTI-based regimen could be regarded as an excellent first-line treatment for newly diagnosed HIV/AIDS patients in Xi’an.
PDR incidence in our study could primarily be caused by the NNRTI resistance mutation V179D/E (8.8%), which could decrease their resistance to NNRTIs such as RPV, EFV, ETR, and NVP.20 While a single polymorphic mutation may not significantly reduce susceptibility, combining two NNRTI-associated mutations can dramatically increase drug resistance.21 Additional research is needed to determine if this single mutation may cause the first-line therapy to fail; however, the high prevalence of single mutations is a cause for concern. Interestingly, NNRTI mutation V179E was discovered in most (80%, 12/15) participants infected with the CRF55_01B strain, confirming that naturally existing HIV-1 genetic diversity plays a crucial role in drug resistance.22 Taking effective measures to limit the CRF55_01B transmission is the need of the hour.23,24 Other dominant NNRTI variants besides V179D/E were K03N and V106I. Both K103N and V106I mutations result in decreased sensitivity to NNRTIs. The K103N mutation is one of the most common NNRTI mutations in China.7,25 It is a long-lasting intra-host persistent mutation that reduces susceptibility EFV by 20-fold and NVP by 50-fold.20,26 Baseline HIV drug resistance has been linked to virological failure and it increases the risk of failure on regimen switch in previous studies.27–29 Consequently, pretreatment of HIV drug resistance in Xi’an calls for change in diagnosis and treatment regimen to prevent the further transmission of resistant viruses.
In this study, we have shown that the HIV-1 genotype could be a probable factor that determined the PDR. Strains with the CRF01_AE and CRF55_01B genotype were more susceptible to harboring PDR. Previous studies have shown genotypes B and C are linked with an elevated likelihood of resistance.9,10 Additional research is required to understand the discrepancies in the results. Further, the study’s sample size is relatively small to draw conclusions; hence, we need to reproduce the study in a larger population to corroborate these findings.
Despite the promising results, the study has a few limitations. The small sample size would end up in a lack of precision or an inability to assess association in the model due to insufficient statistical power. Further, the resistance testing for INSTI was not conducted as part of this investigation. Given the rising prevalence of INSTI in China, it becomes essential to investigate the impact of HIV drug resistance on INSTI. Lastly, no follow-up was conducted, which prevented us from thoroughly evaluating the clinical outcomes of the participants, therefore unable to draw any causal link given the cross-sectional nature of the study.
Conclusion
Our study provides insights into the molecular epidemiological features of HIV-1 and the prevalence of PDR among newly-diagnosed treatment-naive patients in Xi’an, China. A high percentage of participants harbored the resistant virus strains. The identified mutations had a low degree of drug resistance or remained susceptible to NRTI and PI, but not to NNRTI, which is freely provided by the Chinese free HIV ART program. As a result, it is mandatory to include regular baseline genotypic resistance testing is critical in Xi’an. These findings may help optimize the first and second-line antiretroviral regimens for PLWH in various Chinese regions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. UNAIDS. Global HIV & AIDS statistics — fact sheet; 2021. Available from: https://www.unaids.org/en/resources/fact-sheet.
2. Cao W, Hsieh E, Li T. Optimizing treatment for adults with HIV/AIDS in China: successes over two decades and remaining challenges. Curr HIV/AIDS Rep. 2020;17(1):26–34. doi:10.1007/s11904-019-00478-x
3. Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China’s free ART program. Cell Res. 2005;15(11–12):877–882. doi:10.1038/sj.cr.7290362
4. Phillips AN, Stover J, Cambiano V, et al. Impact of HIV drug resistance on HIV/AIDS-associated mortality, new infections, and antiretroviral therapy program costs in Sub-Saharan Africa. J Infect Dis. 2017;215(9):1362–1365. doi:10.1093/infdis/jix089
5. Gupta S, Neogi U. Following the path: increasing trends of HIV-1 drug resistance in China. EClinicalMedicine. 2020;18:100251. doi:10.1016/j.eclinm.2019.100251
6. Bertagnolio S, Beanland RL, Jordan MR, Doherty M, Hirnschall G. The World Health Organization’s response to emerging human immunodeficiency virus drug resistance and a call for global action. J Infect Dis. 2017;216(suppl_9):S801–S804. doi:10.1093/infdis/jix402
7. Kang RH, Liang SJ, Ma YL, et al. Pretreatment HIV drug resistance in adults initiating antiretroviral therapy in China, 2017. Infect Dis Poverty. 2020;9(1):54. doi:10.1186/s40249-020-00668-5
8. Song YX, Xin RL, Li ZC, et al. Prevalence of transmitted drug resistance among HIV-1 treatment-naive patients in Beijing. Epidemiol Infect. 2018;146(3):339–344. doi:10.1017/S0950268817003016
9. Gao L, Xia H, Zeng R, et al. Pre-treatment and acquired antiretroviral drug resistance among people living with HIV in Tianjin, China. HIV Med. 2022;23(Suppl 1):84–94. doi:10.1111/hiv.13252
10. Wang Z, Zhang M, Zhang R, et al. Diversity of HIV-1 genotypes and high prevalence of pretreatment drug resistance in newly diagnosed HIV-infected patients in Shanghai, China. BMC Infect Dis. 2019;19(1):313. doi:10.1186/s12879-019-3927-1
11. Chang W, Zhang M, Ren Q, et al. HIV-1 genetic diversity and recombinant forms among men who have sex with men at a sentinel surveillance site in Xi’an City, China. Infect Genet Evol. 2020;81:104257. doi:10.1016/j.meegid.2020.104257
12. Zeng R, Ren D, Gong X, et al. HIV-1 genetic diversity and high prevalence of pretreatment drug resistance in Tianjin, China. AIDS Res Hum Retroviruses. 2020;36(10):852–861. doi:10.1089/aid.2020.0056
13. de Oliveira T, Deforche K, Cassol S, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21(19):3797–3800. doi:10.1093/bioinformatics/bti607
14. Siepel AC, Halpern AL, Macken C, Korber BT. A computer program designed to screen rapidly for HIV type 1 intersubtype recombinant sequences. AIDS Res Hum Retroviruses. 1995;11(11):1413–1416. doi:10.1089/aid.1995.11.1413
15. Yuan H, Liu Z, Wu X, et al. Evolutionary characteristics and genetic transmission patterns of predominant HIV-1 subtypes among men who have sex with men in China. Int J Infect Dis. 2020;90:125–131. doi:10.1016/j.ijid.2019.10.035
16. Zhang D, Zheng C, Li H, et al. Molecular surveillance of HIV-1 newly diagnosed infections in Shenzhen, China from 2011 to 2018. J Infect. 2021;83(1):76–83. doi:10.1016/j.jinf.2021.04.021
17. Gan M, Zheng S, Hao J, et al. Spatiotemporal patterns of CRF07_BC in China: a population-based study of the HIV strain with the highest infection rates. Front Immunol. 2022;13:824178. doi:10.3389/fimmu.2022.824178
18. Li X, Li Y, Liu H, Trovao NS, Foley BT. The emergence and transmission dynamics of HIV-1 CRF07_BC in Mainland China. Virus Evol. 2022;8(1):veac014. doi:10.1093/ve/veac014
19. WHO. Guidelines on the public health response to pretreatment HIV drug resistance; 2017. Available from: https://www.who.int/hiv/topics/drugresistance/en/.
20. Melikian GL, Rhee SY, Varghese V, et al. Non-nucleoside reverse transcriptase inhibitor (NNRTI) cross-resistance: implications for preclinical evaluation of novel NNRTIs and clinical genotypic resistance testing. J Antimicrob Chemother. 2014;69(1):12–20. doi:10.1093/jac/dkt316
21. Gatanaga H, Ode H, Hachiya A, Hayashida T, Sato H, Oka S. Combination of V106I and V179D polymorphic mutations in human immunodeficiency virus type 1 reverse transcriptase confers resistance to efavirenz and nevirapine but not etravirine. Antimicrob Agents Chemother. 2010;54(4):1596–1602. doi:10.1128/AAC.01480-09
22. Liu Y, Li H, Wang X, et al. Natural presence of V179E and rising prevalence of E138G in HIV-1 reverse transcriptase in CRF55_01B viruses. Infect Genet Evol. 2020;77:104098. doi:10.1016/j.meegid.2019.104098
23. Wei L, Li H, Lv X, et al. Impact of HIV-1 CRF55_01B infection on the evolution of CD4 count and plasma HIV RNA load in men who have sex with men prior to antiretroviral therapy. Retrovirology. 2021;18(1):22. doi:10.1186/s12977-021-00567-z
24. Gan M, Zheng S, Hao J, et al. The prevalence of CRF55_01B among HIV-1 strain and its connection with traffic development in China. Emerg Microbes Infect. 2021;10(1):256–265. doi:10.1080/22221751.2021.1884004
25. Shu Z, Chen Y, Abudureyimu A, et al. Surveillance of HIV-1 drug resistance in Xinjiang: high prevalence of K103N in treatment-naive individuals. Arch Virol. 2018;163(8):2111–2119. doi:10.1007/s00705-018-3825-7
26. Wertheim JO, Oster AM, Johnson JA, et al. Transmission fitness of drug-resistant HIV revealed in a surveillance system transmission network. Virus Evol. 2017;3(1):vex008. doi:10.1093/ve/vex008
27. Kantor R, Smeaton L, Vardhanabhuti S, et al. Pretreatment HIV drug resistance and HIV-1 subtype C are independently associated with virologic failure: results from the multinational PEARLS (ACTG A5175) clinical trial. Clin Infect Dis. 2015;60(10):1541–1549. doi:10.1093/cid/civ102
28. Boender TS, Hoenderboom BM, Sigaloff KC, et al. Pretreatment HIV drug resistance increases regimen switches in sub-Saharan Africa. Clin Infect Dis. 2015;61(11):1749–1758. doi:10.1093/cid/civ656
29. Hermans LE, Hofstra LM, Schuurman R, et al. HIV-1 pretreatment drug resistance negatively impacts outcomes of first-line antiretroviral treatment - week 96 results from the ITREMA trial. AIDS. 2022;36:923–931. doi:10.1097/QAD.0000000000003182
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

