Back to Journals » Infection and Drug Resistance » Volume 16
Genetic Characterization Conferred Co-Resistance to Isoniazid and Ethionamide in Mycobacterium tuberculosis Isolates from Southern Xinjiang, China
Authors Cao B, Mijiti X, Deng LL, Wang Q, Yu JJ, Anwaierjiang A, Qian C, Li M, Fang DA , Jiang Y, Zhao LL, Zhao X, Wan K, Liu H , Li G, Yuan X
Received 23 February 2023
Accepted for publication 2 May 2023
Published 19 May 2023 Volume 2023:16 Pages 3117—3135
DOI https://doi.org/10.2147/IDR.S407525
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Bin Cao,1,2,* Xiaokaiti Mijiti,3,* Le-Le Deng,2,4 Quan Wang,3 Jin-Jie Yu,1,2 Aiketaguli Anwaierjiang,5 Chengyu Qian,2,6 Machao Li,2 Dan-Ang Fang,2,6 Yi Jiang,2 Li-Li Zhao,2 Xiuqin Zhao,2 Kanglin Wan,2 Haican Liu,2 Guilian Li,2 Xiuqin Yuan1
1School of Public Health, University of South China, Hengyang, 421001, People’s Republic of China; 2State Key Laboratory for Infectious Disease Prevention and Control, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, 102206, People’s Republic of China; 3The Eighth Affiliated Hospital of Xinjiang Medical University, Urumqi, 830000, People’s Republic of China; 4National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, People’s Republic of China; 5College of Xinjiang Uyghur Medicine, Hetian, 848000, People’s Republic of China; 6Wenzhou Key Laboratory of Sanitary Microbiology, Key Laboratory of Laboratory Medicine, Ministry of Education, China, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, Zhejiang, 325035, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiuqin Yuan; Guilian Li, Email [email protected]; [email protected]
Background: Ethionamide (ETH), a structural analogue of isoniazid (INH), is used for treating multidrug-resistant tuberculosis (MDR-TB). Due to the common target InhA, INH and ETH showed cross-resistance in M. tuberculosis. This study aimed to explore the INH and ETH resistant profiles and genetic mutations conferring independent INH- or ETH-resistance and INH-ETH cross-resistance in M. tuberculosis circulating in south of Xinjiang, China.
Methods: From Sep 2017 to Dec 2018, 312 isolates were included using drug susceptibility testing (DST), spoligotyping, and whole genome sequencing (WGS) to analyze the resistance characteristics for INH and/or ETH.
Results: Among the 312 isolates, 185 (58.3%) and 127 (40.7%) belonged to the Beijing family and non-Beijing family, respectively; 90 (28.9%) were INH-resistant (INHR) with mutation rates of 74.4% in katG, 13.3% in inhA and its promoter, 11.1% in ahpC and its upstream region, 2.2% in ndh, 0.0% in mshA, whilst 34 (10.9%) were ETH-resistant (ETHR) with mutation rates of 38.2% in ethA, 26.2% in inhA and its promoter, and 5.9% in ndh, 0.0% in ethR or mshA; and 25 (8.0%) were INH-ETH co-resistant (INHRETHR) with mutation rates of 40.0% in inhA and its promoter, and 8% in ndh. katG mutants tended to display high-level resistant to INH; and more inhA and its promoter mutants showed low-level of INH and ETH resistance. The optimal gene combinations by WGS for the prediction of INHR, ETHR, and INHRETHR were, respectively, katG+inhA and its promoter (sensitivity: 81.11%, specificity: 90.54%), ethA+inhA and its promoter+ndh (sensitivity: 61.76%, specificity: 76.62%), and inhA and its promoter+ndh (sensitivity: 48.00%, specificity: 97.65%).
Conclusion: This study revealed the high diversity of genetic mutations conferring INH and/or ETH resistance among M. tuberculosis isolates, which would facilitate the study on INHR and/or ETHR mechanisms and provide clues for choosing ETH for MDR treatment and molecular DST methods in south of Xinjiang, China.
Keywords: cross-resistance, ethionamide, isoniazid, mutation, Mycobacterium tuberculosis
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the leading causes of death worldwide and caused 1.6 million deaths and 10.6 million new cases in 2021.1 Despite decades of efforts to adopt measures to control TB, achieving the goal of ending TB has been delayed due to a variety of reasons, from drug-resistant TB to untimely diagnosis. The proportion of new and previously treated TB cases with multidrug-resistant or rifampicin-resistant tuberculosis (MDR/RR-TB, MDR-TB defined as resistance to at least rifampicin and isoniazid) globally was 3.6% and 18%, respectively, and the combined number of MDR/RR-TB plus extensively drug-resistant tuberculosis in 2021 increased by 6.4% over 2020.1 The persistence of TB and drug-resistant TB was ascribed in part to the paucity of rapid diagnostic techniques; however, the new routes for implementing efficient and rapid diagnosis lie in the knowledge and analysis of the resistance rates and molecular characteristics of different drugs, which varied from different geographical regions.2
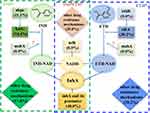
Ethionamide (ETH), a structure analog of the core drug of anti-TB regimens isoniazid (INH), was mostly used in MDR-TB.3,4 As two prodrugs, both INH and ETH were activated by different mycobacterial enzymes for antibacterial effect.5 After being activated by the catalase peroxidase KatG (encoded by katG/Rv1908c)5 and NADPH-specific flavin adenine dinucleotide-containing monooxygenase EthA (encoded by ethA/Rv3854c),6,7 respectively, both INH and ETH reacted with NAD+ to yield INH-NAD or ETH-NAD adducts which were able to bind to and inhibit the same molecular target named NADH-dependent enoyl-acyl carrier protein reductase InhA,8 leading to the disruption of mycolic acid biosynthesis and cell death.9,10 So far, several genes involved in the INH and ETH antibacterial pathway have been found to perform important roles in the acquired resistance of INH and ETH (Figure 1).11
Resistance to INH and ETH in Mycobacterium tuberculosis was mainly attributed to mutations in the activators of the drugs coded by katG and ethA, respectively.11 Mutations in the inhA or its promoter region which lead to the inhibition of InhA enzyme may cause cross-resistance to INH and ETH.8 Mutation in ndh gene is another mechanism explained the cross-resistance between these two drugs. ndh encodes a type-II NADH dehydrogenase which oxidizes NADH into NAD+, mutations in ndh lead to an increase in NADH concentration, which acts as a competitive inhibitor to prevent the binding of INH-NAD and ETH-NAD adducts to InhA and consequently the activity of InhA enzyme is impacted, leading to the co-resistant to INH and ETH.12,13 In addition, mutation in mshA may result in high-level in ETH resistance and low-level INH resistance in biochemical studies.11 The mshA gene encodes the glycosyl transferase which involves in the biosynthesis of mycothiol, a main reducing and detoxifying agent in mycobacterial.14 Finally, there are another two important regions or genes, the upstream region of ahpC and the ethR gene, involved in resistance to INH and ETH, respectively. ahpc encoded an alkyl hydroperoxide reductase, and when the upstream region of ahpC was mutated, it may render M. tuberculosis more susceptible to hydrogen and organic peroxides as ahpC expression increases.11 Mutations in the ahpC were thought to function as compensatory mutations for the loss of KatG activity.15 EthR, a transcriptional repressor, was the negative regulator of ethA,16 and existing studies showed that ethR overexpression increased the resistance to ETH.6
Whole-genome sequencing (WGS) has been applied as a diagnostic, epidemiologic and research tool in the studies of M. tuberculosis since the complete genome sequence of M. tuberculosis was described in 1998.17 Relying on the WGS, not only the prevalence of resistance-causing mutation SNPs was able to be grasped but also the specific deletions and SNPs peculiar to clinical strains could be identified. Additionally, it has the potential to demonstrate more extensive genetic variability.18,19 Hence, it is of interest to investigate the molecular characteristics of INH-resistant and ETH-resistant M. tuberculosis isolates with WGS. However, in previous studies of INH and ETH resistance with WGS, either no cross-resistance studies of INH and ETH were performed or relatively few genetic regions were selected, or the overall resistance characteristics of the geographic region were not combined.20–23 This study aimed to obtain the prevalence data and risk factors of INH and ETH resistance and cross-resistance through drug susceptibility testing (DST) and statistical analysis and investigate mutations in genes of katG, ahpC and its upstream that associated with only INH resistance, ethA and ethR that associated with only ETH resistance, and inhA and its promoter region, ndh and mshA, that associated with INH-ETH cross-resistance in M. tuberculosis isolates from southern region of Xinjiang, China.
Materials and Methods
Ethics Statement
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of the Eighth Affiliated Hospital of Xinjiang Medical University (XJMU8HEC-20161215). Each potential participant was introduced to the nature of the research and provided with an information sheet. Participants were included in this study if their written informed consent was obtained.
Collection of Mycobacterium tuberculosis Clinical Isolates
A total of 312 M. tuberculosis complex isolates from TB patients were collected from four TB special hospitals in charge of the TB control in the southern region of Xinjiang, including Xinjiang Uygur Autonomous Region Chest Hospital, Kashi Lung Hospital, Kuche County Infectious Disease Hospital and Wushi County People Hospital, between Sep 2017 and Dec 2018. All of the pulmonary TB patients aged ≥16 years with positive cultures identified as M. tuberculosis complex (MTBC) and lived in southern Xinjiang, China, were interviewed and enrolled during the study period. The isolates collected from each participant were sub-cultured on the Lowenstein-Jensen (L-J) medium for DST and DNA extraction. The sub-culture, DST, the isolates’ collection and inactivation for DNA extraction were performed in a biosafety level 3 laboratory. The demographic characteristics were collected from the electronic medical record. Mycobacterial species identification was performed using an in-house script by alignment of the WGS assembly data to the 16s rRNA sequence of H37Rv (accession number: NC_000962.3), if identity >99%, the isolates were identified as M. tuberculosis, and all isolates in the present study were identified as M. tuberculosis.
Phenotypical Drug Susceptibility Testing
Each of the 312 M. tuberculosis isolates was tested for susceptibility to 12 anti-TB drugs, using the Sensititre® plates (Thermo Fisher Scientific Inc., Cleveland, Ohio, USA), and all steps were conducted strictly according to the manufacturer’s instructions by trained staff at the national tuberculosis reference laboratory of China. Briefly, the suspensions of M. tuberculosis strains were adjusted to 0.5 McFarland standard with sterile normal saline. The standardized suspensions were diluted 100-fold with Middlebrook 7H9-OADC broth (0.2% glycerol, 10% Middlebrook oleic acid-albumin-dextrose-catalase, and 0.05% Tween 80) for inoculation into the 96-well plates and then incubated at 37°C for 10–21 days to read the results using the Vizion Digital viewing System (Thermo Fisher Scientific Inc., Cleveland, Ohio, USA).24 Minimum inhibitory concentration (MIC) was defined as the lowest concentration without apparent visible bacterial growth compared with positive controls and was measured by two readers.24 The isolates with MICs greater than the following concentrations were identified as resistant: INH 0.25 μg/mL, rifampicin (RIF) 1.0 μg/mL, ethambutol (EMB) 5 μg/mL, streptomycin (SM) 1.0 μg/mL, amikacin (AMK) 1.0 μg/mL, ofloxacin (OFX) 2.0 μg/mL, moxifloxacin (MFX) 0.5 μg/mL, ETH 5.0 μg/mL, rifabutin (RFB) 0.5 μg/mL, kanamycin (KM) 5.0 μg/mL. Cycloserine (CS) and p-aminosalicylic acid (PAS) were excluded from our analysis because phenotypic DST results for these drugs are unreliable.25,26 High level of resistance to INH or ETH was defined as MIC ≥1 μg/mL or ≥20 μg/mL, respectively, while low level of resistance to INH was defined as MIC between 0.25 and 0.5 μg/mL and to ETH was between 5 and 10 μg/mL.27,28 The M. tuberculosis H37Rv (ATCC 27294) strain stored in the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, was used as a susceptible control.
DNA Extraction
The crude genomic DNA was extracted from fresh culture with the cetyltrimethylammonium bromide (CTAB) method as the previous document.29,30 Three to four loops of M. tuberculosis colonies cultured on L-J medium for four weeks were harvested, followed by being heated in a water bath at 80°C for 30 min and then lysed with 0.1 mg/mL lysozyme at 37°C overnight. After treatment with the proteinase K, 10% sodium dodecyl sulfate (SDS), CTAB-NaCl (4.1% NaCl and 10% CTAB) and chloroform-isoamyl alcohol (24:1 [vol/vol]), the samples were centrifuged for 15 min at 13,000 × g. After being incubated at −20°C for 30 min in isopropanol, the genomic DNA samples were washed with 70% ethanol and resuspended with Tris-EDTA (TE, pH8.0). Finally, the DNA pellet was quantified with a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) by measuring the absorption at 260 nm.
Spoligotyping and Data Analysis
Spoligotyping was performed using 43 covalently bound oligonucleotides derived from the spacer sequences of M. tuberculosis H37Rv and Mycobacterium bovis BCG P3 as previously described by Kamerbeek et al.31 The results were entered in binary format into an Excel spreadsheet and compared with data in the SpolDB4 spoligotyping database (http://www.pasteurguadeloupe.fr:8081/SITVITDemo/index.jsp). In the present study, strains with spoligotype patterns characterized by deletion of spacers 1–34 or with additional deletion of one or more of the last nine spacers were classified as Beijing genotype, while others were defined as non-Beijing genotype.
Whole-Genome Sequencing
The gene mutation information in the present study was acquired from WGS data. Sequencing libraries were prepared with genomic DNA using kits as instructed by the manufacturer. DNA libraries were then selected to perform cluster growth and 150 bp paired-end sequencing on DNB SEQ-2000 instrument (Beijing Genomics Institute, Beijing, China). The raw FASTQ sequence reads were filtered by removing the adapter sequences, duplicate reads, and low-quality reads that had a quality score below 20 in more than 30% of the bases. The clean reads were mapped to the genome of H37Rv (accession number, NC_000962.2) using in-house softwares Bowtie2 (Version 2.3.4.1) and samtools (Version 1.7). VarScan (Version 2.4.4) was used for single-nucleotide polymorphism (SNP) finding. All genome-wide SNPs were identified by the VarScan software by parsing the mapping genome sequence data, and then the SNPs related to phylogeny or located in PE/PPE gene family regions were filtered out. An average of 15.4 million sequence reads was acquired per genome at a depth of 500× and with coverage of 98.0%.
According to several published literatures,11,20,27,32,33 eight candidate genes and regions, including katG, ahpC and its upstream (INH), ethA and ethR (ETH), inhA and its promoter region, ndh and mshA (INH and ETH), were selected to compare with the pan-susceptible reference genome (H37Rv, accession number: NC_000962.2) at the level of SNPs in promoter regions or intergenic regions, amino acids in genes, or insertions and deletions. The phenotypic and genotypic results were compared to determine the specificity and sensitivity for each gene with WGS to predict resistance.
Statistical Analysis
SPSS 16.0 (SPSS Inc., Chicago, IL, United States) was used to perform statistical analysis. Pearson chi-square test or Fisher’s exact probability test and logistic regression analysis were used to determine the independent covariable associated with cross-resistance between INH and ETH (INHRETHR), INH resistance, ETH resistance, and MDR group, respectively. A P value less than 0.05 was defined as significant. Sensitivity, specificity, odds ratio (OR) and confidence interval (CI) were calculated using MedCalc statistical software (MedCalc Software, Ostend, Belgium).
Result
Demographic Characteristics
Of the 312 TB patients with M. tuberculosis isolates, 159 (50.96%) were male and 153 (49.04%) were female. Age-wise analysis displayed that the ages of the participants ranged from 16 to 94, with a mean age of 54.47 years. Additionally, 164 (52.56%) were retreated cases (defined as new cases of tuberculosis treated with previous anti-tuberculosis drugs for more than one month, cases of relapse or cases of failure of initial treatment), 148 (47.44%) were new cases (defined as new cases not previously treated with anti-tuberculosis drugs or on drugs for less than one month).
We analyzed the distribution differences of treatment history between gender, age, occupation and education level and found treatment history among the age groups showed statistically significant difference (P < 0.05). We then performed a two-by-two comparison and found that the proportion of new cases was higher than the proportion of retreated cases in the <25 and 25–44 age groups, while the proportion of retreated cases was higher than the proportion of new cases in the 45–65 and >65 age groups. The results are shown in Table 1.
 |
Table 1 Demographic and Genotyping Results of Patients with New Cases and Retreated Cases |
Drug Resistance Profiles in INH and/or ETH-Resistant Isolates
DST on the 312 M. tuberculosis isolates showed that there were 25 (8.0%) INHRETHR, 65 (20.8%) INHRETHS and 9 (2.9%) INHSETHR isolates, and 213 (68.3%) isolates which were susceptible to both drugs. In total, 90 and 34 isolates were resistant to INH and ETH, respectively; 18 (5.8%) pre-extensively drug-resistant TB (Pre-XDR-TB, defined as TB that is resistant to rifampicin and any fluoroquinolone) were found, of which 36 (11.5%) were MDR. Figure 2 shows the numbers of isolates resistant to each drug or special drug combinations.
Genotyping Results
Among the 312 isolates, 185 (58.3%) belonged to the Beijing family, while 127 (40.7%) belonged to the non-Beijing family. Of the Beijing genotypes, 55 (17.6%) were resistant to INH, 18 (5.8%) strains were resistant to ETH, and 14 (4.5%) strains were co-resistant. Meanwhile, 35 (11.2%) and 16 (5.1%) strains of the non-Beijing genotype were resistant to INH and ETH, respectively, and 11 (3.5%) strains were co-resistant.
Factors Associated with INH- and ETH-Resistance, and INH-ETH Co-Resistance
We tried to assess the association between INH, ETH, INH-ETH co-resistance or MDR and demographic characteristics and isolate genotype and only found that INH resistance and MDR were more likely to be associated with retreated cases with OR of 2.00 (95% CI: 1.18–3.40) and 2.53 (95% CI: 1.17–5.47), respectively. The results are shown in Figure 3.
Mutations in katG, inhA and Its Promoter Region, ahpC and Its Upstream Region, mshA and ndh in Isoniazid Resistant Isolates
We found that the mutation located in codon 463 (R463L) in katG was identified as the most prevalent with a frequency of 84.0% (262/312), followed by the A187V mutation in mshA (118/312). These two mutations were lineage markers so were excluded in the present study. In addition, 69 isolates (including 3 INHRETHR, 15 INHRETHS, 3 INHSETHR and 48 INHSETHS isolates) were with a nucleotide substitution of ahpC-88C>A, which was defined as a mutation unrelated to the resistance to INH and ETH according to the WHO document.34 The mutation of ahpC-88C>A and other synonymous mutations except that inhA G-154A (fabG1 L203L) that recorded as INH resistance associated34 were all excluded during the analysis of mutations associated with drug resistance.
Of the 90 INH resistant isolates, 67, 11, 10 and two carried mutations in katG, inhA and its promoter, ahpC and its upstream region, and ndh, respectively. No mutation was detected in mshA (Figure 4A). The most prevalent mutation was observed at katG S315T, inhA −15C>T, ahpC −54C>T, ndh M370I, which were detected in 36, eight, three and two isolates, respectively. Of the 67 katG mutated isolates, 10 combined mutations in the ahpC and its upstream region; five combined mutations in the inhA promoter region (two with −8T>A, three with −15C>T); one carried M370I in ndh (Table 2).
Of the 222 INH susceptible isolates, the most frequent mutated gene was katG (17 isolates, 7.7%), followed by inhA and its promoter region (5 isolates, 2.3%), ahpC and its promoter region (10 isolates, 4.5%), and mshA (6 isolates, 2.7%) (Tables 2 and S1).
There were 11 novel mutations of katG R78P, W668C, L707P, 2186_2191delACAAGG, W728stop, 252dupG, −6575_376del and 553_558delTTCGGC, ahpC −77delT and D22H, and ndh M370I found only in INH resistant isolates, whilst 10 novel mutations found only in INH susceptible isolates: katG K143T and katG D513E, inhA A211T, −124G>A and −301A>G, ahpC V26A, F94C and −75T>G, mshA A187T and P368L (Table S1).
MIC Distributions of Isoniazid in Mycobacterium tuberculosis
MIC values showed that 67 INH resistant isolates were high-level resistant to INH, among which, 59 isolates carried mutations in katG, and eight isolates showed mutations in ahpc and its upstream region (Table 3). The prevalence of katG mutants in high-level INH resistant isolates was significantly higher than that in INH low-level resistant isolates (25.546, P < 0.001). In contrast, mutations in the inhA and its promoter region were statistically higher in low-level resistant isolates than in the high-level ones (8 vs 3 isolates, Fisher’s exact test, P = 0.001). In addition, the most frequent mutation of katG S315T was found in 29 isolates, which all showed high-level resistant to INH (Table 2).
 |
Table 3 The Associations Between Isoniazid Resistance Levels and Four Gene Mutations in Mycobacterium tuberculosis Isolates |
Mutations in ethA, ethR, inhA and Its Promoter Region, mshA and ndh in Ethionamide Resistant Isolates
Among 34 ETH resistant isolates, 13 carried mutations in ethA, 10 in inhA and its promoter, two in ndh (Figure 4B). Ten mutation types were found in the ethA (I34T, D56E, M59I, S266R, P334A, 245_902del, 740delC, 815delT, 1323_1329delCTCGCTG, 1405dupC), three types in the inhA promoter region (−8T>A, −15C>T, −154 G>A), and one in ndh (M37I). No mutation was detected in ethR and mshA in ETH resistant isolates. The most common mutation was inhA −15C>T detected in eight ETH resistant isolates (Table 2).
Of the 278 ETH susceptible isolates, the most frequent mutated gene was ethA (56 isolates, 20.1%), followed by ethR (seven isolates, 2.5%), inhA and its promoter region (six isolates, 2.2%), mshA (six isolates, 2.2%; three with R413N). Mutation of ethA S266R was the most frequent and found in 38 isolates.
Five novel mutations of ethA 815delT, 1405dupC, V439L, M59I and 245_902del were found only in ETH resistant isolates, while five novel mutations E36K, L268T, R306G, E400K, 734_735dupGC in ethA were found only in ETH susceptible isolates; the novel mutations ethA 740delC and P164L were found in both ETH resistant and susceptible isolates (Tables 2 and S1).
MIC Distributions of Ethionamide in Mycobacterium tuberculosis
The combination of mutation information and MIC values showed that among the 34 ETH resistant isolates, 25 were low-level resistant and nine were high-level resistant, and there was no statistical difference in the mutation rates of ethA, ndh or inhA and its promoter between the two resistant levels (Table 4).
 |
Table 4 The Associations Between Ethionamide Resistance Levels and Three Gene Mutations in Mycobacterium tuberculosis Isolates |
Cross-Resistance Profiles Between Isoniazid and Ethionamide in Mycobacterium tuberculosis
There were 25 INHRETHR M. tuberculosis isolates in the present study, 13 (52.0%), nine (36.0%), 10 (40.0%), two (8.0%) and one (4.0%) had mutations in katG, ethA, inhA and its promoter mutations, ndh and ahpC upstream region, respectively (Table 2 and Figure 4C). In detail, of the 25 INHRETHR isolates, the most prevalent mutation was inhA promoter region −15C>T, occurring in 8 isolates. In addition, five out of 25 possessed both mutations in katG and ethA; two had mutation in ndh with a non-synonymous mutation (M370I), one of these two combined with other mutations of katG (R78P) and ahpC (−77delT), another one combined with mutation in ethA (I34T); three isolates only carried mutations in the katG (two with S315T, one with V151F).
To elucidate which genes were associated with both INH and ETH resistance in this study, the differences in mutation rates of each gene between INH or ETH susceptible and resistant isolates were first analyzed by Pearson chi-square test or Fisher’s exact test according to the sample number of isolates. Statistical analysis revealed that inhA and its promoter region and ndh mutations were associated with both isoniazid and ethionamide resistance (Table 5).
 |
Table 5 The Differences in Mutation Rates of Genes Associated with Isoniazid or Ethionamide Resistance |
We further found that mutations in inhA and its promoter region were more frequent in low-level INH or ETH resistant isolates (both found with a ratio of 8/10) (Table 6).
 |
Table 6 The MICs Distribution of Isolates with inhA and Its Promoter Region in Cross-Resistant to INH and ETH |
In the present study, we only found that two isolates carried mutations in ndh M370I, one was high-level resistant to INH and low-level resistant to ETH, the other one was both low-level resistant to INH and ETH.
Prediction of Isoniazid and/or Ethionamide Resistance in Mycobacterium tuberculosis Based on Whole Genome Sequencing
As shown in Table 7, using the phenotypic data as reference, detection of mutation in katG for INH-resistance prediction exhibited a sensitivity of 74.44% and a specificity of 92.34%. When the combination of katG, inhA and its promoter region was evaluated, the sensitivity increased to 81.11%, and the specificity decreased to 90.54%; added with mutations in the ahpC upstream, the sensitivity was not improved, but the specificity was reduced to 88.74%; together with the ndh and mshA mutations, the sensitivity increased to 82.22%, and the specificity decreased to 86.49%.
 |
Table 7 The Prediction Values of Whole Genome Sequencing for Isoniazid Resistance in Mycobacterium tuberculosis Isolates |
Identification of ethA mutations for ETH-resistance prediction revealed a sensitivity of 38.24% and a specificity of 79.86%. While the combination of ethA, ethR, mshA, ndh, inhA and its promoter region mutations was assessed, the sensitivity was enhanced to 61.76% and the specificity was reduced to 75.18%, as shown in Table 8.
 |
Table 8 The Prediction Values of Whole Genome Sequencing for Ethionamide Resistance in Mycobacterium tuberculosis Isolates |
For the prediction of the co-resistance between INH and ETH Detection, mutations in inhA and its promoter region and ndh showed a sensitivity of 48.00% and a specificity of 97.65%, plus that in the mshA showed a sensitivity of 48.00% and a specificity of 95.31%, as shown in Table 9.
 |
Table 9 The Prediction Values of Whole Genome Sequencing for Isoniazid-Ethionamide Co-Resistance in Mycobacterium tuberculosis Isolates |
Discussion
The present study gained a deep insight into the cross-resistance mechanism of INH and ETH, as well as the gene mutation characteristics associated with INH and ETH resistance in M. tuberculosis isolated from the South of Xinjiang, China, by integrating DST and WGS. As expected, high diversity of genetic mutation conferring INH and ETH resistance in M. tuberculosis was identified in our study.
In concordance with previous studies, analysis of INH resistant mutations showed a clear predominance of well-established mutations in katG genes accounting for 74.4% (67/90) in INH-resistant isolates in the present study (Figure 1). In addition, the most common mutation at codon 315 in katG was found in 44.4% (40/90) INH-resistant strains, which varied considerately across the geographic regions, ranging from 97% in South Africa,35 94% in northwestern Russia,36 88% in Colombia,37 46% in Spain,38 28% in Japan.39 The dominance of mutations at codon 315 in katG may be explained by the fact that katG315 mutants retained their catalase-peroxidase activity while showing a lowered ability to activate INH, which probably ensure a sufficient level of oxidative protection to have no bacterial fitness cost and to maintain their virulence and transmissibility.40,41 Notably, there were 29 mono-resistant INH isolates carrying the mutation of katG S315T, and all of these 29 INH-resistant isolates were high-level resistant to INH, which confirmed that mutations at amino acid position 315 of the katG gene were associated with high-level resistance to INH.42,43 It is interesting to note that there were two isolates with mutations of inhA −8T>A combined with katG S315T, which was in line with Jessica’s study conducted in clinical INH resistant isolates,44 suggesting that the diagnostic significance of inhA-8T>A mutation may be compromised as it always co-occurred with katG315. Besides the canonical mutation in katG315, several mutations unreported previously were observed in our study, including three nonsynonymous mutations R78P, W668C, L707P, four frameshift mutations including 6575_376del, 553_558delTTCGGC, 252dupG, 2186_2191delACAAGG, and a silent mutation W728. The fact highlights the concern that a more widespread of use of existing molecular diagnostics that miss these mutations might impose an artificial selection process where the mutants with canonical mutations were detected and eradicated through proper chemotherapy, but those novel mutants could not be captured and continue to spread, probably resulting in the INH-resistant population evolution from a dominant single amino acid site mutation in katG to the whole-gene.
Nearly 42% of ETH-resistant strains harbored mutations in the ethA, in line with many other studies.33,45,46 Five out of 15 ethA mutation types in ETH resistant isolates have not been previously published, this high diversity of ethA mutations associated with ETH resistance supported the idea that genetic mutations in ethA were distributed across the structural gene,11,27 and unlike the dominant mutation katG S315T in INH-resistant variant, also differed from mutations located at a limited area named RR-determining region (RRDR) in RR isolates. The alternative hypothesis for lack of prominent mutations in ETH-resistant strains was that the presence of about 30 monooxygenase homologs in M. tuberculosis could prevent the bacteria from a loss of EthA enzyme activity.6,45 The polymorphism of ethA gene mutation found in our study suggested that the development of a molecular test for rapid identification of ETH-resistance was unsuitable. MIC values for ETH-resistant isolates showed that six isolates with nonsynonymous mutations in ethA were low-level resistant to ETH, we speculated that nonsynonymous mutations in ethA might be associated with low-level resistance to ETH, and further research might be required to confirm their contribution in increasing the MIC for ETH.
There were certain proportions of INH susceptible or ETH susceptible isolates carried mutations in the tested genes in the present study, and some mutations have been reported in INH- or ETH-resistant isolates (Tables 2 and S1). This result may be influenced by the sample sources and geographical regions as well as the methods for DST. Especially, there was a high proportion (20.1%) of ETH susceptible isolates carried mutations in ethA. Despite the exact function of these mutations was uncertain, we speculated that these strains were prothionamide-resistant due to the cross-resistance between ETH and prothionamide, yet we did not perform DST for prothionamide in this study.
Our study demonstrated that mutations in inhA and its promoter region confer 40.0% of INH and ETH co-resistance, similar to several previous reports.27,32,47 The most frequently mutation, inhA promoter −15C>T occurred in 32.0% (8/25) among INHRETHR isolates, consistent with other reports indicating that the proportion of inhA −15C>T ranges from 21.1% to 55.3%,27,32,33 suggesting that inhA promoter −15C>T may serve as a marker of cross-resistance to INH and ETH. When combining the MICs and mutation information, we found that inhA and ndh were indeed associated with cross-resistance in this study, and inhA and its promoter mutations were more prevalent in low-level INH or ETH resistant isolates (both 80%). For the ndh gene mutation, its contribution in cross-resistance between INH and ETH needed to be further investigated due to the limitation of sample size.
Besides the mutations in the canonical genes associated with INH or/and ETH resistance, we also investigated the mutations in ndh, mshA, ahpC and its upstream and ethR. In this study, we detected one unreported mutation in ndh (M370I) in two co-resistant strains. One of the two INHRETHR isolates had the ethA (I34T) mutation to account for ETH resistance, but no other mutation to explain its resistance to INH, and another strain contained mutations both in katG and ahpC associated with INH resistance but no other mutation to explain its resistance to ETH. Thus, we presumed that the mutation M370I in ndh might have potential to define as a new mutation relevant with cross-resistance to INH and ETH. Nevertheless, further research should be conducted to elaborate the underlying mechanism for our observation. Few studies were reported on mshA. In the present study, only a synonymous mutation mshA A92A was found in an INH-resistant isolate; however, it was unlikely to confer resistance since this single-nucleotide polymorphism did not cause change in the structure of the protein. As mentioned before, mutations in ahpC were more likely to appear together with katG mutations, supporting the fact that mutations in ahpC did function as compensatory mutation.15 For ethR, we did not identify any strain with a mutation in ETH-resistant isolates, suggesting that ethR might play a minor role in ETH resistance in M. tuberculosis clinical isolates.11 Notably, one INHRETHS strain displayed a rare mutation N35S in ethR but its effect was unknown. Taken together, the data indicated that the significance and mechanism of ndh, mshA, ahpC and ethR in INH- and ETH-resistance of M. tuberculosis should be discovered, simultaneously emphasizing the complexity of the mechanism underlying the resistance. More importantly, in our study, of the 90 INH-resistant isolates and the 34 ETH-resistant isolates, 14 (15.5%) and 10 (29.4%) showed no detectable mutation in the studied genes, suggesting that alternative mechanisms, such as drug efflux pump and decreased cell wall permeability to drugs, may also be related to drug resistance in M. tuberculosis.48–50
Compared with the phenotypic DST, the sensitivity and specificity for identifying INH-resistant isolates by WGS of combination of katG, inhA and its promoter region were 81.11% and 90.54%, respectively, consistent with data from a systematic review,51 but lower than other findings.32,37 The other combinations showed no superiority but reduced specificity in the present study (Table 7). To date, molecular diagnostics such as Hain GenoType MTBDR plus line probe assay (LPA) have vastly simplified and increased the speed of diagnosing INH-resistant TB.43,52 However, the line-probe solely covered popular mutant loci (katG and inhA) rather than the full spectrum of probes to detect all INH resistance phenotypes, possibly resulted in a consequence of failures to detect resistant strains. Therefore, the combination of katG and inhA and its promoter region can significantly improve the sensitivity and specificity for INH resistance prediction by WGS. For MDR-TB with cross-resistance between INH and ETH, diagnosis of INH-resistant TB only using LPA by detecting INH resistance-associated mutations will likely lead to misuse of ETH in subsequent treatment regimens, ultimately leading to compromised treatment outcomes. Therefore, INH resistance results obtained using LPA should be accompanied by ETH resistance testing to avoid the misuse of ETH and delayed treatment. This fact motivated further exploration of mechanisms of ETH resistance and search for novel specific molecular targets to facilitate the development of specific methods for the detection of ETH resistance in the future.
Imperfectly, for the ETH resistance prediction, we found an optimal combination of ethA, inhA and its promoter and ndh by WGS showed sensitivity of 61.76 and specificity of 76.62%; the sensitivity was lower than the findings from France (78.6%)53 and Colombia (84.2%),37 and a possible explanation was that the isolates resistant to ETH (n = 34) collected in our study were relatively small so that limited the detection of the variety of gene variations. The sensitivity and specificity for detecting cross-resistance between INH and ETH by WGS of the combination of inhA and its promoter and ndh were 48.00% and 97.65%, respectively, which was the same as other findings.27,32 Particularly, the addition of ndh mutations to that in inhA or its promoter region increased the sensitivity by 8.0% to predict cross-resistance between INH and ETH.
Another interesting finding of the present study was that INH resistance and MDR are more likely to be observed in retreated cases, as previously reported, emphasizing the crucial role of early diagnosis and timely, long-term and well-regulated drug administration, especially in patients treated within the hospital system in the region.
One major strength of this study is that we used whole-genome sequencing data to perform analysis based on SNP differences, which enables us to identify mutations more accurately. There are several limitations in our study. First, limited by the small sample size, we only collected a small number of drug-resistant strains so that we did not detect relevant mutations in the genes enrolled in the study. Second, we simply focused on genes known to confer resistance to INH and ETH in M. tuberculosis, and some other genes relevant to drug resistance are overlooked. Lastly, while we detect numerous novel mutations that have not been previously covered, research on the structure of protein in combination with mutagenesis and enzymatic studies directly in M. tuberculosis has not been launched to elucidate the specific molecular mechanisms and their effects on the resistance.
Conclusion
In summary, our results indicated that the prevalence of INH, ETH resistance and co-resistance between INH and ETH in the south of Xinjiang province were 28.9%, 10.9% and 8.0%, respectively. Furthermore, mutations in katG, inhA and its promoter, ahpC and its upstream, and ndh genes accounted for 74.4%, 12.2%, 11.1% and 2.2% of INH resistance, respectively. Mutations in ethA, inhA and its promoter, and ndh genes accounted for 38.2%, 29.4% and 5.9% of ETH resistance, respectively. Mutations in inhA and its promoter and ndh, respectively, conferred 40.0% and 8% INH-ETH co-resistance. The optimal gene combinations by WGS for the prediction of INH and ETH resistance and INH-ETH co-resistance were respectively katG+inhA and its promoter region (sensitivity: 81.11%, specificity: 90.54%), ethA+inhA and its promoter region+ndh (sensitivity, 61.76%, specificity: 76.62%), and inhA and its promoter region+ndh (sensitivity, 48.00%, specificity: 97.65%). Mutations in katG were associated with high-level INH resistance, whilst mutations in inhA and its promoter were associated with both INH and/or ETH low-level resistance. The results found in this study would increase our understanding on the resistance mechanisms of INH and/or ETH and provide clues for choosing ETH for MDR treatment and molecular DST methods in this area.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study obtained approval (XJMU8HEC-20161215) from the Ethics Committee of The Eighth Affiliated Hospital of Xinjiang Medical University. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from individual or guardian participants.
Acknowledgments
We appreciated the staff of Xinjiang Uygur Autonomous Region Chest Hospital, Kashgar, Kuqa and Wushi for supplying strains and collecting data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the project (2017A03006) of the Major science and technology programs of Xinjiang Uygur Autonomous Region from department of Science and Technology, Xinjiang Uygur Autonomous Region and Mega Project of Research on the Prevention and Control of HIV/AIDS, Viral Hepatitis Infectious Diseases (2018ZX10103001-003-012) from the Ministry of Science and Technology, China. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
We have no conflicts of interest to declare.
References
1. Bagcchi S. WHO’s global tuberculosis report 2022. Lancet Microbe. 2023;4:e20. doi:10.1016/S2666-5247(22)00359-7
2. Green KD, Garneau-Tsodikova S. Resistance in tuberculosis: what do we know and where can we go? Front Microbiol. 2013;4:208. doi:10.3389/fmicb.2013.00208
3. Tahaoglu K, Torun T, Sevim T, et al. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med. 2001;345:170–174. doi:10.1056/NEJM200107193450303
4. World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: 2011 Update. Geneva: World Health Organization; 2011.
5. Zhang Y, Heym B, Allen B, et al. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi:10.1038/358591a0
6. Baulard AR, Betts JC, Engohang-Ndong J, et al. Activation of the pro-drug ethionamide is regulated in mycobacteria. J Biol Chem. 2000;275:28326–28331. doi:10.1074/jbc.M003744200
7. Vannelli TA, Dykman A, Ortiz de Montellano PR. The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J Biol Chem. 2002;277:12824–12829. doi:10.1074/jbc.M110751200
8. Banerjee A, Dubnau E, Quemard A, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi:10.1126/science.8284673
9. Vilcheze C, Wang F, Arai M, et al. Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat Med. 2006;12:1027–1029. doi:10.1038/nm1466
10. Vilcheze C, Morbidoni HR, Weisbrod TR, et al. Inactivation of the inhA-encoded fatty acid synthase II (FASII) enoyl-acyl carrier protein reductase induces accumulation of the FASI end products and cell lysis of Mycobacterium smegmatis. J Bacteriol. 2000;182:4059–4067. doi:10.1128/JB.182.14.4059-4067.2000
11. Vilcheze C, Jacobs WR, Hatfull GF, Jacobs Jr. WR. Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: genes, mutations, and causalities. Microbiol Spectr. 2014;2(4):MGM2-0014–2013. doi:10.1128/microbiolspec.MGM2-0014-2013
12. Vilcheze C, Weisbrod TR, Chen B, et al. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother. 2005;49:708–720. doi:10.1128/AAC.49.2.708-720.2005
13. Lee AS, Teo AS, Wong SY. Novel mutations in ndh in isoniazid-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2001;45:2157–2159. doi:10.1128/AAC.45.7.2157-2159.2001
14. Newton GL, Buchmeier N, Fahey RC. Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol Mol Biol Rev. 2008;72:471–494. doi:10.1128/MMBR.00008-08
15. Ng VH, Cox JS, Sousa AO, et al. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol. 2004;52(5):1291–1302. doi:10.1111/j.1365-2958.2004.04078.x
16. Engohang-Ndong J, Baillat D, Aumercier M, et al. EthR, a repressor of the TetR/CamR family implicated in ethionamide resistance in mycobacteria, octamerizes cooperatively on its operator. Mol Microbiol. 2004;51:175–188. doi:10.1046/j.1365-2958.2003.03809.x
17. Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi:10.1038/31159
18. Satta G, Lipman M, Smith GP, et al. Mycobacterium tuberculosis and whole-genome sequencing: how close are we to unleashing its full potential? Clin Microbiol Infect. 2018;24(6):604–609. doi:10.1016/j.cmi.2017.10.030
19. Meehan CJ, Goig GA, Kohl TA, et al. Whole genome sequencing of Mycobacterium tuberculosis: current standards and open issues. Nat Rev Microbiol. 2019;17:533–545. doi:10.1038/s41579-019-0214-5
20. Malinga L, Brand J, Jansen van Rensburg C, et al. Investigation of isoniazid and ethionamide cross-resistance by whole genome sequencing and association with poor treatment outcomes of multidrug-resistant tuberculosis patients in South Africa. Int J Mycobacteriol. 2016;5(Suppl 1):S36–S37. doi:10.1016/j.ijmyco.2016.11.020
21. Roa MB, Tablizo FA, Morado EKD, et al. Whole-genome sequencing and single nucleotide polymorphisms in multidrug-resistant clinical isolates of Mycobacterium tuberculosis from the Philippines. J Glob Antimicrob Resist. 2018;15:239–245. doi:10.1016/j.jgar.2018.08.009
22. Truden S, Sodja E, Zolnir-Dovc M, Sundaramurthy V. Drug-resistant tuberculosis on the balkan peninsula: determination of drug resistance mechanisms with xpert MTB/XDR and whole-genome sequencing analysis. Microbiol Spectr. 2023;11(2):e0276122. doi:10.1128/spectrum.02761-22
23. Welekidan LN, Yimer SA, Skjerve E, et al. Whole genome sequencing of drug resistant and drug susceptible Mycobacterium tuberculosis isolates from Tigray Region, Ethiopia. Front Microbiol. 2021;12:743198. doi:10.3389/fmicb.2021.743198
24. He W, Tan Y, Liu C, et al. Drug-resistant characteristics, genetic diversity, and transmission dynamics of rifampicin-resistant Mycobacterium tuberculosis in Hunan, China, revealed by whole-genome sequencing. Microbiol Spectr. 2022;10:e0154321. doi:10.1128/spectrum.01543-21
25. Liu D, Huang F, Zhang G, et al. Whole-genome sequencing for surveillance of tuberculosis drug resistance and determination of resistance level in China. Clin Microbiol Infect. 2022;28:731e739–731 e715. doi:10.1016/j.cmi.2021.09.014
26. O’Grady J, Maeurer M, Mwaba P, et al. New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Curr Opin Pulm Med. 2011;17:134–141. doi:10.1097/MCP.0b013e3283452346
27. Rueda J, Realpe T, Mejia GI, et al. Genotypic analysis of genes associated with independent resistance and cross-resistance to isoniazid and ethionamide in Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2015;59:7805–7810. doi:10.1128/AAC.01028-15
28. Machado D, Perdigao J, Ramos J, et al. High-level resistance to isoniazid and ethionamide in multidrug-resistant Mycobacterium tuberculosis of the Lisboa family is associated with inhA double mutations. J Antimicrob Chemother. 2013;68:1728–1732. doi:10.1093/jac/dkt090
29. Kigozi E, Kasule GW, Musisi K, et al. Prevalence and patterns of rifampicin and isoniazid resistance conferring mutations in Mycobacterium tuberculosis isolates from Uganda. PLoS One. 2018;13:e0198091. doi:10.1371/journal.pone.0198091
30. van Soolingen D, de Haas PE, Hermans PW, et al. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 1994;235:196–205.
31. Kamerbeek J, Schouls L, Kolk A, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi:10.1128/jcm.35.4.907-914.1997
32. Islam MM, Tan Y, Hameed HMA, et al. Detection of novel mutations associated with independent resistance and cross-resistance to isoniazid and prothionamide in Mycobacterium tuberculosis clinical isolates. Clin Microbiol Infect. 2019;25:1041e1041–1041 e1047. doi:10.1016/j.cmi.2018.12.008
33. Brossier F, Veziris N, Truffot-Pernot C, et al. Molecular investigation of resistance to the antituberculous drug ethionamide in multidrug-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55:355–360. doi:10.1128/AAC.01030-10
34. Walker TM, Miotto P, Koser CU, et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe. 2022;3:e265–e273. doi:10.1016/S2666-5247(21)00301-3
35. Kiepiela P, Bishop KS, Smith AN, et al. Genomic mutations in the katG, inhA and aphC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazulu Natal, South Africa. Tuber Lung Dis. 2000;80(1):47–56. doi:10.1054/tuld.1999.0231
36. Mokrousov I, Narvskaya O, Otten T, et al. High prevalence of KatG Ser315Thr substitution among isoniazid-resistant mycobacterium tuberculosis clinical isolates from Northwestern Russia, 1996 to 2001. Antimicrob Agents Chemother. 2002;46(5):1417–1424. doi:10.1128/AAC.46.5.1417-1424.2002
37. Ferro BE, Garcia PK, Nieto LM, et al. Predictive value of molecular drug resistance testing of Mycobacterium tuberculosis isolates in Valle del Cauca, Colombia. J Clin Microbiol. 2013;51:2220–2224. doi:10.1128/JCM.00429-13
38. Garcia de Viedma D, Del Sol Diaz Infantes M, Lasala F, et al. New real-time PCR able to detect in a single tube multiple rifampin resistance mutations and high-level isoniazid resistance mutations in Mycobacterium tuberculosis. J Clin Microbiol. 2002;40:988–995. doi:10.1128/JCM.40.3.988-995.2002
39. Abe C, Kobayashi I, Mitarai S, et al. Biological and molecular characteristics of Mycobacterium tuberculosis clinical isolates with low-level resistance to isoniazid in Japan. J Clin Microbiol. 2008;46:2263–2268. doi:10.1128/JCM.00561-08
40. Zhao X, Yu H, Yu S, et al. Hydrogen peroxide-mediated isoniazid activation catalyzed by Mycobacterium tuberculosis catalase−peroxidase (KatG) and its S315T mutant. Biochemistry. 2006;45:4131–4140. doi:10.1021/bi051967o
41. Pym AS, Saint-Joanis B, Cole ST. Effect of katG mutations on the virulence of Mycobacterium tuberculosis and the implication for transmission in humans. Infect Immun. 2002;70:4955–4960. doi:10.1128/IAI.70.9.4955-4960.2002
42. van Soolingen D, de Haas PE, van Doorn HR, et al. Mutations at amino acid position 315 of the katG gene are associated with high-level resistance to isoniazid, other drug resistance, and successful transmission of Mycobacterium tuberculosis in the Netherlands. J Infect Dis. 2000;182:1788–1790. doi:10.1086/317598
43. Brossier F, Veziris N, Truffot-Pernot C, et al. Performance of the genotype MTBDR line probe assay for detection of resistance to rifampin and isoniazid in strains of mycobacterium tuberculosis with low- and high-level resistance. J Clin Microbiol. 2006;44:3659–3664. doi:10.1128/JCM.01054-06
44. Torres JN, Paul LV, Rodwell TC, et al. Novel katG mutations causing isoniazid resistance in clinical M. tuberculosis isolates. Emerg Microbes Infect. 2015;4:e42. doi:10.1038/emi.2015.42
45. Morlock GP, Metchock B, Sikes D, et al. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2003;47:3799–3805. doi:10.1128/AAC.47.12.3799-3805.2003
46. Casali N, Nikolayevskyy V, Balabanova Y, et al. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat Genet. 2014;46:279–286. doi:10.1038/ng.2878
47. Bollela VR, Namburete EI, Feliciano CS, et al. Detection of katG and inhA mutations to guide isoniazid and ethionamide use for drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016;20(8):1099–1104. doi:10.5588/ijtld.15.0864
48. Louw GE, Warren RM, Gey van Pittius NC, et al. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother. 2009;53(8):3181–3189. doi:10.1128/AAC.01577-08
49. Vaziri F, Kohl TA, Ghajavand H, et al. Genetic diversity of multi- and extensively drug-resistant Mycobacterium tuberculosis isolates in the capital of Iran, revealed by whole-genome sequencing. J Clin Microbiol. 2019;57. doi:10.1128/JCM.01477-18
50. Zhang Z, Lu J, Wang Y, et al. Prevalence and molecular characterization of fluoroquinolone-resistant Mycobacterium tuberculosis isolates in China. Antimicrob Agents Chemother. 2014;58(1):364–369. doi:10.1128/AAC.01228-13
51. Penn-Nicholson A, Georghiou SB, Ciobanu N, et al. Detection of isoniazid, fluoroquinolone, ethionamide, amikacin, kanamycin, and capreomycin resistance by the Xpert MTB/XDR assay: a cross-sectional multicentre diagnostic accuracy study. Lancet Infect Dis. 2022;22:242–249. doi:10.1016/S1473-3099(21)00452-7
52. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi:10.1056/NEJMoa0907847
53. Maitre T, Morel F, Brossier F, et al. How a PCR sequencing strategy can bring new data to improve the diagnosis of ethionamide resistance. Microorganisms. 2022;10(7):1436. doi:10.3390/microorganisms10071436
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.