Back to Journals » OncoTargets and Therapy » Volume 15
Genetic Analysis and Combined Therapy of Surgery and Chemotherapy for the Progression-Free Survival of a Patient with Ovarian Carcinosarcoma: A Case Report and Literature Review
Authors Guo S , Zhang X , Tang Q, Zhou M , Jiang D, Yu E
Received 16 March 2022
Accepted for publication 14 June 2022
Published 29 June 2022 Volume 2022:15 Pages 717—725
DOI https://doi.org/10.2147/OTT.S363835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Shanshan Guo,1 Xiaoyun Zhang,2 Qianjue Tang,1 Mengyun Zhou,2 Dan Jiang,2 Erkai Yu1
1Departments of Gynecology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, People’s Republic of China; 2Departments of Pathology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, People’s Republic of China
Correspondence: Erkai Yu, Longhua Hospital, 725 Wanping South Road, Xuhui District, Shanghai, People’s Republic of China, Tel/Fax +8618117209488, Email [email protected]
Abstract: Carcinosarcoma, also known as malignant Mullerian mixed tumour, is a rare and aggressive ovarian malignant tumour. The prognosis of ovarian carcinosarcoma is very poor, accounting for the vast majority of ovarian cancer deaths. Due to the rarity of ovarian carcinosarcoma, no unified treatment plan exists at present. Here, we report the case of a 69-year-old patient with stage IC ovarian carcinosarcoma. She underwent right salpingo-oophorectomy and R0 resection, inclouding extrafascial hysterectomy, left salpingo-oophorectomy, omentectomy, appendectomy, right pelvic lymph node dissection and multipoint biopsy. Full-exome sequencing was performed with normal ovarian tissue, cancer tissue, sarcoma tissue and carcinosarcoma tissue, and the results showed that the sarcoma and carcinosarcoma tissue shared more mutated genes. A TP53 mutation occurred in the cancer tissue and carcinosarcoma tissue. By analysing the lychee tree, we found that the sarcoma tissue and carcinosarcoma tissue shared more subclones and determined that they were more closely related; the cancer tissue carried fewer subclones and was the main clone. The sarcoma may have evolved from the cancer tissues. Six rounds of postoperative chemotherapy (carboplatin + paclitaxel + ifosfamide (IFO) (paclitaxel 200 mg, D1 + carboplatin 600 mg, D1 + IFO 2G, D1-D3)) were administered. The patient has been followed up for six years and is currently in good health. In conclusion, the disease was diagnosed in the early stage, and the use of a R0 resection + a three-drug combination chemotherapy may have contributed to the patient’s long-term disease-free survival. The results of the gene study suggested that the sarcoma component may be differentiated from the cancer component. We thus speculated that the origin of this case may have been the fallopian tube.
Keywords: OCS, literature review, gene analysis, origin
Plain Language Summary
A 69-year-old patient with ovarian carcinosarcoma underwent right salpingo-oophorectomy and R0 resection, inclouding extrafascial hysterectomy, left salpingo-oophorectomy, omentectomy, appendectomy, right pelvic lymph node dissection and multipoint biopsy. Six rounds of postoperative chemotherapy (carboplatin + paclitaxel + IFO (paclitaxel 200 mg, D1 + carboplatin 600 mg, D1 + IFO 2G, D1-D3)) were administered. The patient has been followed up for six years and is currently in good health. Full-exome sequencing was performed with normal ovarian tissue, cancer tissue, sarcoma tissue and carcinosarcoma tissue, The results of the gene study suggested that the sarcoma tissue and carcinosarcoma tissue shared more subclones and determined that they were more closely related; the cancer tissue carried fewer subclones and was the main clone. A TP53 mutation occurred in the cancer tissue and carcinosarcoma tissue. The sarcoma may have evolved from the cancer tissues. We thus speculated that the origin of this case may have been the fallopian tube.
Introduction
Ovarian carcinosarcoma (OCS), also known as mixed malignant Mullerian tumour (MMMT), is a rare, biphasic, challenging histologic subtype that accounts for only 1–3% of all ovarian cancers.1 OCSs are aggressive cancers with a similar presentation to epithelial ovarian cancers, although few patients present with disease confined to the ovary, and many will have visceral metastases at presentation. The disease comprises a mixture of two distinct tumour tissues: malignant epithelial components and mesenchymal components. It can be subdivided based on its homogeneity or heterogeneity. The origin of carcinosarcoma is still under study, and immunohistochemical analysis and measurement of serum AFP are important for the establishment of the diagnosis and clarification of the possibility of endometriosis or malignancies derived from endometriosis as precursor factors. The rarity of nonepithelial ovarian cancers in postmenopausal women has the potential to cause initial diagnostic uncertainty and lead to delayed or suboptimal treatment.2 Due to the rarity of OCS, no unified treatment plan exists at present. Although prospective data are lacking, the management of OCS has been extrapolated from the experience of epithelial ovarian cancers, anecdotal evidence or small retrospective published series. Aggressive surgical debulking is still the mainstay therapy and should be followed by adjuvant chemotherapy in advanced-stage disease3.
Here, we report a patient with OCS who underwent surgical treatment and chemotherapy. The patient has been followed up for six years and is currently in good health. Full-exome sequencing was performed with normal ovarian tissue, cancer tissue, sarcoma tissue and carcinosarcoma tissue. We speculated that the origin of this case may have been the fallopian tube based on the results.
Case Report
Description of Illness
This case describes a 69-year-old woman who presented to the Department of Emergency General Surgery, Longhua Hospital, with acute right-sided abdominal pain for 1 day in December 2014. The patient had a normal condition until menopause at 50 years, and she had a medical history of hypertension and tachycardia. On physical examination, right-sided abdominal tenderness and rebound tenderness were identified. Her preoperative labs revealed a CA-125 level of 66.1 U/mL (reference range <25 U/mL) and white blood cell count of 9.41*10^9/l (reference range 3.5–9.5*10^9). The patient underwent exploratory laparotomy for suspicion of acute appendicitis.
Surgical findings revealed a mixed mass that was 10×4 cm in size and was adhered to the right ovary and fallopian tube. The appendix, uterus and left adnexa were intact. After gynaecological consultation, right salpingo-oophorectomy was performed. Frozen sectioning could not be performed because the equipment was out of order. The mass appeared brown and brittle, and the right ovary was 3×4 cm in size. The right fallopian tube appeared to be thickened, with a dark purple, brittle texture.
A diagnosis of homologous carcinosarcoma was confirmed. The patient’s CA-125 level was 41.7 U/mL three days after surgery. Pelvic MRI and chest CT appeared normal. Because the pathological nature of the first operation was unknown, only right salpingo-oophorectomy was performed for this patient. During the operation, ascites was not detected during the operation, and pelvic cleaning fluid examination and pelvic multipoint biopsy were not performed; thus, the thoroughness of the first operation could not be clearly ascertained, and the scope of the operation was expanded. The patient underwent a technically successful R0 resection, including extrafascial hysterectomy, left salpingo-oophorectomy, omentectomy, appendectomy, right pelvic lymph node dissection and multipoint biopsy in January 2015, 18 days after the initial surgery. Multipoint biopsy and lymph node dissection included one sigmoid colon nodule, one lower abdominal wall nodule, one right ovarian nodule, one small intestinal wall nodule, one rectal forearm, one right abdominal wall nodule, one ileocecal part nodule, one right pelvic wall nodule, one right inguinal lymph node and two right extra-iliac lymph nodes. Because no obvious abnormality was found in the left pelvic cavity during the operation, and considering the advanced age of the patient, left pelvic lymph node dissection and aortic dissection were not performed during the operation to avoid postoperative adverse reactions to the greatest extent. The residual neoplastic deposits were less than 1 cm in diameter. The pelvic washings and all excised parts were free of neoplastic involvement.
Results
According to the pathological findings, the right ovarian tumour had ruptured and eroded the fallopian tube, but no obvious tumour invasion was found in other organs. The final diagnosis was homologous OCS classified as stage IC according to the FIGO 2014 guidelines. The patient’s condition was stable after the operation, and she received adjuvant chemotherapy with an ifosfamide, carboplatin and paclitaxel regimen for 6 cycles (paclitaxel 200 mg, first day + carboplatin 600 mg, first day + ifosfamide 2 g, days 1–3). Her CA-125 level was normal before the first chemotherapy session. During chemotherapy, the patient experienced adverse reactions such as hair loss, mild leucopoenia and loss of appetite. The patient has been free of disease for 6 years thus far.
Pathological Analysis
The pathological findings confirmed carcinosarcoma: CK7 (partial +), CK20 (-), Vim (+), PAX8 (partial +), ER (+), PR (-), Villin (-), CAM5.6 (partial +), WT-1 (partial +), P53 (+), AE1/AE3 (partial +), AE1/AE3 C-erBb-2 (-), TTF-1 (-), AFP (±), S-100 (partial +), CDX2 (-/+), CD31 (vessel; +), Ki-67 (60%+), CEA (-), and hepa-1 (-). Intraoperatively, 4 pieces of normal grey–white and grey–red tissue were observed, with a total size of 7×7.5×3 cm. Some areas showed cystic wall-like features, with a size of 4.5×3.5×2 cm. The thickness of the wall was 0.1–0.2 cm, the other tissues appeared cystic and solid, the section was grey and grey–red, the quality was medium to fine, and the suspected area was the umbrella end of the fallopian tube.
The tumour comprised high-grade serous carcinoma and sarcoma cells. The nuclei were multilinear and mitotic (Figure 1). AE1/AE3, p53 and WT-1 positivity was noted in the epithelioid area by EnVision (Figure 1A–C), and Vim-1 was positive in the spindle cell area (Figure 1D).
 |
Figure 1 Typical images of immunohistochemical staining. AE1/AE3, p53 and WT-1 positivity was noted in the epithelioid area by EnVision (A–C), and Vim-1 was positive in the spindle cell area (D). |
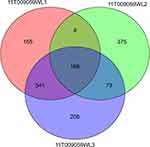 |
Figure 2 Venn diagram showing the number of co-mutated genes detected in the patient. 11T009059WL1: sarcomatous tissue, 11T009059WL2: cancer tissue, 11T009059WL3: carcinosarcoma tissue. |
Genetic Analysis
We performed genetic analysis for this patient to attempt to understand the evolution of carcinosarcoma. We selected normal ovarian tissue, cancer tissue, sarcoma tissue, and carcinosarcoma tissue for full-exome sequencing. In normal ovarian tissue, we only found a variation in BRCA2. The test results in the other three samples are shown in Figure 2. A TP53 mutation occurred in both the cancer and carcinosarcoma tissues but not in the sarcoma tissues. TP53, as an early driver,4 showed that the cancer tissue may have reflected earlier events. We found 241 co-mutated genes in the cancer tissues and carcinosarcoma tissues and 709 mutations in the cancer and sarcoma tissues. The sarcoma and carcinosarcoma tissues shared more mutated genes, and the results showed that the evolutionary relationship between the sarcoma and carcinosarcoma tissues was close. We analysed the evolutionary pedigree of the cancer tissue, using LICHeE to reconstruct multisample tumour phylogenies and tumour subclone decomposition from targeted deep-sequencing SSNV datasets to infer sample heterogeneity. LICHeE is a novel method that automates the phylogenetic inference of cancer progression from multiple somatic samples. It is highly effective in reconstructing phylogenies and uncovering the heterogeneity of previously published datasets and in simulations.5,6 Using this method, we generated a lychee tree (Figure 3) and found that the sarcoma tissue and carcinosarcoma tissue shared more subclones and determined that they were more closely related; the cancer tissue carried fewer subclones and was the main clone. The results also revealed the close relationship between the sarcoma and carcinosarcoma tissues, and the sarcoma may have evolved from the cancer tissues.
Discussion
Genetics and Origin
Three main theories have been debated over the past decade: (1) the collision theory — in which 2 tumour types, epithelial and sarcomatous tumours, evolve independently and then collide; (2) the combination theory — in which a common stem cell precursor gives rise to carcinoma and sarcoma, with both components being derived from a single stem cell undergoing divergent differentiation early in tumour evolution; and (3) the conversion theory — in which the sarcomatous element arises from carcinoma during tumour evolution or an original stem cell differentiates into one cell type, in turn differentiating into a second cell type (metaplasia). Carnevali et al7 reported two cases of TP53 dysfunction in carcinosarcomas. Montalvo-Esquivel et al8 observed a positive reaction for TP53 in carcinomas and sarcomas, indicating the monoclonal origin of the two components. Zhao et al4 analysed the mutational landscape of 68 uterine and ovarian carcinosarcomas by whole-exome sequencing. The results demonstrated that carcinomatous and sarcomatous elements were derived from a common precursor with mutations that were typical of carcinomas. Stable transgenic expression of H2A and H2B in a uterine serous carcinoma cell line demonstrated that mutant, but not wild-type, histones increased the expression of markers of epithelial–mesenchymal transition (EMT) and stimulated the migratory and invasive properties of tumour cells, suggesting a role in sarcomatous transformation. Gotoh et al9 suggested that cancer and sarcoma components share key driving mutations to a large extent and supported the combination or transformation without collision theory for CS histogenesis. The miR-141/200A/200B/200C/429 axis also plays a potential role in the transformation of cancer cells into sarcoma cells in CS tumours.
However, an increasing number of studies have revealed that OCS is often associated with serous intraepithelial carcinoma, suggesting that the disease originates from the fallopian tube.10,11 Serous intraepithelial carcinoma of the fallopian tube (STIC) is the most likely precursor of carcinosarcoma.12,13 Rewsuwan et al14 reported that carcinosarcoma, fallopian tube carcinoma and teratoma coexisted, suggesting that fallopian tube carcinoma may be the origin of OCS through epithelial mesenchymal transition. See et al15 found the same TP53 gene c.376–1G > A splice site mutation in the cancerous and sarcomatous components of OCS and related serous tubal intraepithelial carcinoma. This means that the common source of these three components and OCS may originate from the fallopian tube. Moss et al16 found that most fallopian tube and primary peritoneal tumours were type II tumours (high serous carcinoma, high endometrioid carcinoma, carcinosarcoma and undifferentiated carcinoma) and high-grade serous carcinoma. Regardless of whether the tumour was found in the ovary/fallopian tube/peritoneum, it seemed to be a disease entity, with no significant difference in survival outcome. This finding supports the notion that “tubal ovarian serous carcinoma” should be classified separately. Ardighieri et al17 performed morphological, p53 immunohistochemical and TP53 gene mutation analyses in a single-institution cohort of 16 adnexal carcinosarcomas. Serous tubal intraepithelial carcinoma (STIC) was only observed in patients with high-grade serous features of cancer components, and the same TP53 mutation was found in most PC (pelvic carcinosarcoma)-related STICs, evidencing the clonal relationship between these tumour lesions and suggesting that they originated outside the ovary in STIC.
The genetic analysis of the patient used normal ovarian, cancer, sarcoma, and carcinosarcoma tissues for full-exome sequencing. The result showed that the evolutionary relationship between the sarcoma and carcinosarcoma tissues was close and cancer tissue samples may reflect earlier events. Sarcomas may have evolved from cancer. We did not find the existence of STIC, so we could not perform relevant research on fallopian tube origin. However, according to the literature,16 the origin of epithelial ovarian cancer may be the fallopian tube, and carcinosarcomas also have epithelial components. We thus speculated that the origin of this case may have been the fallopian tube.
Treatment and Prognosis
Due to the rarity of OCS, no unified treatment plan is available at present. The FIGO staging of ovarian cancer is suitable for carcinosarcomas. The 2009 NCCN clinical practice guidelines in oncology (NCCN guidelines) state that carcinosarcoma patients are not suitable for preserving reproductive function. All patients must receive chemotherapy after full surgical staging. This procedure includes hysterectomy, bilateral salpingo-oophorectomy, omentectomy, appendectomy, and para-aortic and pelvic lymphadenectomy to the left renal vein. Peritoneal, paracolic, subdiaphragmatic and minor pelvis biopsies are recommended. For advanced patients, tumour reduction surgery is more difficult.
At present, there is a debate about whether lymph node dissection should be performed in patients in the early stage. Wang et al18 reported that AJCC T2 °CS should be treated with local lymph node dissection, which can improve the survival rate. For AJCC-T1, lymph node metastasis and lymph node dissection have no significant correlation with prognosis, but lymph node metastasis has a trend of malignant survival in patients with earlier stages. Cicin, et al19 reported that whether patients with OCS underwent lymph node dissection did not affect their overall survival. However, Chung et al20 found that the ASA score (American Society of Anesthesiologists score), complete surgery and lymph node dissection were factors influencing the prognosis of non-HGSC ovarian tumours. However, optimal tumour resection during the initial operation is a very important prognostic factor for patients with carcinosarcoma.21 For the chemotherapy regimen, in the study by Kanis et al,22 no difference in PFS (P = 0.42) or OS (P = 0.91) was found between patients receiving the T/C (paclitaxel/carboplatin) regimen and those receiving other chemotherapy regimens. Yalcin et al23 reported that platinum sensitivity was a prognostic factor.
The chemotherapy regimen recommended by the NCCN guidelines is the same as that for epithelial tumours: paclitaxel combined with carboplatin is the standard and preferred regimen for first-line chemotherapy. In addition, the NCCN also recommends other options: cisplatin/ifosfamide (class 2a), carboplatin/ifosfamide (class 2a) and ifosfamide/paclitaxel (class 2b). Follow-up monitoring after treatment is the same as that for epithelial ovarian cancer.
Most of the chemotherapy regimens in retrospective studies are mainly platinum based. Heinzelmann-Schwarz et al24 found that MMMT-O responded better to a combination of carboplatin with anthracyclines than with taxanes (73.9% vs 39.4%, respectively). Boussios et al25 studied the literature on carcinosarcoma up to 2018 and found that platinum was the main chemotherapy for carcinosarcoma, and the addition of paclitaxel or ifosfamide to platinum in first-line treatment should be based on patient factors and associated toxicities. Brackmann et al26 compared platinum + paclitaxel with ifosfamide + paclitaxel and found that a chemotherapy regimen containing platinum can increase PFS but has little effect on carcinosarcomas. Thigpen et al27 found that among the 44 patients with an evaluable response, one showed a complete response (2%), and eight showed partial (18%) responses. An additional 10 (23%) patients exhibited stable disease, while 25 (57%) had progressive disease. Cicin et al19 found that the response rate of 41% in patients treated with chemotherapy compared favourably with the published data with responses rates of 35–47%. The response rate in the 26 patients who received platinum-based chemotherapy was 42%, and for those who received other chemotherapy regimens, it was 33%. Brown et al21 reported that seven out of 17 patients (41%) responded to platinum-based chemotherapy.
In addition, some studies on chemotherapy did not include platinum-based drugs. Glaser et al28 found that an OCS patient-derived xenograft (PDX) showed resistance to carboplatin and paclitaxel (similar to the patient) but exhibited significant sensitivity to ifosfamide and paclitaxel.
Considering that the patient was older and that there was no obvious abnormality on the left side of the pelvic cavity in the second operation, left pelvic lymph node dissection and aortic dissection were not performed during the operation to avoid postoperative adverse reactions to the greatest extent. Because of the rarity and invasiveness of OCS, this patient was treated with carboplatin + paclitaxel + IFO chemotherapy, and no recurrence was noted. The adverse reactions were mild. The BRCA2 gene mutation was found in the follow-up gene testing, which may be the reason why platinum drugs had a significant effect.
Our research has some limitations, as it includes only 1 case study of OCS. Multicentre, randomized, double-blind observational studies are required to verify our treatment outcome and provide more evidence to benefit patients. In addition, our case did not find SITC, and the conclusion of genetic research on whether the OCS originated from the fallopian tube can only be inferred. Relevant research will be further carried out in the future.
Conclusion
This patient’s disease was found in an early stage, and the use of a R0 resection + a three-drug combination chemotherapy regimen may have contributed to the patient’s long-term disease-free survival. The results of the genetic study suggested that the sarcoma component may be differentiated from the cancer component. We thus speculated that the origin of this case may have been the fallopian tube.
Abbreviations
ASA score, American Society of Anesthesiologists score; CS, carcinosarcoma; CT, computed tomography scan; EMT, epithelial–mesenchymal transition; FIGO, International Federation of Gynecology and Obstetrics; IFO, ifosfamide; LICHeE, Lineage Inference for Cancer Heterogeneity and Evolution; LND, lymph node dissection; MMMT, mixed malignant Mullerian tumour; MRI, magnetic resonance imaging; MSI, microsatellite instability; NCCN, National Comprehensive Cancer Network; OCS, ovarian carcinosarcoma; OS, overall survival; PDX, patient-derived xenograft; SSNVs, somatic single nucleotide variants; STIC, serous intraepithelial carcinoma of the fallopian tube; TP53, tumour protein 53; VAF, variant allele frequency.
Data Sharing Statement
The data collected and analysed during this study are included in this review and are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Our patient provided standard written consent for the use of data, pictures and videos for teaching and research purposes at the time of the laparotomy. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. This study protocol was reviewed, and the need for approval was waived by the ethics committee of Longhua Hospital.
Acknowledgments
The authors thank the patient for her participation in this study and for the her agreement to the publication of the report. The authors thank Shanghai OrigiMed Co., Ltd., Shanghai, China for supporting and participating in gene research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Big data research on the diagnosis and treatment of polycystic ovary syndrome was performed by Cao Lingxian, a famous TCM of the Shanghai Science and Technology Plan Project (17401932000).
Disclosure
Shanshan Guo and Xiaoyun Zhang are co-first authors of this study. The authors declare that they have no competing interests. All authors deny any financial or personal relationships with other people or organizations/companies that could inappropriately influence their work.
References
1. Berton-Rigaud D, Devouassoux-Shisheboran M, Ledermann JA, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S55–S60. doi:10.1097/IGC.0000000000000228
2. Boussios S, Attygalle A, Hazell S, et al. Malignant ovarian germ cell tumors in postmenopausal patients: the royal Marsden experience and literature review. Anticancer Res. 2015;35(12):6713–6722.
3. Boussios S, Zarkavelis G, Seraj E, Zerdes I, Tatsi K, Pentheroudakis G. Non-epithelial ovarian cancer: elucidating uncommon gynaecological malignancies. Anticancer Res. 2016;36(10):5031–5042. doi:10.21873/anticanres.11072
4. Zhao S, Bellone S, Lopez S, et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2016;113(43):12238–12243. doi:10.1073/pnas.1614120113
5. Popic V, Salari R, Hajirasouliha I, Kashef-Haghighi D, West RB, Batzoglou S. Fast and scalable inference of multi-sample cancer lineages. Genome Biol. 2015;16(1):91. doi:10.1186/s13059-015-0647-8
6. Miura S, Vu T, Deng J, et al. Power and pitfalls of computational methods for inferring clone phylogenies and mutation orders from bulk sequencing data. Sci Rep. 2020;10(1):3498. doi:10.1038/s41598-020-59006-2
7. Carnevali IW, Cimetti L, Sahnane N, et al. Two cases of carcinosarcomas of the ovary involved in hereditary cancer syndromes. Int J Gynecol Pathol. 2017;36(1):64–70. doi:10.1097/PGP.0000000000000290
8. Montalvo-Esquivel G, Chanona-Vilchis JG, Herrera-Gómez A, Meneses-García AA, Isla-Ortiz D. Primary ovarian carcinosarcoma. Report of eight cases. Ginecol Obstet Mex. 2014;82(7):483–489.
9. Gotoh O, Sugiyama Y, Takazawa Y, et al. Clinically relevant molecular subtypes and genomic alteration-independent differentiation in gynecologic carcinosarcoma. Nat Commun. 2019;10(1):4965. doi:10.1038/s41467-019-12985-x
10. Mikami Y. Paradigm shift in ovarian tumor pathology from the view of genomic abnormalities. Gan To Kagaku Ryoho. 2016;43(3):286–289.
11. Terada KY, Ahn HJ, Kessel B. Differences in risk for type 1 and type 2 ovarian cancer in a large cancer screening trial. J Gynecol Oncol. 2016;27(3):e25. doi:10.3802/jgo.2016.27.e25
12. Weinberger V, Bednarikova M, Cibula D, Zikan M. Serous tubal intraepithelial carcinoma (STIC) – clinical impact and management. Expert Rev Anticancer Ther. 2016;16(12):1311–1321. doi:10.1080/14737140.2016.1247699
13. Seidman JD. Serous tubal intraepithelial carcinoma localizes to the tubal-peritoneal junction: a pivotal clue to the site of origin of extrauterine high-grade serous carcinoma (ovarian cancer). Int J Gynecol Pathol. 2015;34(2):112–120. doi:10.1097/PGP.0000000000000123
14. Rewsuwan S, Satabongkoch N, Suprasert P, Khunamornpong S. Ovarian carcinosarcoma and its association with mature cystic teratoma and primary tubal carcinoma. Case Rep Pathol. 2016;2016:2605045. doi:10.1155/2016/2605045
15. See SHC, Behdad A, Maniar KP, Blanco LZ. Ovarian carcinosarcoma and concurrent serous tubal intraepithelial carcinoma with next-generation sequencing suggesting an origin from the fallopian tube. Int J Surg Pathol. 2019;27(5):574–579. doi:10.1177/1066896919838347
16. Moss EL, Evans T, Pearmain P, et al. Should all cases of high-grade serous ovarian, tubal, and primary peritoneal carcinomas be reclassified as tubo-ovarian serous carcinoma? Int J Gynecol Cancer. 2015;25(7):1201–1207. doi:10.1097/IGC.0000000000000477
17. Ardighieri L, Mori L, Conzadori S, et al. Identical TP53 mutations in pelvic carcinosarcomas and associated serous tubal intraepithelial carcinomas provide evidence of their clonal relationship. Virchows Arch. 2016;469(1):61–69. doi:10.1007/s00428-016-1933-x
18. Wang WP, Li N, Zhang YY, et al. Prognostic significance of lymph node metastasis and lymphadenectomy in early-stage ovarian carcinosarcoma. Cancer Manag Res. 2018;10:1959–1968. doi:10.2147/CMAR.S166524
19. Cicin I, Ozatli T, Turkmen E, et al. Predictive and prognostic factors in ovarian and uterine carcinosarcomas. Balkan Med J. 2016;33(5):517–524. doi:10.5152/balkanmedj.2016.151268
20. Chung YS, Park SY, Lee JY, et al. Outcomes of non-high grade serous carcinoma after neoadjuvant chemotherapy for advanced-stage ovarian cancer: a Korean gynecologic oncology group study (OV 1708). BMC Cancer. 2019;19(1):341. doi:10.1186/s12885-019-5514-7
21. Brown E, Stewart M, Rye T, et al. Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer. 2004;100(10):2148–2153. doi:10.1002/cncr.20256
22. Kanis MJ, Kolev V, Getrajdman J, Zakashansky K, Cohen C, Rahaman J. Carcinosarcoma of the ovary: a single institution experience and review of the literature. Eur J Gynaecol Oncol. 2016;37(1):75–79.
23. Yalcin I, Meydanli MM, Turan AT, et al. Carcinosarcoma of the ovary compared to ovarian high-grade serous carcinoma: impact of optimal cytoreduction and standard adjuvant treatment. Int J Clin Oncol. 2018;23(2):329–337. doi:10.1007/s10147-017-1215-x
24. Heinzelmann-Schwarz V, Kind AB, Vetter M, et al. Should MMMT still be treated with adjuvant taxane-based combination chemotherapy? J Cancer Res Clin Oncol. 2020;146(3):695–704. doi:10.1007/s00432-019-03091-y
25. Boussios S, Karathanasi A, Zakynthinakis-Kyriakou N, et al. Ovarian carcinosarcoma: current developments and future perspectives. Crit Rev Oncol Hematol. 2019;134:46–55. doi:10.1016/j.critrevonc.2018.12.006
26. Brackmann M, Stasenko M, Uppal S, Erba J, Reynolds RK, McLean K. Comparison of first-line chemotherapy regimens for ovarian carcinosarcoma: a single institution case series and review of the literature. BMC Cancer. 2018;18(1):172. doi:10.1186/s12885-018-4082-6
27. Thigpen JT, Blessing JA, DeGeest K, Look KY, Homesley HD. Cisplatin as initial chemotherapy in ovarian carcinosarcomas: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;93(2):336–339. doi:10.1016/j.ygyno.2004.01.007
28. Glaser G, Weroha SJ, Becker MA, et al. Conventional chemotherapy and oncogenic pathway targeting in ovarian carcinosarcoma using a patient-derived tumorgraft. PLoS One. 2015;10(5):e0126867. doi:10.1371/journal.pone.0126867
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.