Back to Journals » Drug Design, Development and Therapy » Volume 13
Generic Tacrolimus (Tacrobell®) Shows Comparable Outcomes to Brand-Name Tacrolimus in the Long-Term Period After Adult Deceased Donor Liver Transplantation
Authors Kim JM , Joh JW , Choi GS, Lee SK
Received 29 August 2019
Accepted for publication 2 December 2019
Published 31 December 2019 Volume 2019:13 Pages 4431—4438
DOI https://doi.org/10.2147/DDDT.S229114
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Jong Man Kim, Jae-Won Joh, Gyu-Seong Choi, Suk-Koo Lee
Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
Correspondence: Jae-Won Joh
Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Irwon-Ro 81, Gangnam-Gu, Seoul 06351, Republic of Korea
Tel +82-2-3410-3466
Fax +82-2-3410-0040
Email [email protected]
Background: Generic tacrolimus (Tacrobell®) is commonly used in liver transplant patients in Korea. No previous studies have assessed the long-term efficacy and safety of generic tacrolimus for adult deceased donor liver transplantation (DDLT) patients. The aim of the present study was to evaluate the long-term efficacy and safety of generic tacrolimus compared to brand-name tacrolimus (Prograf®) in adult DDLT recipients.
Methods: Two hundred sixty-five adult DDLTs were performed in our center between 2003 and 2017. To determine the efficacy and safety of generic tacrolimus, renal function (estimated glomerular filtration rate [eGFR] and creatinine), infectious complications, rejection-free survival rates, and patient survival rates were investigated.
Results: Of 265 patients, 193 were selected and divided into a generic tacrolimus group (n=147) and a brand-name group (n=46). Mean follow-up duration was 63.2 ± 44.3 months. The 1-year, 3-year, 5-year, and 10-year patient survival rates were 89.1%, 86.9%, 84.5%, and 75.2%, respectively, in the generic tacrolimus group and 95.7%, 88.9%, 86.3%, and 83.7% in the brand-name tacrolimus group. There were no statistically significant differences in the infectious complications, new-onset diabetes, and renal dysfunction included mean serum creatinine level or eGFR after DDLT between the two groups. Increased recipient age, continuous renal replacement therapy (CRRT) in the pre-transplant phase, and acute rejection were predisposing factors for patient death.
Conclusion: The present study shows that generic tacrolimus is an alternative comparable to brand-name tacrolimus in adult DDLT patients.
Keywords: liver transplantation, immunosuppression, safety, efficacy
Introduction
Tacrolimus is established as an effective immunosuppressive agent in solid organ transplantation.1 Tacrolimus inhibits the T-cell mediated immune response by blocking transcription of the gene encoding interleukin-2 (IL-2).2
Most countries have attempted to reduce the cost of transplant patient care, but the financial burdens of immunosuppressive therapy remain high.3 Brand-name tacrolimus (Prograf®; Astellas Pharma Inc, Tokyo, Japan) lost its patent in 2008. Since then, generic tacrolimus, which has met all standards for bioequivalence and is therapeutically equivalent to brand-name tacrolimus, has been introduced both locally and internationally.4,5
Tacrobell® (Chong Kun Dang Pharmaceutical Corp, Seoul, Korea) is a generic version of tacrolimus that was approved by the Korea Ministry of Food and Drug Safety in 2004.6 Generic tacrolimus is now widely prescribed for liver transplant recipients in many countries. A 2013 Drug Trend Report from a prescription benefit plan provider estimated that generic tacrolimus captures 30.7% of the total market share of all transplant medications, compared to 7.2% for brand-name tacrolimus.7 Generic tacrolimus is considered safe and effective in liver transplant patients in Korea.6 However, there is little additional information on trends in generic tacrolimus use over time. One study was non-comparative, and another study had a short-term follow up.6,8 A recent study demonstrated the efficacy and safety of generic tacrolimus compared with brand-name tacrolimus in liver transplantation (LT) patients,9 but no previous studies have assessed the long-term efficacy of generic tacrolimus for adult deceased donor liver transplantation (DDLT) patients.
Based on long-term experience with the two formulations, we conducted a retrospective observational study to evaluate the long-term efficacy and safety of generic tacrolimus compared to brand-name tacrolimus in adult DDLT patients.
Methods
Patients
The present retrospective observational study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (SMC-2019-08-033). The IRB waived the requirement for patient consent because this was a retrospective study of data from medical records. The patient data accessed was maintained with confidentiality in present study.
From April 2003 to March 2017, 265 adult DDLTs were performed at our institution. Patients were excluded according to the following criteria: re-transplantation, synchronous multiple organ transplants, pediatric liver transplantation (age <18 years), conversion from each drug, use of mTOR inhibitors, use of cyclosporin, liver graft use in donation after cardiac death, and incomplete medical records. Generic tacrolimus had been in use in our hospital since 2007. The drugs used in the generic tacrolimus and brand-name tacrolimus (Prograf®; Astellas Pharma Inc, Tokyo, Japan) groups were administered according to operator, with no switch between generic tacrolimus and brand-name tacrolimus. No patients used generic and brand-name tacrolimus at the same time. As a result of exclusions, 193 patients were selected and divided into a generic tacrolimus group (n=147) and a brand-name group (n=46). The generic tacrolimus group included 147 patients, and the brand-name tacrolimus group included 46 patients. Mean follow-up duration was 63.2 ± 44.3 months.
To determine the efficacy of generic tacrolimus, we investigated renal function (estimated glomerular filtration rate (eGFR) and creatinine), infectious complications, rejection-free survival rates, and patient survival rates. Rejection was diagnosed based on the Banff schema.
Immunosuppression Regimen
An immunosuppression regimen was introduced in previous papers.10,11 The immunosuppressive regimen included tacrolimus (generic or brand-name) as a component of a double- or triple-drug regimen (the two other drugs were Methylprednisolone [MPD] and mycophenolate mofetil [MMF]). Basiliximab was used as an induction agent. Tacrolimus was administered at a dose of 1mg on the third postoperative day, and the dose was adjusted daily by checking trough levels. MMF was administered at a dose of 500–1000 mg/day to patients of both groups. MPD was administered intravenously in the perioperative period and was gradually tapered to a minimal dose thereafter. Steroids were withdrawn three months after DDLT.
Statistical Analysis
Continuous variables were analyzed using the Mann–Whitney U-test, with results expressed as means ± standard deviations or medians and ranges. Categorical variables are expressed as numbers and percentage of subjects. Chi-square tests or Fisher’s exact tests were conducted to evaluate differences in frequencies of categorical variables between groups. Rejection free-survival rates and patient survival rates were estimated using the Kaplan–Meier method and survival curves were compared with the log rank test. Cox proportional hazards model analysis was used to predict patient death after adult DDLT. Multivariate analysis used significant factors from the univariate analysis. All tests were two-sided, and statistical significance was defined as P < 0.05. All statistical analyses were performed using SPSS ver. 22.0 (SPSS, Inc., IBM Corporation, Armonk, NY, USA).
Results
Baseline Characteristics
A total of 193 patients who underwent adult DDLT from April 2003 March 2017 was reviewed. The generic tacrolimus group (n=147) and brand-name tacrolimus group (n=46) were compared according to demographics and perioperative characteristics.
Sex, age, history of hypertension or diabetes, cause of death, cardiopulmonary resuscitation, use of inotropes, macrosteatosis, microsteatosis, ICU stays, and liver function tests in deceased donors were compared between the two groups. Median deceased donor age was 49 years (range, 8–83 years) in the generic tacrolimus group and 48 years (range, 9–79 years) in the brand-name tacrolimus group. There were no statistically significant differences between the two groups (Table 1). Median recipient age was 53 years (range, 26–77 years) in the generic tacrolimus group and 50 years (range, 19–69 years) in the brand-name tacrolimus group. Sex, age, history of hypertension or diabetes, hepatic encephalopathy, varix bleeding, ascites, spontaneous bacterial peritonitis, and ventilator care during the pre-transplant period in recipients did not significantly differ between the two groups (Table 2). The proportion of hepatitis B virus (HBV) as an indication of DDLT was higher in the brand-name tacrolimus group than in the generic tacrolimus group (71.7% vs. 46.3%). However, the proportion of alcohol as an indication of DDLT was lower in the brand-name tacrolimus group than in the generic tacrolimus group (4.3% vs. 25.2%). The proportion of patients without hepatorenal syndrome was higher in the brand-name tacrolimus group than in the generic tacrolimus group (87.0% vs. 72.1%). Accordingly, the median Model for End-Stage Liver Disease (MELD) score in the brand-name tacrolimus group was lower than that of the generic tacrolimus group (21 vs. 29). No differences were identified in split liver graft, warm ischemic time and cold ischemic time between the two groups.
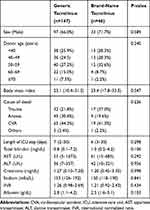 |
Table 1 Baseline Characteristics of Deceased Donors |
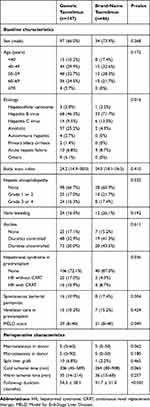 |
Table 2 Baseline Characteristics of Recipients |
Efficacy
The discontinuation rate was 15.6% (n=23) in the generic tacrolimus group and 19.6% (n=9) in the brand-name tacrolimus group in the post-transplant period (P=0.505). No differences were found in mean tacrolimus trough levels during follow-up after DDLT between the two groups (Figure 1). The 1-year, 3-year, and 5-year rejection-free survival rates were 86.3%, 79.7%, and 78.7%, respectively, in the generic tacrolimus group and 75.6%, 64.3%, and 58.7% in the brand-name tacrolimus group. Therefore, rejection-free survival in the brand-name tacrolimus group was significantly lower than in the generic tacrolimus group (P=0.016) (Figure 2A). The causes of death are summarized in the Table 3. Mortality rate was 15.6% in the generic tacrolimus group and 17.4% in the brand-name tacrolimus group, respectively. Graft failure or infection contributed mortality in DDLT patients. The 1-year, 3-year, 5-year, and 10-year patient survival rates were 89.1%, 86.9%, 84.5%, and 75.2% in the generic tacrolimus group and 95.7%, 88.9%, 86.3%, and 83.7% in the brand-name tacrolimus group. No significant difference in patient survival rate was identified between the generic tacrolimus and brand-name tacrolimus groups (P=0.518) (Figure 2B).
 |
Table 3 Causes of Death After DDLT |
 |
Figure 1 Mean tacrolimus trough levels in generic and brand-name tacrolimus (mean and 95% confidence interval at each point). |
 |
Figure 2 (A) Patient survival rate and (B) rejection-free survival rate. |
Safety
We summarized several complications in Table 4. The incidences of pneumonia, cytomegalovirus infection, herpes zoster, and fungal infection were not significantly different between the two groups. Nor were there statistically significant differences in de novo hypertension, new-onset diabetes, and de novo malignancy between the two groups. Additionally, no differences were found between the two groups in mean serum creatinine level and mean eGFR after DDLT (Figure 3). However, five patients in the generic tacrolimus group (3.4%) and four patients in the brand-name tacrolimus group (8.7%) required hemodialysis because of chronic renal failure. Finally, two patients in the generic tacrolimus group underwent kidney transplantation.
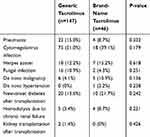 |
Table 4 Safety Between the Generic Tacrolimus and Brand-Name Tacrolimus Groups |
 |
Figure 3 (A) Mean serum creatinine level. (B) Mean eGFR (95% confidence interval at each point). |
Risk Factors for Patient Survival
Univariate analysis showed that recipient age, history of continuous renal replacement therapy (CRRT) in the recipient, and acute rejection were closely associated with patient survival (Table 5). Multivariate analysis showed that increased recipient age, CRRT in the pre-transplant period, and acute rejection were predisposing factors for patient death.
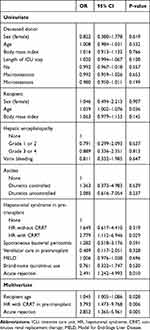 |
Table 5 Risk Factors for Patient Death |
Discussion
Previous studies reported that the 5-year efficacy and safety of generic tacrolimus (Tacrobell®) and brand-name tacrolimus (Prograf®) are similar in LT patients including living donor liver transplantation (LDLT) and DDLT. DDLT cases usually have a higher MELD score compared with LDLT cases because hepatocellular carcinoma (HCC) patients with lower MELD scores tend to undergo LDLT for curative treatments. Therefore, we focused only on DDLT cases, which had higher MELD scores. This study identified the long-term efficacy and safety of generic tacrolimus compared with brand-name tacrolimus in adult patients undergoing DDLT.
The present study demonstrated that there was no difference in long-term patient survival rates between generic tacrolimus and brand-name tacrolimus groups, although rejection-free survival rate was lower in the generic tacrolimus group. The cause of death was not different between the two groups. There was no group effect in multivariate analysis. No differences in serum creatinine or eGFR were evident between the two groups during the long follow-up period. Events of infectious complications, new-onset diabetes after transplantation, and end-stage renal disease in the generic tacrolimus group were not different from those of the brand-name tacrolimus group.
Generic drugs have a cost-saving effect. Generic tacrolimus has met all standards for demonstrating bioequivalence and is therapeutically equivalent to brand-name tacrolimus.5 However, the transplant community has expressed concerns about using generic tacrolimus instead of brand-name tacrolimus.3,4,12 In addition, patients may not believe that generic tacrolimus is equivalent to brand-name tacrolimus. Our study included a mean follow-up duration longer than five years in adult DDLT patients. The present study showed that, although rejection-free survival rate in the brand-name group was higher than in the generic tacrolimus group, patient survival rates and events of infectious complications and renal dysfunction in the generic tacrolimus group were similar to those in the brand-name tacrolimus group during long-term follow up. A previous study reported that lymphocyte subsets with generic tacrolimus were comparable to those with brand-name tacrolimus. Only the level of CD4+Foxp3+ T cells was higher in the brand-name tacrolimus group than in the generic tacrolimus group after LT.13 The present study again demonstrated that the efficacy and safety of generic tacrolimus were not different from those of brand-name tacrolimus.
Tacrolimus has a narrow therapeutic index and is subject to routine therapeutic drug monitoring and dose adjustment. High peripheral blood concentrations of tacrolimus are associated with nonspecific consequences of over-immunosuppression and specific effects of calcineurin inhibition, notably nephrotoxicity, neurotoxicity, and thrombotic microangiopathy.14 There has been some reluctance amongst clinicians and patients to consider changing tacrolimus formulations.3 The present study showed that mean serum creatinine level and eGFR in the generic tacrolimus group were not different from those of the brand-name tacrolimus group; mean tacrolimus trough level was also not different between the two groups. A previous study already showed that the efficacy of generic tacrolimus was not different from that of brand-name tacrolimus.6 This study again shows that generic tacrolimus can be safely and effectively used.
Although the generic drug approval process has a long-term successful track record, concerns remain regarding approval of narrow therapeutic index generic immunosuppressants, such as tacrolimus, in transplant recipients. Post-marketing surveillance studies are recommended to obtain additional safety data.15
Our center has used the brand-name tacrolimus since 2000 and generic tacrolimus since 2007. Therefore, follow-up duration was significantly different between the two groups. Patients who underwent adult DDLT between 2000 and 2007 are all in the brand-name tacrolimus group. The brand-name tacrolimus group may have a higher rejection rate because there was less experience with immunosuppressive medication after liver transplantation in the early 2000s.
We already reported using the data from the Korean National Health Insurance System that recipient age is an important factor for in-hospital mortality after DDLT.16 A single-center study showed that patients aged ≥70 years should not be excluded from LT or even LDLT based solely on recipient age. This implies that careful selection of recipients and donors and meticulous surgical technique are necessary for successful results.17 However, our study again shows that elderly patients have increased mortality after liver transplantation.
Our study had several limitations. First, this study was retrospective; therefore, unknown or unmeasured confounding variables are possible. However, the present study provides outcomes over a relatively long follow-up period. Second, no cost saving effect was analyzed because there were no available data. Third, there were several significant differences between the two groups, including etiology for DDLT, hepatorenal syndrome, MELD score, and observation period. Lastly, our study included a small number of DDLT cases. Western countries have many DDLT cases, where the deceased donation rate is higher than in our country and in Eastern Asia. We cannot know the appropriate sample size necessary to draw a conclusion, but we think that our data sufficiently support our conclusion.
In conclusion, generic tacrolimus is an alternative comparable to brand-name tacrolimus after adult DDLT. However, the results of the present study cannot be generalized to other generic formulations of tacrolimus.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Group USMFLS. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331(17):1110–1115. doi:10.1056/NEJM199410273311702
2. Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6(10):577–583. doi:10.1038/nrneph.2010.101
3. Molnar AO, Fergusson D, Tsampalieros AK, et al. Generic immunosuppression in solid organ transplantation: systematic review and meta-analysis. BMJ. 2015;350:h3163. doi:10.1136/bmj.h3163
4. Taube D, Jones G, O’Beirne J, et al. Generic tacrolimus in solid organ transplantation. Clin Transplant. 2014;28(5):623–632. doi:10.1111/ctr.2014.28.issue-5
5. Spence MM, Nguyen LM, Hui RL, Chan J. Evaluation of clinical and safety outcomes associated with conversion from brand-name to generic tacrolimus in transplant recipients enrolled in an integrated health care system. Pharmacotherapy. 2012;32(11):981–987. doi:10.1002/phar.2012.32.issue-11
6. Yu YD, Lee SG, Joh JW, et al. Results of a Phase 4 trial of Tacrobell(R) in liver transplantation patients: a multicenter study in South Korea. Hepatogastroenterology. 2012;59(114):357–363. doi:10.5754/hge11472
7. Liu Q, Smith AR, Park JM, et al. The adoption of generic immunosuppressant medications in kidney, liver, and heart transplantation among recipients in Colorado or nationally with Medicare part D. Am J Transplant. 2018;18(7):1764–1773. doi:10.1111/ajt.14722
8. Park K, Kim YS, Kwon KI, Park MS, Lee YJ, Kim KH. A randomized, open-label, two-period, crossover bioavailability study of two oral formulations of tacrolimus in healthy Korean adults. Clin Ther. 2007;29(1):154–162. doi:10.1016/j.clinthera.2007.01.016
9. Choi HJ, Kim DG, Kwak BJ, Han JH, Hong TH, You YK. Comparison of the long-term efficacy and safety of generic tacrolimus, Tacrobell, with Prograf in liver transplant recipients. Drug Des Devel Ther. 2018;12:295–301. doi:10.2147/DDDT
10. Kim JM, Kwon CHD, Joh JW, et al. ABO-incompatible living donor liver transplantation with rituximab and total plasma exchange does not increase hepatocellular carcinoma recurrence. Transplantation. 2018;102(10):1695–1701. doi:10.1097/TP.0000000000002154
11. Kim JM, Kwon CHD, Joh JW, Choi GS, Kang ES, Lee SK. Comparative peripheral blood t cells analysis between adult Deceased Donor Liver Transplantation (DDLT) and Living Donor Liver Transplantation (LDLT). Ann Transplant. 2017;22:475–483. doi:10.12659/AOT.903090
12. van Gelder T. What is the future of generics in transplantation? Transplantation. 2015;99(11):2269–2273. doi:10.1097/TP.0000000000000782
13. Kim JM, Kwon CHD, Joh JW, et al. Differences in peripheral blood lymphocytes between brand-name and generic tacrolimus used in stable liver transplant recipients. Med Princ Pract. 2017;26(3):221–228. doi:10.1159/000455861
14. Ball S. Bioequivalence of twice-daily oral tacrolimus in transplant recipients: more evidence for consensus? PLoS Med. 2017;14(11):e1002429. doi:10.1371/journal.pmed.1002429
15. Harrison JJ, Schiff JR, Coursol CJ, et al. Generic immunosuppression in solid organ transplantation: a Canadian perspective. Transplantation. 2012;93(7):657–665. doi:10.1097/TP.0b013e3182445e9d
16. Gil E, Kim JM, Jeon K, et al. Recipient age and mortality after liver transplantation: a population-based cohort study. Transplantation. 2018;102(12):2025–2032. doi:10.1097/TP.0000000000002246
17. Kwon JH, Yoon YI, Song GW, et al. Living donor liver transplantation for patients older than age 70 years: a single-center experience. Am J Transplant. 2017;17(11):2890–2900. doi:10.1111/ajt.14355
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
