Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Generalized Granuloma Annulare Associated with Sjogren’s Syndrome: A Case Report
Authors Wang H , Wang Y, Zheng Z, Cui Y
Received 6 December 2022
Accepted for publication 8 February 2023
Published 18 February 2023 Volume 2023:16 Pages 453—456
DOI https://doi.org/10.2147/CCID.S399782
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Huijing Wang,1 Ying Wang,2 Zhancai Zheng,2 Yong Cui2
1Graduate School, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Dermatology, China-Japan Friendship Hospital, Beijing, People’s Republic of China
Correspondence: Ying Wang; Zhancai Zheng, Department of Dermatology, China-Japan Friendship Hospital, 2 Yinghua East Street, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +8618911977681 ; +8613501186450, Email [email protected]; [email protected]
Abstract: Granuloma annulare (GA) is an uncommon disease in dermatology. Here, we report a case of generalized GA combined with Sjogren’s syndrome (SS) in a 65-year-old woman. To our knowledge, generalized GA combined with SS has not been reported before.
Keywords: combination, case report, granuloma annulare, generalized skin lesions, Sjogren’s syndrome
Introduction
Granuloma annulare (GA) is a chronic skin disease characterized by annular erythema, which mainly occurs histologically in the dermis and subcutaneous tissue.1 The etiology and pathogenesis of GA are not well understood. Epidemiology shows that GA can occur at any age, but the incidence and prevalence of it are highest in the fifth decade of life.2 GA may have psychiatric comorbidities3 and has shown to be significantly associated with autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).4 However, generalized GA combined with Sjogren’s syndrome (SS) has not been reported before. Herein, we report a case of such a combination and successfully treated with a low dosage of oral steroid.
Case Report
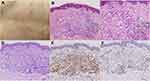
A 65-year-old woman presented with a 4-year history of asymptomatic irregular annular plaques on her trunk and lower limbs, and the number of lesions increased significantly in the recent 6 months. She also complained of dry eyes and mouth since the onset of the skin lesions but did not experience dysphagia, vaginal dryness, dyspareunia, Raynaud’s phenomenon, xerosis, vasculitis, peripheral neuropathy, or psychiatric diseases. Over the years, the patient has intermittently used Chinese herbal ointment and halometasone cream, but the effect is poor. Physical examination revealed domed-shaped, mild indurated erythematous papules with smooth surfaces coalesced into irregular annular plaques measuring about 1~5 cm in diameter. The circinate papules were remarkably seen on the periphery and tended to resolve in the center of plaques which were distributed on the trunk and extremities (Figure 1A).
Skin biopsy revealed granulomatous infiltration including mainly histiocytes, focally accumulated or dispersed between collagen bundles, with a few multinucleated giant cells. Some perivascular lymphocytes and accidental eosinophils were seen (Figure 1B and C). Special stains revealed AB-PAS (+focally and weakly) (Figure 1D), acid fast (-), and silver stain (-). Immunohistochemistry revealed KP-1(+) (Figure 1E), CD3(+) (Figure 1F), and CD20(-). These histopathological findings were consistent with GA.
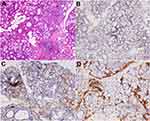
Abnormal laboratory tests include white blood cells (2.07*10^9/L; normal: 3.5~9.5*10^9/L), antinuclear antibodies (1:80; normal <1:40), anti-SSA antibodies (+), anti-Ro52 antibodies (+), anti-dsDNA antibodies (59 IU/mL; normal <20 IU/mL), IgG (1750 mg/dl; normal: 694~1620 mg/dl), IgA (381.00 mg/dl; normal: 68.00~378.00 mg/dl), IgM (299.0 mg/dl; normal: 60.0~263.0 mg/dl), C4 (14.10 mg/dl; normal: 16.00~47.00 mg/dl), RF (53.8 IU/mL; normal <20 IU/mL), other laboratory parameters, including tumor markers and lupus anticoagulants were within normal limits. Ultrasonography of superficial lymph nodes revealed enlargement of the bilateral neck and supraclavicular lymph nodes. Schirmer test (right, 0 mm; left, 0 mm) was positive. Labial salivary gland histopathology revealed focal periductal localized lymphocytic infiltrates in exocrine glandular tissue along with otherwise intact acinar. Interstitial infiltration and focal aggregation of plasma cells were seen (3 focus, ≥50 cells/focus). Other findings include a decrease in the number and some atrophy of glandular acini, dilation of the part glandular duct, and mild increase of fat tissues. Immunohistochemistry revealed CD20 (+ focally and sparsely), CD3 (+ focally and sparsely), CD38 (+ focally and sparsely), and IgG4 (-) (Figure 2). These findings are consistent with the diagnosis of SS.
Thus, the final diagnosis was generalized GA combined with SS. Prednisone acetate 15 mg per day orally and halometasone cream twice a day externally were given for the treatment. Two weeks later, the lesion faded. At the follow-up visit two months later, the lesions had completely disappeared, and the symptoms of SS, such as the dry mouth and dry eyes, significantly improved too.
Discussion
GA is significantly associated with autoimmune diseases, such as RA and SLE.4 However, up to now, only three studies have been reported about GA combined with SS.5–7 As in our case, all the reported patients previously were elderly females. Not like our case, all the skin lesions of these patients were localized on the forearm, neck, hands, thighs, fingers, and elbows, respectively. Generalized GA combined with SS has not been reported before. There is accumulating evidence that both GA and SS are T-cell–mediated diseases,8,9 indicating that similar underlying immune pathogenesis may contribute to the combination of GA and SS.
Clinically, the generalized papular lesions and annular plaques of GA combined with SS should be differentiated from a number of skin diseases such as classical GA, sarcoidosis, secondary syphilis, etc. Skin biopsy can make the diagnosis. Histologically, it should be differentiated from interstitial granulomatous dermatitis (IGD) and necrobiosis lipoidica (NL). IGD which has various clinical presentations such as purplish red plaques usually has a negative mucin staining.10 Skin lesions of NL usually begin as red-brown papules or nodules, most frequently in the lower legs, then progress to yellow atrophic and telangiectatic plaques. It is histologically characterized by collagen degeneration, granuloma formation, and endothelial swelling.
In past reported cases, topical corticosteroids with or without oral hydroxychloroquine or methotrexate were used for the treatment. The response differed, from partial improvement to complete cure. We use prednisone acetate 15 mg per day orally and got a good response for the lesions as well as for the symptoms of SS. Thus, the oral steroids may be a good treatment choice for this combination, and the dosage should be based on the need of SS. Furthermore, dermatologists should be aware of the association of GA with autoimmune illnesses, which may include SS, RA, SLE, etc.
Conclusion
We reported the generalized lesions of GA combined with SS and found that oral steroids for this combination had a significant improvement. Though it is a rare condition, the possibility of SS should also be considered while making a diagnosis of GA.
Ethics Approval and Informed Consent
The written informed consent was signed by the patient to have the case details and any accompanying images published. Publication of the case details was approved by China-Japan Friendship Hospital.
Acknowledgments
We thank the patient and physicians for participating in our study.
Funding
This study was supported by Elite Medical Professionals project of China-Japan Friendship Hospital (NO.ZRJY2021-QM04).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xue J, Lam JM. Granuloma annulare. Paediatr Child Health. 2019;24:366–367. doi:10.1093/pch/pxz003
2. Barbieri JS, Rodriguez O, Rosenbach M, et al. Incidence and prevalence of granuloma annulare in the United States. JAMA Dermatol. 2021;157(7):1–8. doi:10.1001/jamadermatol.2021.1847
3. Joshi TP, Chen V, Dong JL, et al. Psychiatric comorbidities associated with granuloma annulare: a case control study in the all of us database. J Am Acad Dermatol. 2022;87(3):e119–e120. doi:10.1016/j.jaad.2022.05.056
4. Barbieri JS, Rosenbach M, Rodriguez O, et al. Association of granuloma annulare with type 2 diabetes, hyperlipidemia, autoimmune disorders, and hematologic malignant neoplasms. JAMA Dermatol. 2021;157(7):817–823. doi:10.1001/jamadermatol.2021.1805
5. Sumikawa Y, Ansai S, Kimura T, et al. Interstitial type granuloma annulare associated with Sjogren’s syndrome. J Dermatol. 2010;37:493–495. doi:10.1111/j.1346-8138.2010.00865.x
6. Sakiyama T, Hirai I, Konohana A, et al. Interstitial-type granuloma annulare associated with Sjögren syndrome. J Dtsch Dermatol Ges. 2014;12(5):415–416.
7. Corrà A, Quintarelli L, Verdelli A, et al. Granulomatous dermatitis and systemic disease: an association to consider. Biomed Res Int. 2020;2020:3281380. doi:10.1155/2020/3281380
8. Pontarini E, Verstappen GM, Grigoriadou S, et al. Blocking T cell co-stimulation in primary Sjögren’s syndrome: rationale, clinical efficacy and modulation of peripheral and salivary gland biomarkers. Clin Exp Rheumatol. 2020;126:222–227.
9. Min MS, Wu JN, He H, et al. Granuloma annulare skin profile shows activation of T-helper cell type 1, T-helper cell type 2, and Janus kinase pathways. J Am Acad Dermatol. 2020;83(1):63–70. doi:10.1016/j.jaad.2019.12.028
10. Wang Y, Wu YT, Zheng ZC, et al. Interstitial granulomatous dermatitis associated with primary biliary cirrhosis. J Dermatol. 2018;45(1):112–113. doi:10.1111/1346-8138.13778
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.