Back to Journals » Clinical Epidemiology » Volume 12
General Practitioner Attendance in Proximity to HPV Vaccination: A Nationwide, Register-Based, Matched Case–Control Study
Authors Lützen TH , Rask CU, Plana-Ripoll O , Bech BH , Krogsgaard LW, Rolving N , Rytter D
Received 8 May 2020
Accepted for publication 15 July 2020
Published 8 September 2020 Volume 2020:12 Pages 929—939
DOI https://doi.org/10.2147/CLEP.S253429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Irene Petersen
Tina Hovgaard Lützen,1 Charlotte Ulrikka Rask,2,3 Oleguer Plana-Ripoll,4 Bodil Hammer Bech,1 Lene Wulff Krogsgaard,1 Nanna Rolving,5,6 Dorte Rytter1
1Research Unit for Epidemiology, Department of Public Health, Aarhus University, Aarhus C 8000, Denmark; 2Research Unit, Department of Child and Adolescent Psychiatry, Aarhus University Hospital, Aarhus N 8200, Denmark; 3Department of Clinical Medicine, Aarhus University, Aarhus N 8200, Denmark; 4National Centre for Register-Based Research, Department of Economics and Business Economics, Aarhus University, Aarhus V 8210, Denmark; 5University Clinic of Innovative Patient Pathways, Diagnostic Centre, Silkeborg Regional Hospital, Silkeborg 8600, Denmark; 6DEFACTUM, Corporate Quality, Central Denmark Region, Aarhus N 8200, Denmark
Correspondence: Tina Hovgaard Lützen Research Unit for Epidemiology, Department of Public Health
Aarhus University, Bartholins Allé 2, Aarhus C 8000, Denmark
Tel +45 22410722
Email [email protected]
Aim: This study aimed to describe and compare general practitioner (GP) attendance patterns in the years surrounding HPV-vaccination among cases suspected to experience adverse events and their matched controls in order to determine if a potential larger change in GP attendance among cases was observed in temporal relation to vaccination.
Methods: Register-based, matched case–control study. Cases were defined as women referred to specialized hospital settings (HPV-centers) for suspected adverse event between June 1st 2015 and December 31st 2015 (n=1458). Information on referral was obtained from the HPV-centers directly. Each case was matched with five controls on age at vaccination, region, time of first vaccine registration and total number of doses, resulting in a total study population of 8670 girls and women. Negative binomial regression analyses were used (i) to estimate mean yearly GP contacts among cases and controls, and (ii) to further investigate the relative difference in change in GP attendance following vaccination between cases and controls.
Results: Overall, cases displayed higher GP attendance than controls from five years before vaccination up until five years after. Compared to controls, cases increased their GP attendance more in the years following HPV vaccination, corresponding to a 40% increase in the incidence rate ratios (IRR) from before to after vaccination (ratio-IRR = 1.40 [1.36; 1.44]). The change occurred in close proximity to vaccination, and the pattern was the same independently of the year of vaccination. However, for the later vaccination years cases displayed an additional increase in their GP attendance around time of extensive media attention.
Conclusion: Girls and women being referred for suspected adverse events after HPV-vaccination changed their GP attendance pattern in close proximity to their first HPV-vaccination and not solely in temporal linkage to the onset of public debate.
Keywords: HPV vaccination, general practice attendance, adverse effects, case–control
Plain Language Summary
In 2015, the Danish human papilloma virus (HPV) vaccination coverage experienced a significant drop due to reports on suspected adverse events, with about 2000 affected women referred to HPV-centres. Even though the coverage is now rising, many women remain ill and the cause(s) unclear. Several studies have not found associations between HPV-vaccines and serious adverse events, and with only about 0.3% of all vaccinated women affected, other factors besides vaccination may play a role when experiencing illness. Previous studies have found that referred women more often had psychiatric conditions, hospital diagnoses and higher GP attendance before vaccination, suggesting a higher level of pre-existing morbidity compared to other HPV-vaccinated women. This could indicate that the extensive media coverage on suspected adverse events may have caused them to link the onset of pre-existing symptoms to time of vaccination. Through data retrieved from various national registries, this study identified general practitioner (GP) attendance in the years surrounding vaccination, and considered changes in attendance as a sign of illness. Through this, we aimed to determine whether possible changes correlated to time of vaccination or the onset of public debate. The study found that, irrespectively of vaccination year, referred women change their GP attendance pattern in a temporal link to the first vaccination. These results may have several explanations including coincidence or triggering of bodily distress and functional disorders. Regardless they underline the need of special attention to a possible vulnerable group where other factors potentially in combination with vaccination may increase symptoms and/or illness.
Introduction
Human papilloma virus (HPV) has been identified as a necessary cause for the development of cervical cancer, the fourth most common cancer among women worldwide.1 Vaccination against HPV-infection was included in the Danish Child Vaccination Program in 2009, and has since been offered free of charge to all 12-year-old girls. In addition, two catch-up programs with free vaccination have been available, one in 2008–2010 for women born from 1993 to 1995 and the second in 2012–2013 for women born after 1984.2
The Danish program is non-mandatory, and has first offered the quadrivalent vaccine (Gardasil, Merck & Co., Whitehouse Station, NJ, USA), secondly the bivalent (Cervarix, GSK Biologicals, Rixensart, Belgium), and since 2017 the nonavalent vaccine has been available (Gardasil 9). The efficacy and safety of the vaccines have been examined extensively and found to be highly protective from HPV-infection with no increased risk of severe adverse events including several autoimmune and neurological diseases.3–8 However, in 2013 the otherwise high vaccination coverage started decreasing drastically in Denmark, from above 90% to a mere 47%.9 This decrease occurred at the same time as extensive media attention on girls and women experiencing various unspecific symptoms and illness following vaccination.9,10 The symptoms were heterogeneous and included dizziness, fatigue and nausea among others.11 Consequently, five specialized hospital settings (hereafter HPV-centres) opened in June 2015, one in each region of Denmark. Their purpose was to examine girls and women referred from their general practitioner (GP) on the criteria of having unspecified or unexplained physical symptoms occurring in proximity to the HPV vaccination, and by December 2017, approximately 2000 had been referred. In recent years, the number of referrals has declined extensively leading to a closure of the HPV-centres in 2019, and today regular hospital departments are executing the tasks of the HPV-centres.
Previous research has focused on predictors for being referred to an HPV-centre and found that referred girls and women had significantly more GP contacts and were more likely to have had hospital contacts due to psychiatric disorders and to have redeemed psychiatric medication prior to vaccination compared with other HPV-vaccinated women.12 Higher GP attendance prior to vaccination was also found in another study examining girls and women with reports of possible side effects registered in the Danish Medicine Agency.13 These studies indicate that affected girls and women had increased morbidity or had a different healthcare-seeking behavior, already prior to the time of vaccination.
Now, the vaccination coverage has stabilized around 80% for the first dose, but the birth cohorts from 2002 to 2005 still seem less likely to complete the full vaccine regimen.14 Regardless of the coverage stabilizing, the question remains what causes the increased experience of unspecific symptoms and illness in this group of referred patients. Extensive research on the safety of the vaccines concludes no link to their reported symptoms, and with a relatively low proportion of affected girls and women, it is possible that other factors such as morbidity prior to vaccination may affect their susceptibility.12,13,15 The increased morbidity and GP attendance in referred girls already prior to vaccination, may indicate that the extensive media coverage may have caused them to link the onset of pre-existing symptoms to the time of vaccination. It is therefore important to examine whether there is a temporal link between vaccination and symptom experience, considering changes in GP attendance frequency in close proximity to vaccination, as a proxy for illness. Therefore, this study aims to describe and compare GP attendance patterns in referred females and HPV-vaccinated controls before and after vaccination, and to examine whether a potential larger change in GP attendance frequency among referred females occurs in a temporal link to vaccination.
Materials and Methods
Study Design and Source Population
The study was conducted as a register-based, matched case-control study. All Danish citizens are given a personal identification number at birth (Civil Personal Registration [CPR]). This CPR number was used to identify the source population and to link personal information across national registries. The source population consisted of women born from 1975 to 2008, who had received the first HPV-vaccination dose between 2008 and 2015 (N=544,712). Criteria for being identified as HPV-vaccinated included having ≥1 service code registration for vaccine injection in the Danish National Health Insurance Service Register (service codes 8328–8330 or 8334–8336) or ≥1 registration of redeeming a prescription for the vaccines in the Danish Register of Medicinal Product Statistics (Anatomic Therapeutic Chemical [ATC] code J07BM*).
Study Population
We defined cases as HPV-vaccinated women, who had been referred by their GP to HPV-centres between June 1st 2015 and December 31st 2015. We obtained CPR numbers on 1592 cases from the HPV-centres and merged these with personal information from the Danish Register of Medicinal Product Statistics, the Danish National Patient Register, the Danish National Health Insurance Service Register and Statistics Denmark. A total of 134 cases (8.4%) were excluded due to missing HPV-vaccine registration (n=95), being vaccinated prior to the national vaccine introduction (n=29), and when multiple prescription and GP registrations made it difficult to determine time of first HPV-vaccine and number of doses received (n=10), resulting in 1458 included cases.
For each case, five HPV-vaccinated controls were randomly selected from the source population, matched on age at first vaccine registration (whole years), time of first vaccine registration (-/+2 months), region of residence and total number of doses received. The final study population consisted of 8670 women, equivalent to 1458 cases and 7212 controls, of which 26 cases had less than five controls, and 72 controls were matched more than once.
Exposure
Information on GP attendance was obtained from the Danish National Health Insurance Service Register from 2004 to 2016. GP attendance was defined as number of face-to-face, telephone/email and out-of-office contacts (service codes 0101, 0105, 0201, 0501, 0471).
Covariates
A study, describing socioeconomic characteristics of this case population, found that cases were different from controls regarding maternal socioeconomic status.16 From Statistics Denmark, we therefore obtained information on maternal educational level, maternal employment status as well as maternal age at time of vaccination and included these in the study as potential confounders, in addition to matching variables. Maternal educational level was divided into five categories according to DISCED-15; primary school, upper secondary school, short cycle/vocational training, bachelor/vocational bachelor and master/PhD program.17 Maternal employment status was grouped as employee/lowest income, employee/middle income, employee/highest income, self-employed, outside workforce/student, retirement/pension and employee/unspecified. Information on these covariates was obtained for one year prior to the first vaccine registration. Maternal age at time of vaccination was divided into quartiles according to data.
As previous studies have shown cases to have a higher GP attendance prior to vaccination, we also included chronic somatic conditions diagnosed up to five years prior to the first vaccine registration as a covariate in order to adjust for GP contacts related to chronic illness. Information on chronic somatic conditions was obtained from the Danish National Patient Registry via the following ICD-10 codes: DG40 (epilepsy), DM08 (juvenile arthritis), DN0-DN39 (renal disease), DE10-DE14 (diabetes mellitus), DK50-51 (Crohn disease and ulcerative colitis), DK900 (coeliac disease), DH54 (visual impairment), DH90-91 (hearing loss), DG80 (cerebral palsy), DQ05 (spina bifida), DG71 (muscular disease), DI* (heart diseases). In addition, we included asthmatic conditions separately as asthmatic children often are followed in general practice. Redemption of prescription medication for asthmatic conditions were obtained from the Danish Register of Medicinal Product Statistics using ATC codes R03A, R03B, R03C and R03D, and asthmatic conditions were defined as redemption of ≥2 prescriptions for β2-agonists or steroids (R03A, R03B and R03C), or ≥1 prescription for leukotriene receptor antagonists (R03DC), or having an asthma diagnosis (ICD-10 code DJ45) in the National Patient Registry.18
Previous results have also shown that cases more often had psychiatric comorbidity prior to vaccination compared to controls, and psychiatric morbidity diagnosed up to five years prior to the first vaccine registration was therefore also included as a covariate in order to adjust for GP contacts related to this.12 Psychiatric comorbidity was defined according to redeemed prescriptions or hospital contacts due to psychiatric morbidity in the five years prior to the first vaccine registration. Redeemed prescriptions were identified through ATC codes in the Danish Register of Medicinal Product Statistics, and the following codes were included: N06A* (antidepressants), N05A* (antipsychotics), N06BA* (attention deficit hyperactivity disorder [ADHD]), N05BA* (anxiolytics), N05BB* (anxiolytics), N03AX16 (anxiolytics) and N05BE01 (anxiolytics). The ATC codes N06AA (tricyclic antidepressants) and N06AX12 (bupropion) were excluded due to their frequent use for insomnia, smoking cessation and as pain medication.19,20 In addition, psychiatric diagnoses were identified in the National Patient Registry with the following ICD-10 codes: DF20-29 (psychotic disorders), DF30-39 (affective disorders), DF40-49 (nervous and stress-related disorders), DF50 (eating disorder) and DF90 (ADHD).
Statistical Analyses
Characteristics for cases and controls on matching variables, covariates and exposures were summarized using numbers and percentages. In order to visualize and examine possible changes in GP attendance from five years prior to and up to five years after vaccination, negative binomial regression analysis was used to estimate mean yearly GP contacts. In addition, an interaction term between case/control status and a time variable indicating before or after vaccination was added in order to estimate the relative difference in a possible change in GP attendance following vaccination between cases and controls. The models were adjusted for prior chronic somatic condition (yes/no), prior asthmatic condition (yes/no), prior psychiatric condition (yes/no) and maternal covariates as categorized in Table 1.
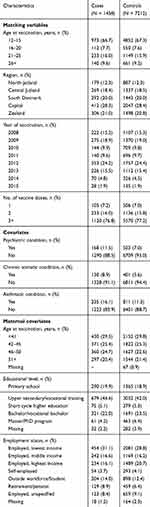 |
Table 1 Characteristics of Matching Variables and Covariates for Cases and Controls |
In addition to the main analyses, sub-analyses restricted GP attendance to 12 months prior to and after vaccination (divided into intervals of three months) in order to more closely examine the vaccination proximity of a possible change in GP attendance. Furthermore, sub-analyses were also conducted stratified on year of vaccination (2008–2009, 2010–2012 and 2013–2015) and age at vaccination (-/+15 years). In order to examine whether possible changes in GP attendance after vaccination differs across years of receiving the vaccination, the age-group analyses were further stratified into the three vaccination year groups. Finally, we performed a sensitivity analysis for residents in the Capital region, as their registration of out-of-office primary care was changed in 2014, and since then these contacts from the Capital region have not been included in the Danish National Health Insurance Service Register. The results are presented as incidence rate ratios (IRR) for before and after vaccination and as a ratio of these (R-IRR) with 95% confidence intervals (95% CI), which can be interpreted as the change in IRR from before to after vaccination. Data were analyzed using STATA 13.1® statistical software (StataCorp LP, TX, USA).
Results
Characteristics for cases and controls are presented in Table 1. Overall, most cases were from the Capital Region, most were vaccinated in 2009 and 2012 and most at the ages of 12–15 and 21–25 years. About 77% had received the full vaccine regime of three doses, whereas 16% and 7% had received only two and one dose, respectively. In line with previous findings, cases were more likely to have a chronic somatic condition (8.9% vs 5.6%), an asthmatic condition (16.1% vs 11.3%) and pre-existing psychiatric conditions (11.5% vs 7%) prior to vaccination compared to controls. For the maternal covariates, cases and controls were similar regarding maternal age at vaccination, however, cases were more likely to have a mother with shorter education and lower-income compared to controls (Table 1).
For the entire study population, cases display a stable, but 33% higher GP attendance pattern in the years prior to vaccination compared to controls (IRR= 1.33 [1.28; 1.38]) (Figure 1 and Table 2). Beginning in the first year after vaccination, cases present an increase in mean yearly GP contacts that continues in the following years, whereas controls only present a minor rise in the year following vaccination and then return to their natural slope. In the last year observed, cases show an additional increase in GP attendance compared to controls (Figure 1). On average, cases increased their GP attendance to having 86% more GP contacts after vaccination compared to controls (IRR= 1.86 [1.79; 1.93]). This corresponds to a ratio of IRR from before to after vaccination of 40% between cases and controls (R-IRR= 1.40 [1.36; 1.44]) (Table 2).
Stratifying on vaccination year yielded similar results. Hence, for all strata, cases displayed a higher GP attendance prior to vaccination, and cases changed their pattern in the years after vaccination (Figure 2A–C and Table 2). In all strata, this change began in the first year after vaccination. Particularly cases vaccinated in 2013–2015 displayed a substantial rise, whereas cases vaccinated in 2010–2012 displayed a less clear tendency. The additional increase in GP attendance among cases in the last year observed after vaccination, as seen in the main result, is now only present in 2010–2012 (Figure 2A–C).
For the age-stratified sub-analyses, both age groups show a similar pattern to the main analyses in relation to the years prior to vaccination. Following vaccination, both cases and controls in the youngest age group change their GP attendance pattern, however, cases present a much steeper rise than controls beginning in the first year after vaccination. The ratio of IRR from before to after vaccination however remained similar to the main results (R-IRR= 1.44 [1.39; 1.49]) (Figure 3A and B; Table 2). For the oldest age group, controls remain stable after vaccination with only a minor rise in the first year, whereas cases after vaccination display a steep rise in the first year and then again in the latter year. Again, the ratio of IRR from before to after vaccination remained similar to the main results (R-IRR= 1.32 [1.27; 1.38]) (Figure 3A and B; Table 2). In the age group analyses stratified by vaccination year, the younger age group independent of vaccination year, and the older age group vaccinated in 2013–2015, showed similar patterns as in Figure 2A–C (Suppl. Figure 1ABC and 2C). However, the older age group showed different GP attendance patterns in the remaining vaccination year groups (Suppl. Figure 2AB). For those vaccinated in 2008–2009, both cases and controls have a steep rise in the first year after vaccination, however controls return to their slope and cases continue to rise thereafter (Suppl. Figure 2A). For 2010–2012, controls are stable throughout the study period, and although cases increase their GP attendance after vaccination, the change in pattern begins already prior to vaccination (Suppl. Figure 2B).
Further sub-analyses restricted to the 12 months prior to and after vaccination, show that the increase in GP contacts in cases started 0 to 3 months after vaccination, whereas GP contacts in controls are close to stable throughout the period (Figure 4). Supplementary data stratifying for year of vaccination also display the rise to occur within the first 3 months in all three vaccination year groups (Suppl. Figure 3ABC). Finally, the sensitivity analysis performed on residents in the Capital region regarding out-of-office contacts from 2014 and onwards, found no difference in the GP attendance pattern compared to the main results (data not shown).
Discussion
Principal Findings
Compared with other vaccinated women, cases generally displayed a significant increase in GP contacts in close proximity to HPV-vaccination, a pattern that overall does not differ when stratified by year of vaccination or age group. The increase in GP contacts begins in the first year after vaccination, and within this year, sub-analyses suggests the increase to occur in the first three months.
Comparison with Other Research
To our knowledge, this study is the first to examine GP attendance after vaccination for this specific population of girls and women referred to HPV-centres. In a previous study from our research group, we compared GP attendance among vaccinated and non-vaccinated girls included in the Danish National Childhood Vaccination Program.21 Girls vaccinated with the HPV-vaccine displayed an increase in GP attendance following vaccination. However, the same increase was observed among girls only receiving the MMR-vaccine, indicating that this reflected differences in health-care seeking patterns between vaccinated and non-vaccinated girls, rather than specific adverse events following HPV-vaccination. A recent population-based, cohort study, examining hospital diagnoses on non-specific symptoms such as fatigue and pain in the year following HPV-vaccination, found no increased incidence among HPV-vaccinated girls compared to matched, unvaccinated girls, indicating no causal link.22
Possible Explanations for the Findings
This study finds a substantial increase in GP contacts for cases in the first year after vaccination across all vaccination years. This increase suggest that GPs have referred according to the established clinical guidelines, specifically the main criteria of reporting unspecific symptoms in a temporal link to vaccination, and thereby contributes to a valid case definition in this study. The most distinct change in GP attendance occurs in cases vaccinated in 2013–2015. However, for these years of vaccination it is not possible to separate effects of the vaccination from the influence of media attention on GP attendance.
An increase in the first year after vaccination is also evident in the age group analyses, although the tendency only remains clear in the younger age group, when stratifying for vaccination year. The older age group showed diverse patterns across vaccination years. This could potentially be due to differences in the age range between vaccination year groups caused by the different recipients of the catch-up programs. The results could therefore display heterogeneous use of GP, rather than providing a clear description of patterns prior to and after vaccination in a homogenous group. The considerable increase in the year after vaccination among both cases and controls in 2008–2009, may be explained by GPs registering a face-to-face contact in addition to the vaccination registration, a practice perhaps occurring to a higher degree in the introduction period of the vaccine.
Across analyses, controls also show a minor rise in the year after vaccination and then return to their natural slope. This slight increase may be due to demasking, where a GP contact for HPV-vaccination results in another GP contact shortly after regarding issues arisen during the first consultation. This will have been the same for both cases and controls. The additional increase in the last year observed after vaccination, as found in the main analyses, was not present in 2008–2009 but occurs again in 2010–2012, indicating an association with the opening of the HPV-centres in 2015, as cases would have had to visit their GP in order to be referred.
The associations established in this study may have several possible explanations. Experiencing symptoms after vaccination may relate to prior illness or they may have been present to some extent also before vaccination, suggesting that GPs may have considered the HPV-centres as a new option for referring these long-term ill patients for further examination and possible diagnosis. However, our results does not support this, as the observed increase in GP attendance would then only have been present in the latter years, where media has brought attention to the HPV-vaccine as a possible explanation for experiencing illness.
Another explanation could be that the symptoms are of temporal coincidence and would have occurred irrespectively of the vaccination. The experienced symptoms are of known occurrence among girls and younger women and when introducing a new vaccine across age groups (eg, catch-up programs), it may be expected that these symptoms present themselves in proximity to the time of vaccination for some girls simply by coincidence.23
The symptoms may also be a consequence of a “biosocial contagion”, a theory that describes how a phenomenon can spread within a population, where the uprising in mainly negative media attention created a foundation for this.24 The vaccination coverage has recovered in recent years, yet referrals to HPV-centres have declined immensely, which may support the conclusion that the discrepancy between clinical experience and safety studies was driven by extensive media attention and “biosocial contagion”. However, this study evidently shows a significant change occurring after vaccination independently of vaccination year and thereby contradicting the narrative that increased GP attendance was solely due to the onset of public debate.
Lastly, the potential adverse events have been suggested to be similar to other conditions characterized as bothersome bodily distress with medically unexplained symptoms, also known as functional disorders.13,25 These disorders are suggested to develop through complex, multifactorial pathways conceivably triggered by various factors such as trauma, infection and/or personal stress.26,27 As of now, there is no evidence to support vaccination as a trigger; it cannot however be ruled out that the presence of the abovementioned factors in combination with vaccination may have reinforced preexisting, unspecific symptoms or induced bodily distress.
Strengths and Limitation
As this is conducted as a register-based study, participation was almost complete. CPR-numbers for a total of 1592 cases were obtained from the HPV-centres. However, 95 (6%) cases were excluded due to missing HPV-vaccine registration. Missing HPV-vaccine registration is possibly due to register error or having received the vaccination elsewhere (eg, fitness centers offered the vaccine for a short period). A total of ten (0.6%) cases were excluded as their combination of self-purchased vaccines and vaccination by their GP made it difficult to determine the timing of the first vaccination and/or the number of doses. Both missing and flawed registration is considered independent of later referral and is therefore not expected to have caused selection bias.
Our study may however be subject to Neyman’s bias with the mix of prevalent cases, who may have been ill for several years, and incident cases, who may have had symptoms for a shorter period. Especially for those vaccinated during the first years of HPV-vaccination, some of the less severe cases may have recovered since their vaccination, leaving only the more severe cases to be enrolled in the study. We tried to accommodate this potential bias by stratifying the analyses by year of vaccination, as Neyman’s bias would be expected to be of less importance in the latter vaccination years. Stratification led to similar results in all year strata, indicating that Neyman’s bias was of limited importance.
Information on GP attendance obtained from the National Health Insurance Service Register is considered accurate and of high validity as GP’s rely on correct registration at each visit in order to receive remuneration.28 For psychiatric and somatic conditions, information on an individual level was retrieved from the Register of Medicinal Product Statistics, the Danish Psychiatric Central Research Register and the Danish National Patient Registry, all data sources with high validity and positive predictive values with minimal risk of information bias.29–31 The study may however be subject to residual confounding, as registries do not contain information on less severe forms of the included conditions, which are neither diagnosed nor receiving medication, but may attend the GP more frequently. All covariates were recorded prior to vaccination and entered in national registries by independent bodies, resulting in a low risk of recall bias.
The analyses have been adjusted for potential confounders, which did not change the results substantially. Unknown variables may however still have confounded the associations.
Implications and Future Research
Overall, this study was conducted in a Danish context. However, the results may be replicable in other developed countries, where HPV-vaccination has been introduced using catch-up programs alongside childhood vaccination programs. The results may be considered useful in clinical practice, suggesting GPs to be attentive to reactions in girls and women with vulnerabilities, and potentially prevent these by providing them with clear information about the vaccine and current evidence regarding adverse events. Although this study design does not allow a conclusion on direct causality between the vaccine and potential adverse events, this study suggests that more research is needed on vaccines as potential triggers for bodily distress in individuals with preexisting vulnerabilities or predispositions.
Conclusion
Girls and women being referred to an HPV-center increase their GP attendance pattern in close proximity to their first HPV-vaccination and not solely in temporal linkage to the onset of public debate.
Ethics
The Danish Data Protection Agency (journal number 2015–57-0002) and the Danish Patient Safety Authority approved the study. According to Danish legislation, ethical approval of registry studies is not required. According to Danish legislation, data availability is restricted to researchers listed in the approvals.
Acknowledgments
Collected data: Dr. Med. Erik Østergaard, Department of Woman-Child and Urology, Aalborg University Hospital, Dr. Med. Michael Nielsen, Department of Neurology, Aalborg University Hospital, Dr. Med. Svend Stenvang Pedersen, Department of Infectious Diseases, Odense University Hospital, Dr. Med. Niels Fisker, H.C. Andersen Children’s Hospital, Odense University Hospital, Dr. Med. Martin Faber Boxill, Department of Pediatrics, Regional Hospital of Viborg, Dr. Med. Dan Pradsgaard, Department of Pediatrics, Regional Hospital of Viborg, Dr. Med. Vibeke Neergaard Sørensen, Diagnostics Centre, Regional Hospital of Silkeborg, Dr. Med. Reinar Bue Falck Juvik, Diagnostics Centre, Sjælland University Hospital Roskilde, Dr. Med. Lise Heilmann Jensen, Department of Pediatrics, Sjælland University Hospital Roskilde and Dr. Med. Jesper Mehlsen, Frederiksberg Syncope Centre, Frederiksberg Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Human papillomavirus (HPV) and cervical cancer; 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer.
2. Statens Serum Institut. Vaccination mod livmoderhalskræft. [Vaccination against cervical cancer]; 2020. Available from: https://www.ssi.dk/vaccinationer/boernevaccination/vaccination-mod-livmoderhalskraeft.
3. Feiring B, Laake I, Bakken IJ, et al. HPV vaccination and risk of chronic fatigue syndrome/myalgic encephalomyelitis: a nationwide register-based study from Norway. Vaccine. 2017;35(33):4203–4212. doi:10.1016/j.vaccine.2017.06.031
4. Frisch M, Besson A, Clemmensen KKB, Valentiner-Branth P, Molbak K, Hviid A. Quadrivalent human papillomavirus vaccination in boys and risk of autoimmune diseases, neurological diseases and venous thromboembolism. Int J Epidemiol. 2018;47(2):634–641. doi:10.1093/ije/dyx273
5. Hviid A, Svanstrom H, Scheller NM, Gronlund O, Pasternak B, Arnheim-Dahlstrom L. Human papillomavirus vaccination of adult women and risk of autoimmune and neurological diseases. J Intern Med. 2018;283(2):154–165. doi:10.1111/joim.12694
6. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases-a systematic review and meta-analysis. Pharmacol Res. 2018;132:108–118. doi:10.1016/j.phrs.2018.04.007
7. Arnheim-Dahlstrom L, Pasternak B, Svanstrom H, Sparen P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347:f5906.
8. Scheller NM, Svanstrom H, Pasternak B, et al. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA. 2015;313(1):54–61. doi:10.1001/jama.2014.16946
9. The Danish Board of Health. Børnevaccinationsprogrammet. Årsrapport 2016. [The Danish Child Vaccination Program. Annual Report 2016]. 2018:35–36.
10. Suppli CH, Hansen ND, Rasmussen M, Valentiner-Branth P, Krause TG, Molbak K. Decline in HPV-vaccination uptake in Denmark - the association between HPV-related media coverage and HPV-vaccination. BMC Public Health. 2018;18(1):1360. doi:10.1186/s12889-018-6268-x
11. Brinth L, Theibel AC, Pors K, Mehlsen J. Suspected side effects to the quadrivalent human papilloma vaccine. Dan Med J. 2015;62(4):A5064.
12. Lützen TH, Bech BH, Mehlsen J, et al. Psychiatric conditions and general practitioner attendance prior to HPV vaccination and the risk of referral to a specialized hospital setting because of suspected adverse events following HPV vaccination: a register-based, matched case-control study. Clin Epidemiol. 2017;9:465–473. doi:10.2147/CLEP.S135318
13. Molbak K, Hansen N, Valentiner-Branth P. Pre-vaccination care-seeking in females reporting severe adverse reactions to HPV vaccine. A registry based case-control study. PLoS One. 2016;11(9):e0162520. doi:10.1371/journal.pone.0162520
14. Statens Serum Institut. Overvågning i tal, grafer og kort. HPV færdigvaccineret, vaccinationstilslutning. [surveillance in numbers, graphs and geographical maps - HPV completed vaccination, vaccine uptake]; 2020. Available from: https://statistik.ssi.dk//sygdomsdata#!/?vaccination=6&show=Graph&datatype=Vaccination.
15. Krogsgaard LW, Bech BH, Plana-Ripoll O, Thomsen RW, Rytter D. Hospital contacts and diagnoses five years prior to HPV vaccination among females referred for suspected adverse vaccine effects: a Danish nationwide case-control study. Vaccine. 2019;37(13):1763–1768. doi:10.1016/j.vaccine.2019.02.029
16. Weye N, Fonager K, Lützen T, Rytter D. Socioeconomic predictors of referral to a diagnostic centre on suspected adverse events following HPV vaccination. Eur J Public Health. 2018;28(6):1109–1113. doi:10.1093/eurpub/cky088
17. Statistics Denmark. Classification of educational levels; 2019. Available from: http://www.dst.dk/extranet/uddannelsesklassifikation/Niveau_Level.html.
18. Moth G, Vedsted P, Schiotz P. Identification of asthmatic children using prescription data and diagnosis. Eur J Clin Pharmacol. 2007;63(6):605–611. doi:10.1007/s00228-007-0286-4
19. Hartmann-Boyce J, Aveyard P. Drugs for smoking cessation. BMJ. 2016;352:i571. doi:10.1136/bmj.i1717
20. Katon W, Pedersen HS, Ribe AR, et al. Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry. 2015;72(6):612–619. doi:10.1001/jamapsychiatry.2015.0082
21. Krogsgaard LW, Vestergaard CH, Plana-Ripoll O, et al. Health care utilization in general practice after HPV vaccination-a Danish nationwide register-based cohort study. PLoS One. 2017;12(9):e0184658. doi:10.1371/journal.pone.0184658
22. Thomsen RW, Ozturk B, Pedersen L, et al. Hospital records of pain, fatigue, or circulatory symptoms in girls exposed to human papillomavirus vaccination: cohort, self-controlled case series, and population time trend studies. Am J Epidemiol. 2020. doi:10.1093/aje/kwz284
23. Rasmussen TA, Jorgensen MR, Bjerrum S, et al. Use of population based background rates of disease to assess vaccine safety in childhood and mass immunisation in Denmark: nationwide population based cohort study. BMJ. 2012;345(sep17 1):e5823. doi:10.1136/bmj.e5823
24. Meinert L, Seeberg J. Can epidemics be noncommunicable? - reflections on the spread of ‘noncommunicable’ diseases. Med Anthropol Theory. 2015;2(2):54–71.
25. Cramon C, Poulsen CL, Hartling UB, Holden IK, Johansen IS. Possible adverse effects of the quadrivalent human papillomavirus vaccine in the Region of Southern Denmark: a retrospective, descriptive cohort study. Dan Med J. 2017;64(7).
26. Fink P, Rosendal M, Toft T. Aetiology. In: Fink P, Rosendal M, editors. Functional Disorders and Medically Unexplained Symptoms. Aarhus: Aarhus University Press; 2015:63–67.
27. Lievesley K, Rimes KA, Chalder T. A review of the predisposing, precipitating and perpetuating factors in chronic fatigue syndrome in children and adolescents. Clin Psychol Rev. 2014;34(3):233–248. doi:10.1016/j.cpr.2014.02.002
28. Andersen JS, Olivarius Nde F, Krasnik A. The Danish national health service register. Scand J Public Health. 2011;39(7 Suppl):34–37. doi:10.1177/1403494810394718
29. Kildemoes HW, Sorensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi:10.1177/1403494810394717
30. Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7 Suppl):54–57. doi:10.1177/1403494810395825
31. Vestergaard M, Obel C, Henriksen TB, et al. The Danish national hospital register is a valuable study base for epidemiologic research in febrile seizures. J Clin Epidemiol. 2006;59(1):61–66. doi:10.1016/j.jclinepi.2005.05.008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





