Back to Journals » Cancer Management and Research » Volume 10
Gene expression of hENT1, dCK, CDA, dCMPD and topoisomerase IIα as an indicator of chemotherapy response in AML treated with cytarabine and daunorubicin
Authors Kulsoom B , Shamsi TS , Afsar NA
Received 24 July 2018
Accepted for publication 3 October 2018
Published 9 November 2018 Volume 2018:10 Pages 5573—5589
DOI https://doi.org/10.2147/CMAR.S181299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xueqiong Zhu
Bibi Kulsoom,1,2 Tahir Sultan Shamsi,1 Nasir Ali Afsar3
1Center of Excellence in Molecular Medicine, National Institute of Blood Diseases and Bone Marrow Transplantation, Karachi, Pakistan; 2Department of Biochemistry, Jinnah Medical and Dental College, Karachi, Pakistan; 3Department of Pharmacology, Jinnah Medical and Dental College, Karachi, Pakistan
Purpose: Acute myeloid leukemia patients are commonly treated with cytarabine (Ara-C) and anthracyclines but the sustained remission rate is not very promising. We explored the role of drug-metabolizing enzymes and transporters in the therapeutic response.
Patients and methods: Bone marrow and peripheral blood samples of 90 newly diagnosed acute myeloid leukemia patients treated with standard 3+7 regimen were analyzed through real-time PCR for expression of human equilibrative nucleoside transporter 1, deoxycytidine kinase, cytidine deaminase (CDA), deoxycytidine monophosphate deaminase (dCMPD) and topoisomerase IIα (Topo-IIa). The expression of these markers was studied in relationship with good (persistent remission) and poor therapeutic response (relapse/resistance).
Results: High Topo-IIa expression in peripheral blood was associated with good response (P=0.006). Relapse was higher among low expressors of Topo-IIa in peripheral blood (OR: 26.25). Bone marrow Topo-IIa expression followed a similar trend but did not reach statistical significance. In contrast, patients with high bone marrow dCMPD expression had poor response (OR: 3; P=0.043). One-year disease-free survival (DFS) was better among those with high bone marrow Topo-IIa (P=0.04) or CDA (P=0.03) expression. High bone marrow Topo-IIa expression also had better DFS at 6 months (P=0.04) and at 12 months (P=0.04).
Conclusion: High expression of Topo-IIa in peripheral blood is a favorable indicator of persistent remission, good therapeutic response and DFS. High dCMPD and low CDA expression in bone marrow is associated with poor therapeutic outcome.
Keywords: topoisomerase IIα, hENT1, dCMPD, CDA, dCK, survival, AML, antimetabolites, anthracyclines
Introduction
Acute myeloid leukemia (AML) is mainly treated with chemotherapy regimen comprising of cytarabine and anthracyclines.1 Treatment of AML is often complicated by resistance to conventional chemotherapy, which reflects the survival of tumor cells despite exposure to cytotoxic chemotherapeutic agents.2,3 This has been a major problem in effective cancer treatment.4 Therapeutic response depends on availability of drug at the target tissue where it inflicts damage to the malignant cells. Therefore, sensitivity and resistance to a drug could be influenced by the expression of drug transporters, drug metabolizing enzymes, and thus availability of sufficient drug at target receptors.
Human equilibrative nucleoside transporter 1 (hENT1) or solute carrier family 29 member 1 is a transmembrane glycoprotein present in cell and mitochondrial membranes. It is primarily involved in importing nucleosides for salvage pathway of purine and pyrimidine nucleosides. Cytotoxic nucleoside analogs, such as cytarabine (Ara-C), also utilize this transporter to enter cells.5 Thus, efficiency of Ara-C influx can determine the intracellular drug level and thereby response to chemotherapy.6
Deoxycytidine kinase (dCK) is the rate-limiting enzyme of salvage pathway that phosphorylates nucleoside analogs, including Ara-C and gemcitabine to respective monophosphates. These monophosphates are phosphorylated by various kinases to diphosphates and triphosphates, which are in turn utilized for DNA synthesis. These Ara-C nucleotides inhibit DNA replication and transcription by inhibiting DNA and RNA polymerases.7 Ara-C is converted to its inactive metabolite Ara-uridine through cytidine deaminase (CDA) while Ara-CMP is converted to an inactive metabolite, Ara-UMP by deoxycytidine monophosphate deaminase (dCMPD).8 Both in vitro and in vivo studies have shown that changes in the cellular levels of dCK, CDA and dCMPD are associated with changes in intracellular drug concentrations. Thus, the efficacy of a specific dose of Ara-C may also vary depending upon expression of these enzymes as they may alter the availability of drug at the target.9–12
Topoisomerases, such as topoisomerase IIα (Topo-IIa), are enzymes required for relieving DNA supercoils during replication, transcription and chromosome condensation.13 Topo-IIa expression starts increasing during S phase of cell cycle and continues to increase in G2 and M phases, thus being highly expressed in rapidly dividing cells. Some anticancer drugs such as anthracyclines, etoposide and mitoxantrone act by targeting Topo-IIa and preventing it from religating nicks in DNA, thus introducing strand breaks.14,15 Topo-IIa expression has been found to correlate with clinical outcome in various malignancies, but the findings of various studies are contradictory, where high Topo-IIa expression is correlated with a favorable outcome in some studies,16–19 while having an adverse outcome in others.20–23 For example, in acute lymphoblastic leukemia (children n=65), an increased Topo-IIa expression correlated with daunorubicin resistance as part of the FRALLE-93 protocol.20 Thus, it is imperative that the chemotherapy outcome should be studied in relation to molecular markers given above to draw a holistic picture. Hence, this study was designed to comprehensively analyze the role of hENT1, dCK, CDA, dCMPD and Topo-IIa gene expression in response to standard 3+7 AML chemotherapy regimen.
Materials and methods
Patient induction, sample collection and real-time/quantitative PCR
A total of 90 patients diagnosed with AML were recruited at National Institute of Blood Disease & Bone Marrow Transplantation (NIBD&BMT) Karachi, during September 2011 to March 2017. All patients, who received cytarabine 200 mg/m2/day for 7 days and daunorubicin 45 mg/m2/day for 3 days, were included. Complete remission (CR) was defined as blast cells <5% in bone marrow and no blast cells in peripheral blood. Patients remained under observation and were assessed for CR documentation during the days 21–28 after the first cycle of induction chemotherapy.
The study was approved by the Ethical Review Board at NIBD&BMT in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in this research. Any participant under the age of 18 had parental or legal guardian written informed consent confirmed.
Patient bone marrow and peripheral blood samples were collected separately and were used to study the expression of hENT1, dCK, CDA, dCMPD and Topo II. Detailed information about sample collection and storage, RNA extraction, reverse transcription reaction and quantitative real-time PCR (qPCR) were previously reported.24 Briefly, we enriched the leukemia blasts with the Ficoll gradient method.24 The peripheral blood mononuclear cells layer yielded cells overwhelmingly populated by blast cells. Other mononuclear cells could be seen only sporadically, as confirmed by morphology and flow cytometry. Primers and probes used are: for hENT1 forward 5′-TGTTTCCAGCCGTGACT-3′, reverse 5′-CAGGCCACATGAATACAG-3′ and probe 5′-/56-FAM/CA GCA CCT G/ZEN/G GAA CGTTAC TT/3IABkFQ/-3′; for dCK forward 5′-TGCAGGGAAGTCAACATT-3′, reverse 5′-TCCCACCATTTTTCTGAG-3′ and probe 5′-/56-FAM/TA AAC AAT T/ZEN/G TGT GAA GATTGG GAA G/3IABkFQ/-3′; for CDA forward 5′-GGAGGCCAAGAAGTCAG-3′, reverse 5′-GACGGCCTTCTGGATAG-3′ and probe 5′-/56-FAM/CA ACA TAG A/ZEN/A AAT GCC TGCTAC CC/3IABkFQ/-3′; for dCMPD forward 5′-AATGGGTGCAGTGATGAC-3′, reverse 5′-CTTAGCGCATTCATTACAAG-3′ and probe 5′-/56-FAM/AT CAT GAA C/ZEN/A AAA ATT CGACCG AT/3IABkFQ/-3′; for Topo-IIa forward 5′-AGTCGCTTTCAGGGTTCTTGAG-3′, reverse 5′-TTTCATTTACAGGCTGCAATGG-3′ and probe 5′-/56-FAM/CC CTT CAC G/ZEN/A CCG TCA CCATGG A/3IABkFQ/-3′, and for GAPDH forward 5′-GAAGGTGAAGGTCGGAGTCA-3′, reverse 5′-GAAGATGGTGATGGGATTTC-3′, and probe 5′-(FAM)/56-JOEN/CC GAC TCT T/ZEN/G CCC TTCGAA C/3IABkFQ/ (TAMRA)-3′.25,26
Statistical analyses
Statistical analyses were done using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Data are presented as frequencies and percentages, or median and interquartile ranges where applicable. Chi-squared tests were used to analyze the differences between the groups. Spearman’s correlations (rs) were computed to determine the relationships between different gene expressions. The Kaplan–Meier survival analysis (log-rank test) was carried out to explore the survival according to gene expression. Only a P<0.05 was considered significant.
Results
Baseline characteristics
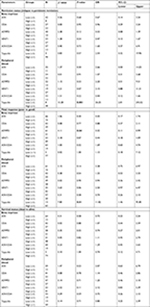
Ninety AML patients were analyzed in this study. Baseline data, such as age, gender and ethnic distribution, AML subtype, myeloperoxidase status, mutations, translocations and karyotyping are given in Table 1, which shows that CR was achieved by 56 patients while 34 were resistant to chemotherapy. A relapse was reported in 19 patients who had achieved CR. Thus, 37 patients who achieved remission and did not relapse during the study period (persistent remission) were labeled as good responders to therapy (41%), whereas all those patients who showed either little or no remission after chemotherapy or relapsed later were grouped together as poor responders (n=53; 59%). One-year disease-free survival (DFS) and overall survival (OS) status was calculated. Since gene expression data were not normally distributed, median and IQRs are provided according to various groups, as shown in Table S1.
  | Table 1 Baseline characteristics of the study population (n=90) Abbreviations: AML, acute myeloid leukemia; MPO, myeloperoxidase. |
Chi-squared and Fisher exact tests
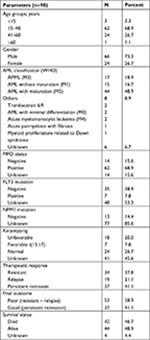
Grouping variables were dichotomized for meaningful analysis, ie, gender (male and female), AML classification (APML being good prognostic, all others being poor prognostic), myeloperoxidase status (negative, positive), remission status (relapse, persistent remission), final therapeutic response (poor, good) and survival status (dead, alive). Nonparametric variables were compared by chi-squared or Fisher’s exact test (Table 2). AML other than APML had a higher relapse rate (P=0.012), poor final therapeutic response (P<0.001) and a trend of higher mortality (P=0.05). In addition, patients with relapse and poor therapeutic outcome (resistant + relapsed) had higher mortality (P<0.001 for both groups). FLT3-positive patients had a poor therapeutic outcome when compared with FLT3-negative patients (P=0.03).
  | Table 2 Comparison between groups according to baseline characteristics Note: aBecause all relapse in poor and all remission in good category. All chi-squared values df =1 |
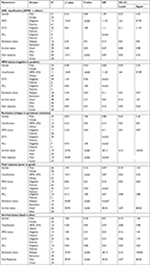
Since the gene expression data were not normally distributed, we stratified it as either low or high expression. If the relative gene expression was up to one-fold, the patients were labeled low expressers, and if more than one, high expressers. Chi-squared or Fisher’s exact test was done to compare low or high expression between relapse vs remission, poor vs good therapeutic response and dead vs alive (survival) groups (Table 3). Among good responders, a trend of high hENT1 expression (bone marrow, P=0.07; peripheral blood, P=0.05) but lower marrow dCMPD expression (P=0.043) was observed when compared with poor responders. Although bone marrow Topo-IIa showed a trend of being higher among good responders, it did not reach significance. However, such significance for Topo-IIa expression was achieved in peripheral blood samples of good responders (P=0.006). Subgroup analysis showed that peripheral blood Topo-IIa expression was higher in persistent remission vs relapse group (P=0.001).
No significant difference was found in the gene expression of dCK and CDA among these clinical outcome groups.
OR was computed, which showed that patients with low Topo-IIa expression in peripheral blood relapsed more often (OR: 26.25) and had poorer therapeutic response (OR: 11.8) than those with higher expression. Patients with lower dCMPD expression in bone marrow were likely to have good response (OR: 3.1).
Nonparametric correlation – Spearman’s Rho (rs)
Correlation was computed among the expression of genes in bone marrow and peripheral blood (Table 4). A positive correlation was observed between the expression in bone marrow and peripheral blood for each gene. There was a positive correlation between dCK and dCMPD as well as hENT1 in respective bone marrow and peripheral blood samples. Both bone marrow and peripheral blood dCK expression positively correlated with dCK/CDA ratio in both bone marrow and peripheral blood. CDA expression positively correlated with hENT1 and Topo-IIa expression in respective sample types. dCMPD expression positively correlated with hENT1 and Topo-IIa expression in respective sample types. In addition, Topo-IIa correlated positively with dCK/CDA ratio.
OS
OS was calculated for 6 and 12 months by Kaplan–Meier analysis and is presented in Figure 1. Better OS at 12 months was noted for APML (P=0.031), whereas poor OS at 6 and 12 months was seen in those with FLT3 mutation when compared with those without it (P=0.003 and P=0.001, respectively). A trend of higher Topo-IIa expression in peripheral blood with better DFS was observed (P=0.09). No significant differences were observed for higher and lower expression of other genes regarding 6- or 12-month OS.
DFS
Figure 2 shows better 12-month DFS among high CDA expressors (P=0.032) in bone marrow. Better DFS was found with high bone marrow Topo-IIa expression at both 6 (0.04) and 12 months (P=0.04). A trend of high Topo-IIa expression in peripheral blood with better DFS was also observed (P=0.08).
Discussion
We observed that high Topo-IIa expression is strongly associated with a better CR rate in AML patients treated with standard dose 3+7 induction chemotherapy comprising Ara-C and daunorubicin, and thus translates into low relapse and better DFS, whereas high dCPMD expression was associated with poor chemotherapy response. In agreement with other studies, we observed that patients with APML and absent FLT3 mutation had better therapeutic response and survival.
Although WHO and European Leukemia Net classifications have included many factors determining the clinical outcome in terms of response and survival, the picture is far from complete. Remission rates remain poor and many patients do not survive, pointing to the possibility of incompletely treated disease with chemotherapy. Multiple mechanisms can potentially be attributed to a partial or absent response to chemotherapy. These may include alterations in influx and efflux of the chemotherapeutic drugs, activation and inactivation of the drugs, drug target availability and ultimately the efficiency of apoptotic machinery. These factors are not yet part of any AML therapeutic guideline partly due to conflicting data. The current study is an attempt to clarify the role of these mechanisms in association with therapeutic outcome in AML patients treated with Ara-C and daunorubicin.
Expression of Ara-C transporter hENT1 and metabolizing enzymes dCK, CDA and dCMPD
Ara-C is transported inside the cell by hENT15 and increased retention of Ara-C in leukemic cells has been associated with longer duration of CR in patients with AML.27 Low and high expression of hENT1 have been reported as a predictor of Ara-C resistance in AML10,28 and sensitivity in acute lymphocytic leukemia,9 respectively. Some solid organ tumors have shown interesting results. For example, some degree of hENT1 protein expression has been found associated with increased OS and DFS in pancreatic cancer treated with gemcitabine (a pyrimidine nucleoside analog) when compared with no hENT1 expression.29–31 It has also been shown in vitro that hENT1 expression positively correlated with IC50 of gemcitabine, suggesting a direct role of hENT1 expression in cancer cell sensitivity in a non-small-cell lung cancer cell line exposed to gemcitabine.32 Greenhalf et al31 inferred that pancreatic cancer patients with no hENT1 expression should not be treated with gemcitabine. Similarly, higher expression of hENT1 has been found associated with prolonged survival or better therapeutic response in patients with advanced biliary tract cancer who were treated with gemcitabine.33
In a recent study of hematological malignancy, high-risk Myelodysplastic syndrome patients treated with decitabine (another pyrimidine analog) also suggested a similar outcome.34 We observed that in AML patients treated with the standard 3+7 regimen, a trend of higher hENT1 expression was seen among good responders, although it did not reach statistical significance (bone marrow, P=0.07; peripheral blood, P=0.05). This inability to reach statistical significance could be partly explained based on sample size, because another study with a higher sample size reported a shorter DFS among hENT1-deficient AML patients (n=123) treated with Ara-C dosage similar to our study.28
Once Ara-C is inside the cells, dCK, a cytoplasmic enzyme, activates Ara-C by phosphorylating it, which is then incorporated in the newly synthesized DNA, while CDA inactivates it. Abraham et al35 have described Ara-C resistance index, which is denoted as ΔCt (DCK × ENT1)/ΔCt CDA. They found that resistance index values were significantly higher in resistant patients compared with sensitive patients. In contrast, we have observed that dCK and CDA expression was not associated with response to chemotherapy; however, we had a smaller sample size but more rigorous method of gene expression calculation (ΔΔCt), which might explain the difference. Regarding the inactivators of Ara-C, we have shown that expression of bone marrow dCMPD was significantly higher in poor therapeutic response, with OR=3 (Table 3). Paradoxically, a higher CDA expression in bone marrow (instead of anticipated lower expression) was found significantly associated with better DFS. At present, the significance of this isolated finding is unclear. This could be a chance finding because no other subgroup analysis could support this observation. Hence, it needs to be explored further.
In contrast, Achiwa et al32 did not find any correlation of dCK expression with IC50 of gemcitabine in a non-small-cell lung cancer line. Similarly, many studies have shown no significant association of the gene expression of dCK, CDA and hENT1 with clinical outcome or survival in AML (n=123 and 42)28,36 as well as a variety of other cancers such as myelodysplastic syndrome (n=98)34 and biliary tract cancer (n=28).33 Our study shows a similar trend of dCK or CDA expression with regard to patient survival or chemotherapy outcome.
We observed that bone marrow and peripheral blood hENT1 expression correlated with each other and with dCK, CDA, dCMPD and Topo-IIa positively in respective bone marrow and peripheral blood samples (Table 4). These observations might indicate a synchronized regulation of expression of these genes suggesting differential transcriptomic regulation.
Role of anthracycline target Topo-IIa
The expression of Top IIα, being a target molecule for anthracyclines, seems imperative in assessing the response to anthracycline-based chemotherapy. In our study, AML patients were treated with daunorubicin 45 mg/day as part of the standard regimen described above. We observed that higher peripheral blood Topo-IIa expression was associated with persistent remission as compared with resistance (P=0.047) or relapse (P<0.001) (Mann–Whitney U-test, data not provided). In addition, patients with higher peripheral blood Topo-IIa expression had significantly better DFS at 6 and 12 months. There was no effect of Topo-IIa expression on OS. Patients with low Topo-IIa expression in peripheral blood had higher odds to develop relapse (OR >26) and poor therapeutic response (OR >11). Thus, availability of Topo-IIa is important for a better therapeutic response. Topo-IIa expression in peripheral blood was found positively and strongly correlated with hENT1 expression. This correlation may be explained by a possible upregulation of Topo-IIa in response to the drug entering the cell, although the transporter for daunorubicin entry is not well characterized. Some studies have shown, in agreement with our findings that higher Topo-IIa expression could predict better outcome in breast cancer patients treated with anthracyclines. In one study, patients were treated with five cycles of 5-flurouracil, epirubicin, cyclophosphamide, which included an anthracycline called epirubicin 75–100 mg/m2.18 In another study, it was reported that Topo-IIa expression in Her2/neu expressing breast cancer positively influenced response to anthracycline-based chemotherapy.17 A systematic review of breast cancer patients receiving anthracycline-based chemotherapy has also supported this observation.16 As described earlier, Topo-IIa expression increases during S, G2 and M phases of the cell cycle, and is thus highly expressed in rapidly dividing cells.14,15 Chemotherapy may kill the cancer cells and lead to senescence of normal tissue.37 Thus, it can be hypothesized that if Topo-IIa is highly expressed it may not only provide a better drug target in malignant cells but also help in subsequent DNA repair in normal tissue damaged by chemotherapy.
However, high expression may be a double-edged sword, as it may dilute the therapeutic effect of anthracyclines by providing abundant topoisomerase function to evade the drug action. Currently, little is known about this intricate balance and much seems to depend on pathology, tissue involved and anthracycline dosing, among other factors. For example, in contrast to our results, in small cell lung cancer patients (n=93) receiving doxorubicin and etoposide, high Topo-IIa expression was associated with poor therapeutic outcome and low OS.21 Similarly, another study conducted on Hodgkin’s lymphoma patients receiving anthracycline (doxorubicin or epirubicin; n=238) reported that high expression of Topo-IIa was associated with adverse prognosis, whereas low expression had no effect.38 In patients with colorectal carcinoma (n=228), Topo-IIa overexpression was found associated with advanced tumor stage and resistant disease.23
However, some studies could not find any significant association of Topo-IIa expression with outcome or survival among AML (n=123, semiquantitative RT-PCR)28 and breast cancer (n=232) treated with anthracyclines.39
Similarly, in some studies of AML, Topo-IIa expression was not found associated with clinical outcome at all.28,40 Conflicting results may be due to the use of techniques with different sensitivities. Immunohistochemistry was the technique used in most of these studies to assess Topo-IIa expression, but more sensitive techniques are now available.
The prognostic role of MPO has remained controversial.41 There could be multiple reasons for that. For example, it is important to only consider studies with similar criteria for MPO positivity while exploring its role related to therapeutic outcome. An interesting observation of our study was the association of MPO positivity with poor therapeutic response.
Others have reported that MPO positivity is a favorable prognostic factor, though the effect size is small.41 A recent study suggests that MPO status may, in fact, be a reflection of DNA methylation status in blast cells, and thus could be an indirect marker that should be interpreted with caution.42 Thus, the MPO controversy needs further exploration in depth.
The main strength of our study included adoption of a uniform treatment and follow-up protocol as well as inclusion of both bone marrow and peripheral blood. Our study also had limitations. It was a single-center study and may not be representative of the entire population of AML patients. We recommend that this study should be replicated with a larger sample size and inclusion of other hematological malignancies to validate our results regarding gene expression.
Conclusion
AML patients with APML subtype had better CR rate and better OS. FLT3 mutation is a known bad prognostic marker. All patients positive for this mutation could not achieve CR and all were resistant to chemotherapy. A higher hENT1 expression tended to be associated with better CR rate in these patients. However, this observation may be confirmed by use of a larger sample size. CDA and dCK expression did not correlate with CR rate. Higher dCPMD expression was associated with poor chemotherapy response. A high Topo-IIa expression is strongly associated with better CR rate in AML patients treated with standard dose 3+7 induction chemotherapy comprising Ara-C and daunorubicin, and thus translates into low relapse and better DFS.
Acknowledgments
The authors acknowledge Alfaisal University, Riyadh, Saudi Arabia, for the financial support through grant IRG-302111603131 for partial support of this project. They also thank Dr Peter M. B. Cahusac, Associate Professor of Pharmacology and Biostatistics, Alfaisal University, for his kind support in reviewing the article for English language.
Author contributions
All authors met authorship criteria and made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. | ||
Lerch E, Espeli V, Zucca E, et al. Prognosis of acute myeloid leukemia in the general population: data from southern Switzerland. Tumori. 2009;95(3):303–310. | ||
Bahl A, Sharma A, Raina V, et al. Long-term outcomes for patients with acute myeloid leukemia: a single-center experience from AIIMS, India. Asia Pac J Clin Oncol. 2015;11(3):242–252. | ||
Shtil AA. Signal transduction pathways and transcriptional mechanisms as targets for prevention of emergence of multidrug resistance in human cancer cells. Curr Drug Targets. 2001;2(1):57–77. | ||
Zhang J, Visser F, King KM, Baldwin SA, Young JD, Cass CE. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007;26(1):85–110. | ||
Mata JF, Scrideli CA, Queiroz RP, et al. Cytosine arabinoside-metabolizing enzyme genes are underexpressed in children with MLL gene-rearranged acute lymphoblastic leukemia. Braz J Med Biol Res. 2006;39(11):1417–1423. | ||
Hazra S, Ort S, Konrad M, Lavie A. Structural and kinetic characterization of human deoxycytidine kinase variants able to phosphorylate 5-substituted deoxycytidine and thymidine analogues. Biochemistry. 2010;49(31):6784–6790. | ||
Chabner BA, Amrein PC, Druker BJ. Antineoplastic Agents. Texbook of Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. Brunton LL, Lazo JS, Parker KL, editors. New York: The McGraw-Hill Companies Inc; 2006:1315–1403. | ||
Stam RW, den Boer ML, Meijerink JP, et al. Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood. 2003;101(4):1270–1276. | ||
Kanno S, Hiura T, Ohtake T, et al. Characterization of resistance to cytosine arabinoside (Ara-C) in NALM-6 human B leukemia cells. Clin Chim Acta. 2007;377(1–2):144–149. | ||
Lamba JK, Crews K, Pounds S, et al. Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther. 2007;323(3):935–945. | ||
Rathe SK, Largaespada DA. Deoxycytidine kinase is downregulated in Ara-C-resistant acute myeloid leukemia murine cell lines. Leukemia. 2010;24(8):1513–1515. | ||
Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17(5):421–433. | ||
Pogorelčnik B, Perdih A, Solmajer T. Recent developments of DNA poisons–human DNA topoisomerase IIα inhibitors–as anticancer agents. Curr Pharm Des. 2013;19(13):2474–2488. | ||
Bower JJ, Karaca GF, Zhou Y, Simpson DA, Cordeiro-Stone M, Kaufmann WK. Topoisomerase II alpha maintains genomic stability through decatenation G(2) checkpoint signaling. Oncogene. 2010;29(34):4787–4799. | ||
Miyoshi Y, Kurosumi M, Kurebayashi J, et al. Predictive factors for anthracycline-based chemotherapy for human breast cancer. Breast Cancer. 2010;17(2):103–109. | ||
Wang J, Xu B, Yuan P, et al. TOP2A amplification in breast cancer is a predictive marker of anthracycline-based neoadjuvant chemotherapy efficacy. Breast Cancer Res Treat. 2012;135(2):531–537. | ||
Mukherjee A, Shehata M, Moseley P, Rakha E, Ellis I, Chan S. Topo2α protein expression predicts response to anthracycline combination neo-adjuvant chemotherapy in locally advanced primary breast cancer. Br J Cancer. 2010;103(12):1794–1800. | ||
Nikolényi A, Sükösd F, Kaizer L, et al. Tumor topoisomerase II alpha status and response to anthracycline-based neoadjuvant chemotherapy in breast cancer. Oncology. 2011;80(3-4):269–277. | ||
Grandgirard N, Ly-Sunnaram B, Ferrant D, et al. Impact of Topoisomerase II alpha and spermine on the clinical outcome of children with acute lymphoblastic leukemia. Leuk Res. 2004;28(5):479–486. | ||
Dingemans AM, Witlox MA, Stallaert RA, van der Valk P, Postmus PE, Giaccone G. Expression of DNA topoisomerase II alpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res. 1999;5(8):2048–2058. | ||
Materna V, Pleger J, Hoffmann U, Lage H. RNA expression of MDR1/P-glycoprotein, DNA-topoisomerase I, and MRP2 in ovarian carcinoma patients: correlation with chemotherapeutic response. Gynecol Oncol. 2004;94(1):152–160. | ||
Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008;8(7):790–797. | ||
Kulsoom B, Shamsi TS, Afsar NA, Memon Z, Ahmed N, Hasnain SN. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: are we ready for Bcl-2-directed therapy? Cancer Manag Res. 2018;10:403–416. | ||
Burger H, Foekens JA, Look MP, et al. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res. 2003;9(2):827–836. | ||
Suzuki K, Kazui T, Yoshida M, et al. Drug-induced apoptosis and p53, BCL-2 and BAX expression in breast cancer tissues in vivo and in fibroblast cells in vitro. Jpn J Clin Oncol. 1999;29(7):323–331. | ||
Rustum YM, Preisler HD. Correlation between leukemic cell retention of 1-beta-D-arabinofuranosylcytosine 5’-triphosphate and response to therapy. Cancer Res. 1979;39(1):42–49. | ||
Galmarini CM, Thomas X, Calvo F, et al. In vivo mechanisms of resistance to cytarabine in acute myeloid leukaemia. Br J Haematol. 2002;117(4):860–868. | ||
Spratlin J, Sangha R, Glubrecht D, et al. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10(20):6956–6961. | ||
Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136(1):187–195. | ||
Greenhalf W, Ghaneh P, Neoptolemos JP, et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014;106(1):djt347. | ||
Achiwa H, Oguri T, Sato S, Maeda H, Niimi T, Ueda R. Determinants of sensitivity and resistance to gemcitabine: the roles of human equilibrative nucleoside transporter 1 and deoxycytidine kinase in non-small cell lung cancer. Cancer Sci. 2004;95(9):753–757. | ||
Murata A, Amano R, Yamada N, et al. Prognostic predictive values of gemcitabine sensitivity-related gene products for unresectable or recurrent biliary tract cancer treated with gemcitabine alone. World J Surg Oncol. 2013;11:117. | ||
Wu L, Shi W, Li X, et al. High expression of the human equilibrative nucleoside transporter 1 gene predicts a good response to decitabine in patients with myelodysplastic syndrome. J Transl Med. 2016;14:66–74. | ||
Abraham A, Varatharajan S, Karathedath S, et al. RNA expression of genes involved in cytarabine metabolism and transport predicts cytarabine response in acute myeloid leukemia. Pharmacogenomics. 2015;16(8):877–890. | ||
Veuger MJ, Honders MW, Willemze R, Barge RM. Deoxycytidine kinase expression and activity in patients with resistant versus sensitive acute myeloid leukemia. Eur J Haematol. 2002;69(3):171–178. | ||
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;21(355):1253–1261. | ||
Doussis-Anagnostopoulou IA, Vassilakopoulos TP, Thymara I, et al. Topoisomerase II alpha expression as an independent prognostic factor in Hodgkin’s lymphoma. Clin Cancer Res. 2008;14(6):1759–1766. | ||
Arriola E, Moreno A, Varela M, et al. Predictive value of HER-2 and Topoisomerase II alpha in response to primary doxorubicin in breast cancer. Eur J Cancer. 2006;42(17):2954–2960. | ||
Gieseler F, Glasmacher A, Kämpfe D, et al. Topoisomerase II activities in AML blasts and their correlation with cellular sensitivity to anthracyclines and epipodophyllotoxines. Leukemia. 1996;10(Suppl 3):S46–S49. | ||
Kim Y, Yoon S, Kim SJ, Kim JS, Cheong JW, Min YH. Myeloperoxidase expression in acute myeloid leukemia helps identifying patients to benefit from transplant. Yonsei Med J. 2012;53(3):530–536. | ||
Itonaga H, Imanishi D, Wong YF, et al. Expression of myeloperoxidase in acute myeloid leukemia blasts mirrors the distinct DNA methylation pattern involving the downregulation of DNA methyltransferase DNMT3B. Leukemia. 2014;28(7):1459–1466. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.