Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Gender-specific association of functional prodynorphin 68 bp repeats with cannabis exposure in an African American cohort
Authors Yuferov V, Butelman ER, Kreek MJ
Received 15 December 2017
Accepted for publication 7 February 2018
Published 16 April 2018 Volume 2018:14 Pages 1025—1034
DOI https://doi.org/10.2147/NDT.S159954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Vadim Yuferov,* Eduardo R Butelman,* Mary Jeanne Kreek
Laboratory of the Biology of Addictive Diseases, The Rockefeller University, New York, NY, USA
*These authors contributed equally to this work
Background: Cannabis use disorders (CUDs) cause substantial neuropsychiatric morbidity and comorbidity. There is evidence for gender-based differences in CUDs, for instance, a greater prevalence in males than in females. The main active component of cannabis is delta 9-tetrahydrocannabinol (delta 9-THC), a partial agonist of the cannabinoid type 1 receptor. Preclinical studies show that genetic or pharmacological manipulation of the kappa opioid receptor/dynorphin system modulates the effects of delta 9-THC.
Methods: In this case-control study of adult African Americans (n=476; 206 females, 270 males), we examined the association of the functional prodynorphin 68 bp (PDYN 68 bp) promoter repeats with categorical diagnoses of cannabis dependence (Diagnostic and Statistical Manual of Mental Disorders-IV criteria), as well as with a rapid dimensional measure of maximum lifetime cannabis exposure (the Kreek–McHugh–Schluger–Kellogg cannabis scale).
Results: The PDYN 68 bp genotype (examined as short–short [SS], short–long [SL], or long–long [LL], based on the number of repeats) was not significantly associated with categorical cannabis-dependence diagnoses, either in males or in females. However, in males, the PDYN 68 bp SS+SL genotype was associated with both greater odds of any use of cannabis (p<0.05) and earlier age of first cannabis use, compared to the LL genotype (ie, 15 versus 16.5 years of age; p<0.045). Males in the SS+SL group also had greater odds of high lifetime exposure to cannabis, compared to the LL group (p<0.045). Of interest, none of the aforementioned genetic associations were significant in females.
Conclusion: This study provides the first data on how the PDYN 68 bp genotype is associated with gender-specific patterns of exposure to cannabis. Overall, this study shows that PDYN 68 bp polymorphisms affect behaviors involved in early stages of nonmedical cannabis use and potentially lead to increasing self-exposure. These data may eventually lead to improvements in personalized medicine for the prevention and treatment of highly prevalent CUDs and neuropsychiatric comorbidities.
Keywords: cannabis, gender, prodynorphin, gene polymorphism, dimensional phenotype
Introduction
Nonmedical use of cannabinoid compounds, obtained from cannabis plants, is associated with considerable morbidity, as well as with comorbidity due to psychiatric and other substance-use disorders.1–3 The main active component of cannabis is delta 9-tetrahydrocannabinol (delta 9-THC), a partial agonist of the cannabinoid type 1 receptor (CB1) receptor (CB1-r).4 It can be hypothesized that the health-related consequences of cannabis use are related to various dimensional aspects of cannabis exposure (eg, the amount, pattern, and duration of use), in addition to environmental and genetic factors.
Among the major neurobiological systems that are known to interact with CB1-r is the kappa opioid receptor (KOR) system, and its cognate ligands, the dynorphins (encoded by the PDYN gene).5 Preclinical studies show that pharmacological and genetic modulation of the kappa receptor and PDYN systems causes robust changes in the behavioral effects of CB1-r ligands, including delta 9-THC.6,7 The kappa receptor/PDYN system is also known to be strongly involved in the mediation of the rewarding and aversive effects of other drugs of abuse, as well as the expression of dysphoria and depression- and anxiety-like effects.8–12 It is also known that dynorphins and other kappa receptor agonists cause a decrease in striatal extracellular dopamine concentrations in vivo.13,14
The human prodynorphin (PDYN) gene consists of four exons. Exons 1 and 2 encode the 5′-untranslated region of the mRNA, while exons 3 and 4 encode the translated region.15 Numerous mutations have been identified in the human PDYN gene, including a tandem repeat polymorphism in the promoter region. This polymorphism consists of one to five tandem repeats of 68 bp length.15,16 The 68 bp repeat has been the subject of several functional analyses and genetic association studies. An in vitro reporter gene expression assay showed that one and two copies of the repeat cause lower drug-induced increases in expression of the reporter gene than do three and four copies.17,18 However, a more recent in vitro expression study using a construct with a longer PDYN promoter sequence,19 as well as in vivo studies, demonstrated a complex relationship between the polymorphisms and PDYN expression, which is influenced by many factors such as brain region, gender, and cell type.18–20
Studies in rats demonstrated gender- and strain-related differences in self-administration of the synthetic CB1-r agonist WIN 55,212-2,21 with female rats showing a higher self-administration than males. A recent review has focused on gender-based differences in terms of specific effects of cannabis, including subjective effects and signs of withdrawal.22
The PDYN 68 bp repeat polymorphism has been investigated extensively in association studies for several drug-dependence phenotypes but with inconsistent results across studies. Several studies showed that three or four copies of the repeat constitute a risk factor for the development of cocaine/alcohol codependence and heroin-dependence diagnoses.16,23,24 Other studies reported no association of this polymorphism with drug use phenotype.17,25,26 However, to our knowledge, there have not been reports on the association of the PDYN 68 bp polymorphism with nonmedical cannabis use.
This study examined the association of variations of the PDYN 68 bp polymorphism with dimensional measures of cannabis exposure in African Americans, based on two reasons: 1) the frequency of the PDYN alleles is different between African American and Caucasian populations;19 2) prior studies reported an association of this PDYN polymorphism with heroin dependence in African Americans only, and not in Caucasians or Hispanics.16,23 Of interest, a recent epidemiological report showed greater odds of moderate or severe cannabis use disorders (CUDs) in African Americans, compared to other groups in the USA, including Caucasians.1 These differences could be due to behavioral, genetic, socioeconomic, environmental, or biomedical factors, or a combination thereof.
Recent trends in psychiatric research and genetic association studies have increasingly focused on “dimensional” phenotypic measures, as opposed to only on categorical diagnoses.27,28 Dimensional measures are those that are based on quantification of specific attributes and may be especially useful for clinical presentations that vary along some form of a continuum.29 One such dimensional measure, studied herein with a rapid instrument (the Kreek–McHugh–Schluger–Kellogg [KMSK] scale), is the “maximum lifetime exposure”, operationally defined as the maximal frequency, amount, and duration of the heaviest use in a person’s lifetime, up to the date of ascertainment.30,31
This study was therefore designed to examine the association of PDYN 68 bp repeats with both categorical diagnoses of cannabis dependence, as well as with dimensional measures of cannabis exposure, in African American men and women.
Methods
Participants
This was a case-control study, with a cohort of sequentially ascertained African American participants, examined as outpatients in a research hospital setting. “Cases” are defined as participants with an addictive disease diagnosis (Diagnostic and Statistical Manual of Mental Disorders-IV [DSM IV] criteria), and “controls” are participants without an addictive disease diagnosis. This study is a part of a project on the association of genetic markers with specific drug addictions. The total sample reported herein consisted of 487 consecutively ascertained African American participants. After exclusion of relatives and subjects with African genetic contribution below a set threshold (<50%), 476 participants, 354 African American cases with cannabis-, alcohol-, cocaine-, or opioid-dependence diagnoses and 122 African American normal volunteers (controls) were used for further analyses (Table 1). The mean age at the time of ascertainment was 44.0 (SD ±10.0) years in the cases and 30.9 (SD ±9.6) years in the controls.
  | Table 1 Demographics of the African American cohort in the study |
Assessment of ancestry contribution, genome sharing, familial relationship, and duplicates in the study cohort
As mentioned earlier, African American ancestry was determined from questionnaires on family origin and confirmed by analysis using the software Structure 2.232 of 155 ancestral informative markers, as described previously.33 Five self-reported African American participants with <50% African ancestry contribution were excluded. Familial relationships were assessed via pairwise identity by descent analyses using Plink version 1.9 software, as part of a different study.33 Six participants with >20% genome sharing (PI_HAT >0.2) with other participants in this cohort were excluded, as they were relatives.
Recruitment, inclusion and exclusion criteria
Participants were recruited through posted notices and newspaper advertisements in the New York City area; these notices and the overall protocol were approved by the Rockefeller University Hospital Institutional Review Board.
Study inclusion criteria
Participants had to be ≥18 years of age as well as competent enough to understand procedures and to sign and understand the informed consent in English.
Study exclusion criteria
Participants with uncontrolled schizophrenia or other psychotic disorders, which prevented them from understanding study procedures or informed consent, were excluded.34
Participants were excluded from the control category if they had any DSM IV addictive disease diagnosis (of abuse or dependence) or any of the following: 1) current or continuing abuse of alcohol, or at least one instance of drinking to intoxication during the previous 30 days; 2) any use of illicit drugs, including opiates, cocaine, and amphetamines (but excluding cannabis), during the 30 days prior to ascertainment; 3) if they had used illicit drugs (with the exception of cannabis) for at least three times a week for a period of at least 1 month, in their lifetime; and 4) if they had used cannabis on >12 days in the 30 days prior to ascertainment.34
As mentioned earlier, participants were also recruited through postings at several addictive disease clinics in New York City. Cases with addictive diseases were also ascertained as part of community recruitment in the same urban area. All ascertainments were completed during a standardized face-to-face interview with a trained licensed PhD psychologist, medical doctor, nurse practitioner, or registered nurse.
Measures
Structured Clinical Interview for DSM IV Axis I Disorders semistructured interview for DSM IV criteria35 and the KMSK scales for maximal lifetime exposure to specific drugs (focusing on cannabis in this study) were used.30 The cannabis KMSK scale is an ordinal integer measure of maximal self-exposure (scale range: 0–14) and can be rapidly determined in a clinical interview. A KMSK score of zero indicates that the participant had no lifetime use of cannabis, and this score increases ordinally up to a score of 14. The cannabis KMSK score is the composite sum of three subscores, characterizing the time in a participant’s life when use was the heaviest. These subscores are as follows: a) frequency of use (eg, per day or week; score range: 0–6); b) duration of use (eg, in months or years; score range: 0–3); and c) amount of use in a sitting (eg, in “joints”; score range: 0–5). The full text of the scale can be freely accessed at: http://lab.rockefeller.edu/kreek/kmsk. The scale has been used to measure drug exposure in different clinical populations and also as a dimensional phenotype in genetic association studies.31,36,37 A recent recalculation of the receiver operating characteristic (ROC) curve was carried out for a large sample in this urban area, for cannabis KMSK scores and the DSM IV cannabis-dependence diagnosis. This ROC analysis resulted in a cannabis KMSK score ≥10 as the “cut point” for optimal concurrent validity (unpublished data). The KMSK questionnaires also collected the age of first use and the age of onset of heaviest use for a substance (in whole years).
Genotyping of the PDYN 68 bp tandem repeat polymorphism
Genomic DNA was extracted from peripheral blood lymphocytes using a salt precipitation-based DNA extraction procedure. Approximately 100 ng of genomic DNA was amplified by polymerase chain reaction (PCR) using oligonucleotide primers for the promoter region of the prodynorphin gene. The primer sequences were as follows: forward primer 5′-CTGTGTATGGAGAGGCTGAGT-3′; and reverse primer 5′-AGGCGGTTAGGTAGAGTTGTC-3′; these flank the PDYN 68 bp tandem repeat region. A step-down PCR was performed for 35 cycles of 30 seconds at 94°C, 30 seconds at 66°C, 30 seconds at 63°C, 30 seconds at 60°C, 30 seconds at 57°C, 30 seconds at 52°C, and 30 seconds at 72°C, followed by 6 minutes at 72°C using an AmpliTaq Gold kit (Applied Biosystems, Foster City, CA, USA). PCR products were electrophoresed on a 2.5% agarose gel. PDYN genotypes were determined according to the size and number of PCR DNA fragments.
The lengths of the 68 bp tandem repeat PCR DNA fragments are expected to be 273 bp (one copy of the repeat), 341 bp (two copies), 406 bp (three copies), and 473 bp (four copies). For further analysis, genotypes were grouped as short/short “SS” (1,1; 1,2; 2,2 copies), short/long “SL” (1,3; 1,4; 2,3; 2,4 copies), and long/long “LL” (3,3; 3,4; 4,4 copies) repeat alleles.16 Several prior studies have used an “L recessive” model in genotype analyses for the PDYN 68 bp, ie, SS+SL versus LL.38
Statistical analyses
Contingency analysis for categorical diagnoses of cannabis dependence and PDYN genotype were carried out with Fisher’s exact test. Cannabis KMSK scores (scale range: 0–14) were analyzed with Mann–Whitney U-tests, as they were not normally distributed. Cannabis KMSK scores were also cumulated into three simple “bins”, divided into scores of 0–4, 5–9, and 10–14. These “bins” thus provide a simplified ordinal measure of cannabis exposure as “low”, “medium”, and “large”, respectively, and were examined with chi-square analysis. Moreover, the “high”-exposure bin contains the participants with cannabis KMSK scores greater than or equal to the cut point for optimal concurrent validity with the DSM IV cannabis-dependence diagnosis (ie, cannabis KMSK scores ≥10; mentioned earlier). Logistic regressions were used to examine the ORs of cannabis KMSK scores in the “high-exposure” bin, using genotype as a predictor, with and without gender as covariate. All statistical analyses were carried out with Prism (Graphpad Software, Inc., La Jolla, CA, USA) or with Statistica (Tibco) software. The alpha level of significance was set at p≤0.05.
Results
Participant demographics
All 476 participants were African Americans, 270 males and 206 females, sequentially ascertained from April 4, 2002 to August 1, 2013. The demographic data of the cohort are shown in Table 1.
Cannabis KMSK scores in cases with cannabis dependence versus controls
As expected, cases with cannabis-dependence diagnoses (total n=110, KMSK data available from n=106) had higher cannabis KMSK scores than controls (normal volunteers, n=122). Median cannabis KMSK scores for cases with cannabis dependence were 14 (interquartile range [IQR]: 12–14) and for controls, they were zero (IQR: 0–0) (Mann–Whitney U=304.5; p<0.0001).
Ages of onset of heaviest use of cannabis and other drugs, in cases with cannabis dependence
In the cases with cannabis-dependence diagnosis, the age of onset of heaviest use of cannabis was in adolescence (mean: 16.8 years [95% confidence limits {CL}: ±2.03 years]), and preceded that for the other major drugs studied in this cohort (ie, alcohol, cocaine, and heroin), which occurred in early adulthood, from age 21 years onward (Friedman’s analysis of variance [ANOVA] statistic=72.83; p<0.0001 and Dunn’s post hoc tests; data not shown).
PDYN genotype and cannabis dependence as a categorical diagnosis
Genotypic frequencies in the control group (n=122) were 44% for LL and 22% for SS genotypes and, in the cannabis-dependent group (n=110), the frequencies were 36% and 29%, respectively. Chi-square analyses conducted either for the total group, or separately for men and women, did not show significant association of the PDYN 68 bp repeat genotype with cannabis-dependence diagnosis (Table S1).
Cannabis KMSK scores in males versus females
In the whole cohort, the median cannabis KMSK score was higher for males (median=11, IQR: 4–14; total n=270 with cases and controls combined; KMSK data available for n=268) than for females (median=3, IQR: 0–12; total n=206; KMSK data available for n=200) (Mann–Whitney U=18,659; p<0.0001). All the following genetic association analyses were therefore carried out after gender stratification.
Age of first use of cannabis and the PDYN 68 bp genotype in males
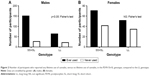
Using an “L recessive” model, we analyzed the ages of first use of cannabis in males with the SS+SL genotypes, versus the LL genotype, using a survival analysis. A log-rank test (Mantel–Cox) was significant (p<0.045), and the analysis indicated that the median age of cannabis first use for male participants with the SS+SL genotype was earlier than for those with the LL genotype (15 years versus 16.5 years of age) (Figure 1). The survival curves indicated that the occurrence of first use of cannabis after age of 25 years was uncommon for either genotype.
Age of onset of heaviest use of cannabis and PDYN 68 bp genotype in males
Using a similar rationale, we then compared the age of onset of heaviest cannabis use across genotypes. The result of this analysis was nonsignificant (not shown).
Genotype analysis of male participants who had never used cannabis, versus those who had used cannabis
We analyzed the PDYN genotype for participants who had never used cannabis (ie, cannabis KMSK score=0) versus those who had at least some use of cannabis in their life time (ie, cannabis KMSK score 1–14). A Fisher’s exact test for this analysis was significant (p<0.05), showing a greater proportion of participants who had ever used cannabis in the SS+SL versus the LL genotype (Figure 2).
“Low”, “medium”, and “high” cannabis exposure bins and the PDYN genotype in males
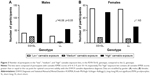
We divided the cannabis KMSK scale into three bins, denoting “low”, “medium”, and “high” exposure (ie, scores 0–4, 5–9, and 10–14, respectively). As mentioned earlier, the high-exposure bin (KMSK scores 10–14) also contains all scores greater than or equal to the cut point for optimal concurrent validity with the cannabis-dependence diagnosis. A contingency analysis of these three cannabis exposure bins and the PDYN genotype (ie, SS+SL versus LL) was significant; χ2=6.08 (p<0.05) (Figure 3). The proportion of participants in the high cannabis exposure group was greater in the SS+SL versus LL genotype.
Genotype analyses in females
Similar genotype analyses were carried out in females. None of these analyses reached significance when examining the female participants in this cohort (Figures 1–3).
Logistic regressions
The SS+SL genotype was a positive predictor of odds of having a cannabis KMSK score in the “high”-exposure bin, compared to the LL genotype as the reference group (OR: 1.47 [95% CL: 1.01–2.13]; p<0.045). However, this effect of genotype was not significant when gender was included as a covariate (p=0.142; not significant [ns]). In this regression analysis, males had greater odds of a cannabis KMSK score in the “high”-exposure bin, compared to females as the reference group (OR: 2.76 [95% CL: 1.90–4.03]; p<0.0001).
Discussion
We found that the PDYN 68 bp repeat genotype was not associated with odds of categorical diagnoses of cannabis dependence, in either males or females, in this cohort composed of African Americans. However, we found that in males, the PDYN 68 bp genotype was associated with a specific pattern of cannabis first use and of maximum lifetime exposure. Thus, male participants with the LL genotype had a lower probability of any use of cannabis, compared to those with the SS+SL genotype. Furthermore, when cannabis use did commence in males with the LL genotype, it did so at a later age, compared to those with the SS+SL genotype. We also found that males with the LL genotype had a decreased probability of reaching “high” cannabis lifetime exposure, compared to those with the SS+SL genotype. Studies have shown that earlier first use of cannabis is associated with greater risk of heavy use of this substance.39,40 Of note, none of the above genetic associations were detected in females. This study therefore provides the first data on genetic associations of the functional 68 bp PDYN genotype, suggesting that this genotype influences the early stages of cannabis use in males and, potentially, the progression to heavy exposure.41–43
As mentioned earlier, dynorphins are endogenous KOR agonists and have countermodulatory roles on dopaminergic neurotransmission, a major neurobiological mechanism underlying the rewarding effects of drugs of abuse (including cannabis) and natural reinforcers.13,44–48 KOR/dynorphin systems also mediate other behavioral and mood functions,8,49 which could also underlie particular stages of cannabis use. In vitro and postmortem brain studies have demonstrated an effect of PDYN 68 bp repeat polymorphisms on PDYN mRNA levels.17–20 Earlier in vitro studies have shown that gene reporter constructs containing three or four copies of this 68 bp tandem repeat (compared to one or two copies) led to higher expression of the reporter gene in mouse neuroblastoma cells (NG108-15) when stimulated by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate.17 Subsequently, we found cell-specific effects of the 68 bp repeats on the transcriptional activity of the PDYN promoter.19 Specifically, in human neuronal SK-N-SH and H69 cells, three or four repeats led to lower expression of luciferase than did one or two repeats. The opposite effect was found in the kidney-derived HEK293 cells.19 Interestingly, in previous studies, three or four copies of the PDYN repeats were found to be a risk factor for the development of cocaine-dependence diagnoses and dual cocaine/alcohol-dependence diagnoses.16,23 In contrast, in this study, we found that one or two copies of the repeats (SS genotype), presumably yielding higher levels of endogenous dynorphin peptides, are associated with increased probability of any use of cannabis, as well as earlier first use of cannabis, in males. A different genetic association study found that persons with one or two copies of the repeats exhibited an intermediate phenotype that included greater propensity for disinhibited behavior.38 Based on the latter study, it may be hypothesized that disinhibited behavior in the SS genotype could manifest as increased probability of any cannabis use, or earlier first use of cannabis, as found herein. Alternatively, it may be hypothesized that high PDYN expression can result in changes in mood and dysphoria, in addition to potentially increasing the risk of early cannabis use and more regular cannabis use.
Methodological considerations and limitations
This study had a relatively small cohort; however, this limitation may be mitigated by the fact that all participants were ascertained in a homogeneous setting, in face–to-face interviews with trained licensed clinicians. We also note that, as is common in this clinical field, many participants also had other DSM IV substance-dependence diagnoses, in addition to cannabis dependence.50 However, we found that the age of onset of heaviest cannabis use (in adolescence) preceded that for alcohol, cocaine, or heroin in this cohort (from young adulthood onward). Therefore, genetic associations to cannabis exposure found herein were unlikely to be confounded by heavy use of these other drugs, since this occurred later in the life span. Overall, the KMSK scores characterize the period in a participant’s life when use of a specific drug is heaviest, and this occurred for cannabis prior than for any of the other major drugs used by cases in this cohort. The age at ascertainment of cases was older than that of controls herein (44 and 30.9 years, respectively). However, herein, we found that first use of cannabis was uncommon after 25 years of age. Therefore, it is unlikely that the observed age-related differences between cases and controls would have resulted in miscategorizing of individuals who would have developed a diagnosable CUD later in their life span.
All the present associations were found in males but not in females, and this suggests that there are gender-specific factors that play a role in the impact of this functional PDYN polymorphism on cannabis use. It cannot be excluded that this lack of association in females was due to the lower number of female-versus-male cases with cannabis dependence found herein. Sex-specific effects of the KOR/PDYN and CB1-r receptor systems have been reported preclinically.49,51
Conclusion
This is the first study to examine the impact of functional PDYN 68 bp repeat polymorphisms on nonmedical use of cannabis. We did not detect genetic associations with categorical diagnoses of cannabis dependence, but only with specific aspects of cannabis use, including first use, and differential levels of exposure. Furthermore, in our study, these associations were observed only in males. This suggests that these PDYN 68 bp polymorphisms may affect behaviors involved in early stages of nonmedical cannabis use and potentially lead to increasing self-exposure. These data may eventually lead to improvements in personalized medicine for the prevention and treatment of highly prevalent CUDs.1,3
Acknowledgments
We thank all the clinical staff of the Laboratory of the Biology of Addictive Diseases for recruitment and participant ascertainment. We thank Dr Orna Levran for analyses of ancestry and familial relationship in the cohort studied. Mr Matthew Randesi is gratefully acknowledged for help in PDYN polymorphism genotyping. This paper was funded by the Dr Miriam and Sheldon G Adelson Medical Research Foundation and by a National Institutes of Health – Clinical and Translational Science Awards (NIH-CTSA) grant to the Rockefeller University Hospital (1UL1TR001866).
Disclosure
The authors report no conflicts of interest in this work.
References
Kerridge BT, Pickering R, Chou P, Saha TD, Hasin DS. DSM-5 cannabis use disorder in the national epidemiologic survey on alcohol and related conditions-III: gender-specific profiles. Addict Behav. 2018;76:52–60. | ||
Blanco C, Hasin DS, Wall MM, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a us national longitudinal study. JAMA Psychiatry. 2016;73(4):388–395. | ||
Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73(3):292–297. | ||
Grim TW, Morales AJ, Gonek MM, et al. Stratification of cannabinoid 1 receptor (CB1R) agonist efficacy: manipulation of CB1R density through use of transgenic mice reveals congruence between in vivo and in vitro assays. J Pharmacol Exp Ther. 2016;359(2):329–339. | ||
Katia B. Interactions of the opioid and cannabinoid systems in reward: insights from knockout studies. Front Pharmacol. 2015;6:6. | ||
Zimmer A, Valjent E, Konig M, et al. Absence of delta-9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. J Neurosci. 2001;21(23):9499–9505. | ||
Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22(3):1146–1154. | ||
Reed B, Fang N, Mayer-Blackwell B, et al. Chromatin alterations in response to forced swimming underlie increased prodynorphin transcription. Neuroscience. 2012;220:109–118. | ||
Spangler R, Unterwald EM, Kreek MJ. “Binge” cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19(4):323–327. | ||
Carlezon WA Jr, Beguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther. 2009;123(3):334–343. | ||
Butelman ER, Yuferov V, Kreek MJ. Kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35(10):587–596. | ||
Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4(5):543–546. | ||
Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244(3):1067–1080. | ||
Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl). 2004;172(4):422–429. | ||
Horikawa S, Takai T, Toyosato M, et al. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983;306(5943):611–614. | ||
Williams TJ, LaForge KS, Gordon D, et al. Prodynorphin gene promoter repeat associated with cocaine/alcohol codependence. Addict Biol. 2007;12(3–4):496–502. | ||
Zimprich A, Kraus J, Woltje M, Mayer P, Rauch E, Hollt V. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J Neurochem. 2000;74(2):472–477. | ||
Babbitt CC, Silverman JS, Haygood R, Reininga JM, Rockman MV, Wray GA. Multiple functional variants in cis modulate PDYN expression. Mol Biol Evol. 2010;27(2):465–479. | ||
Rouault M, Nielsen DA, Ho A, Kreek MJ, Yuferov V. Cell-specific effects of variants of the 68-base pair tandem repeat on prodynorphin gene promoter activity. Addict Biol. 2011;16(2):334–346. | ||
Yuferov V, Ji F, Nielsen DA, et al. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34(5):1185–1197. | ||
Fang J, Yin L, Cao S, Liao Y, Xue C. Dye-sensitized Pt@Tio2 core-shell nanostructures for the efficient photocatalytic generation of hydrogen. Beilstein J Nanotechnol. 2014;5:360–364. | ||
Liu Y, Kieslich CA, Morikis D, Liao J. Engineering pre-SUMO4 as efficient substrate of SENP2. Protein Eng Des Sel. 2014;27(4):117–126. | ||
Dahl JP, Weller AE, Kampman KM, et al. Confirmation of the association between a polymorphism in the promoter region of the prodynorphin gene and cocaine dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;139B(1):106–108. | ||
Wei SG, Zhu YS, Lai JH, Xue HX, Chai ZQ, Li SB. Association between heroin dependence and prodynorphin gene polymorphisms. Brain Res Bull. 2011;85(3–4):238–242. | ||
Ray R, Doyle GA, Crowley JJ, et al. A functional prodynorphin promoter polymorphism and opioid dependence. Psychiatr Genet. 2005;15(4):295–298. | ||
Nikoshkov A, Drakenberg K, Wang X, Horvath MC, Keller E, Hurd YL. Opioid neuropeptide genotypes in relation to heroin abuse: dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proc Natl Acad Sci U S A. 2008;105(2):786–791. | ||
Sloan ME, Gowin JL, Yan J, et al. Severity of alcohol dependence is associated with the fatty acid amide hydrolase Pro129thr missense variant. Addict Biol. 2018;23(1):474–484. | ||
Savage JE, Sawyers C, Roberson-Nay R, Hettema JM. The genetics of anxiety-related negative valence system traits. Am J Med Genet B Neuropsychiatr Genet. 2017;174(2):156–177. | ||
Liang PW, Liao CY, Chueh CC, et al. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv Mater. 2014;26(22):3748–3754. | ||
Kellogg SH, McHugh PF, Bell K, et al. The Kreek-McHugh-Schluger-Kellogg Scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69(2):137–150. | ||
Crystal HA, Hamon S, Randesi M, et al. A C17t polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African American women. Addict Biol. 2012;17(1):181–191. | ||
Zhou Z, Yuan Q, Mash DC, Goldman D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc Natl Acad Sci U S A. 2011;108(16):6626–6631. | ||
Levran O, Randesi M, Li Y, et al. Drug addiction and stress-response genetic variability: association study in African Americans. Ann Hum Genet. 2014;78(4):290–298. | ||
Bond C, LaForge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95(16):9608–9613. | ||
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-Tr Axis I Disorders, Research Version, Patient Edition (SCID-I/P; Version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. | ||
Jackson CB, Varon J, Ho A, Marks KM, Talal AH, Kreek MJ. Identification of substance use and dependence among patients with viral hepatitis. Dig Liver Dis. 2010;42(9):650–656. | ||
Tang YL, Khoury L, Bradley B, Gillespie CF, Ressler KJ, Cubells JF. Substance use disorders assessed using the Kreek-Mchugh-Schluger-Kellogg (KMSK) scale in an urban low-income and predominantly African American sample of primary care patients. Am J Addict. 2011;20(3):292–299. | ||
Flory JD, Pytte CL, Hurd Y, Ferrell RE, Manuck SB. Alcohol dependence, disinhibited behavior and variation in the prodynorphin gene. Biol Psychol. 2011;88(1):51–56. | ||
Behrendt S, Wittchen HU, Hofler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol Depend. 2009;99(1–3):68–78. | ||
Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addict Behav. 2009;34(3):319–322. | ||
Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8(11):1450–1457. | ||
Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction. 2009;104(4):518–532. | ||
Egervari G, Jutras-Aswad D, Landry J, et al. A functional 3′UTR polymorphism (rs2235749) of prodynorphin alters microrna-365 binding in ventral striatonigral neurons to influence novelty seeking and positive reward traits. Neuropsychopharmacology. 2016;41(10):2512–2520. | ||
Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89(6):2046–2050. | ||
Zernig G, Pinheiro BS. Dyadic social interaction inhibits cocaine-conditioned place preference and the associated activation of the accumbens corridor. Behav Pharmacol. 2015;26(6):580–594. | ||
Adamantidis AR, Tsai HC, Boutrel B, et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31(30):10829–10835. | ||
Knoll AT, Muschamp JW, Sillivan SE, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70(5):425–433. | ||
Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276(5321):2048–2050. | ||
Chartoff EH, Mavrikaki M. Sex differences in kappa opioid receptor function and their potential impact on addiction. Front Neurosci. 2015;9:466. | ||
McCabe SE, West BT, Jutkiewicz EM, Boyd CJ. Multiple DSM-5 substance use disorders: a national study of us adults. Hum Psychopharmacol. Epub 2017 Jul 27. | ||
Rubino T, Vigano D, Realini N, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33(11):2760–2771. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.