Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Gender differences in the T-cell profiles of the airways in COPD patients associated with clinical phenotypes
Authors Forsslund H, Yang M, Mikko M, Karimi R, Nyrén S, Engvall B, Grunewald J, Merikallio H, Kaarteenaho R , Wahlström J, Wheelock ÅM, Sköld CM
Received 26 May 2016
Accepted for publication 22 July 2016
Published 20 December 2016 Volume 2017:12 Pages 35—48
DOI https://doi.org/10.2147/COPD.S113625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Helena Forsslund,1 Mingxing Yang,1 Mikael Mikko,1 Reza Karimi,1 Sven Nyrén,2 Benita Engvall,1 Johan Grunewald,1 Heta Merikallio,1,3 Riitta Kaarteenaho,3–5 Jan Wahlström,1 Åsa M Wheelock,1 C Magnus Sköld1
1Department of Medicine Solna and Centre for Molecular Medicine, Respiratory Medicine Unit, 2Department of Molecular Medicine and Surgery, Karolinska Institutet and Karolinska University Hospital Solna, Stockholm, Sweden; 3Respiratory Research Unit and Medical Research Center Oulu, University of Oulu and Oulu University Hospital, Oulu, Finland; 4Unit of Medicine and Clinical Research, Pulmonary Division, University of Eastern Finland, 5Center for Medicine and Clinical Research, Division of Respiratory Medicine, Kuopio University Hospital, Kuopio, Finland
Abstract: T lymphocytes are believed to play an important role in the pathogenesis of chronic obstructive pulmonary disease (COPD). How T cells are recruited to the lungs and contribute to the inflammatory process is largely unknown. COPD is a heterogeneous disease, and discriminating disease phenotypes based on distinct molecular and cellular pathways may provide new approaches for individualized diagnosis and therapies. Bronchoalveolar lavage (BAL) and blood samples were obtained from 40 never-smokers, 40 smokers with normal lung function, and 38 COPD patients. T-cell chemokine receptor expression was analyzed with flow cytometry, and soluble BAL cytokines and chemokines were measured using a cytokine multiplex assay. Correlations with gender and clinical characteristics including lung imaging were investigated using multivariate modeling. Th1/Tc1- and Th2/Tc2-associated soluble analytes and T-cell chemokine receptors were analyzed as cumulative Th1/Tc1 and Th2/Tc2 immune responses. A higher expression of chemokine receptor CCR5 on CD8+ T cells in BAL and higher percentage of CXCR3+CD8+ T cells in blood was found in female smokers with COPD compared to those without COPD. CCR5 expression on CD4+ and CD8+ T cells was lower in BAL from male smokers with COPD compared to those without COPD. Among female smokers with COPD, Th1/Tc1 immune response was linked to BAL macrophage numbers and goblet cell density, and Th2/Tc2 response was associated with the measures of emphysema on high-resolution computed tomography. The highly gender-dependent T-cell profile in COPD indicates different links between cellular events and clinical manifestations in females compared to males. Our findings may reveal mechanisms of importance for the difference in clinical course in female COPD patients compared to males.
Keywords: bronchoalveolar lavage, chemokines, cytokines, Th1/Th2
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease characterized by airflow limitation that is not fully reversible. In the industrialized world, the major risk factor for COPD is tobacco smoking. Historically considering a males’s disease, females have now surpassed males in COPD prevalence and hospitalizations.1–3 A large number of studies report the existence of gender differences in the manifestations of the disease, including a faster decline in lung function and worse symptoms among females, who also seem to be more susceptible to the toxic effects of cigarette smoke.4,5
The inflammation in COPD is characterized by increased number of macrophages, neutrophils, and T lymphocytes in the lung. The T lymphocytes are mainly CD8+ T-cytotoxic cells (Tc), but CD4+ T-helper (Th) cells are also increased in more severe disease. In the initial response to cigarette smoke, airway epithelial cells and macrophages induce airway inflammation, both through direct effects and through the recruitment of T cells from the periphery. CD8+ cells are able to destroy lung parenchyma through cytolytic activities while CD4+ cells recruit and activate other immune cells, including neutrophils, perpetuating the sustained inflammatory process. As T cells respond following antigen recognition, they differentiate in response to the inflammatory milieu to mount a response with a specific cytokine profile.6–9
A large body of evidence indicates a skewing toward a Th1/Tc1 response in COPD.6 These cells typically produce cytokines such as interleukin-2 (IL-2), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). However, there are studies that advocate that Th2/Tc2 cells, characterized by their expression of IL-4 and IL-13, can also be involved.10–12
T cells and other immune cells are directed to the site of inflammation by the binding of chemokines to cell-surface chemokine receptors. Th1/Tc1 cells can express CXCR3 and CCR5, which bind specifically to CXCL9–11 and CCL3–5, respectively. Th2/Tc2 cells can express CXCR4 binding specifically to CXCL12.13 The resulting chemokine-orchestrated cell recruitment is vital for the protection against the cigarette smoke-induced events, which also contributes to disease development. Consequently, a substantial interest in developing receptor antagonists or chemokine blockers for new therapeutic solutions has evolved.14
In this study, factors of plausible importance for the recruitment of T cells to the lung were studied. To do this, T cells and soluble analytes in bronchoalveolar lavage (BAL)15 and blood from COPD patients, healthy smokers, and never-smokers were analyzed. It was hypothesized that patterns of inflammatory markers involved both in the response to cigarette smoke and in COPD in the lower airways may help reveal mechanistic gender differences and characterize disease phenotypes.
Materials and methods
Study subjects and patients
The subjects included in this study were part of the Karolinska COSMIC cohort (www.ClinicalTrials.gov/ct2/show/study/NCT02627872), consisting of 40 never-smokers, 40 smokers with normal lung function (smokers), and 38 COPD patients with GOLD stage I–II. Detailed characteristics of the subjects have been summarized in Table 116,17 and also in the Supplementary materials. High-resolution computed tomography (HRCT) was performed by inspiratory and expiratory scans as described previously.18 All participants provided written informed consent, and the study was approved by the Regional Ethics Committee in Stockholm on October 26, 2006 (ref: 2006/959-31/1).
Bronchoscopy with BAL, processing of BAL and blood cells, and cytospin preparations from bronchial brushings were performed as described previously.19–21 BAL characteristics, including BAL-cell differential counts, have been described elsewhere.16
Flow cytometric analysis of T cells
BAL and blood cells were analyzed for their expression of the cell surface molecules CD3, CD4, CD8, CXCR3, CCR5, CXCR4, and CD69 using multicolor flow cytometry. Prior to monoclonal antibody staining, macrophage depletion from the BAL cells was performed as described previously.22 BAL cells (1×106/100 μL cell wash) and 100 μL of blood were stained with monoclonal antibodies and analyzed with flow cytometry as described in the Supplementary materials. For statistical purposes, at least 50 detected events in the final gates for a specific analyte, as well as a clear separation between the positive and negative cell populations in the median fluorescence intensity (MFI) analysis were required for inclusion.
Multiplex analysis of inflammatory mediators
In order to investigate the recruitment of peripheral T cells to the lungs in smokers and COPD patients, combined analyses of selected chemokine receptors on T cells and chemokines they produce were performed, and the Th1/Th2 balance between chemokine receptors, cytokines and chemokines typically associated with the Th1/Tc1 and Th2/Tc2 profiles, respectively, were investigated. In addition to the Th1/Th2 panels, a set of variables directly associated with macrophage and epithelial processes in COPD was selected. Macrophage panel: IL-1β, CXCL9, and vascular endothelial growth factor (VEGF). These mediators can be produced and released by macrophages and have been postulated to play an important role in the pathophysiology of COPD. The epithelial cell panel is transforming growth factor-β2 (TGF-β2), TGF-β3, and IL-1β. IL-1β and TGF-β are important products of epithelial cells and are indicated to have important roles in the peripheral airways in COPD patients. TGF-β is increased in the airway epithelial cells in COPD patients and is suggested to be a mediator of peripheral airway fibrosis in COPD. As such, concentrated BAL supernatants were measured for the chemokines CXCL8, CXCL9, CXCL10, CXCL12, CCL3, CCL4, CCL5, the cytokines IL-1β, IL-4, IL-12 (p70), IL-13, IFN-γ, TNF-α, and the growth factors VEGF and TGF-β1, 2, and 3 using multiplex magnetic bead-based assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA) through LUMINEX® xMAP® technology. For further information, see Supplementary materials.
Statistical analyses
Univariate analyses were performed with GRAPHPAD PRISM® version 5.02 (GraphPad Software Inc., San Diego, CA, USA). Comparisons between two independent groups were performed by using Mann–Whitney U-test, and for more than two groups, one-way analysis of variance (ANOVA) test followed by Dunn’s multiple comparison test for non-parametric data (P<0.05) was performed.
Multivariate data analyses were performed on all T-cell variables and soluble analytes using SIMCA® version 14.0 (Umetrics AB, Umeå, Sweden) both by unsupervised (principal component analysis [PCA]) and supervised (orthogonal projections to latent structures [OPLS])23 approaches (for more details, see Supplementary materials). Model performance is reported in terms of cumulative correlation coefficients (R2), predictive performance based on seven-fold cross validation (Q2), and cross-validated ANOVA (CV-ANOVA) P-values for the OPLS models. Variable selection for model optimization was performed iteratively on the basis of the scaled loadings of the predictive component of the OPLS models (P[corr]).23
Pathway-based correlation analyses were performed on clinical data (for information on included clinical data, see www.ClinicalTrials.gov/ct2/show/study/NCT02627872) and immune cell pathways using partial least squares regression. Soluble analytes and T-cell chemokine receptors were structured into four groups; variables primarily related to Th1/Tc1 cell, Th2/Tc2 cell, macrophage, and epithelial cell processes, respectively (Table 2).
Results
T-cell chemokine receptor expression
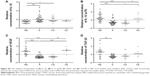
Univariate analysis revealed higher median CXCR3 percentages among CD8+ T cells than among CD4+ T cells in BAL in all study groups. The percentage of CXCR3+CD4+ T cells in BAL was significantly lower in smokers compared to never-smokers (P<0.01) (Figure 1A). No differences in the proportions of CXCR3+CD8+ T cells were seen between the study groups (Figure 1B).
The expression of CCR5 on CD4+ T cells in BAL was significantly higher in smokers compared to never-smokers (P<0.01) (Figure 1C). No significant differences between the groups were observed in the CD8+ T-cell population (Figure 1D). In blood, the only significant difference was a decreased expression of CCR5 on CD8+ T cells from smokers compared to never-smokers (P<0.01) (data not shown).
No differences between the study groups were seen with regard to the expression of the Th2/Tc2 cell marker CXCR4 on CD4+ and CD8+ BAL T cells (Figure 1E and F).
Inflammatory mediators in BAL supernatant
The relative concentration of the Th2/Tc2-associated cytokine IL-13 was lower in smokers and COPD smokers compared to never-smokers (P<0.01 and P<0.001, respectively), as were the Th2/Tc2-associated chemokine CXCL12 in smokers compared to never-smokers (P<0.05) (Figure 2A and B). There were no significant differences between the study groups in IL-4 levels, neither in the Th1/Tc1-associated TNF-α, IFN-γ, CCL3, -4, -5, or CXCL9, -10 (Figure S1A–H).
Compared to never-smokers, the relative concentration of the pro-inflammatory cytokine IL-1β was higher in BAL from smokers (P<0.001) and COPD smokers (P<0.05). Levels of IL-12 (p70), by contrast, were lower in both the groups of smokers compared to both never-smokers (P<0.001) and COPD ex-smokers (P<0.05) (Figure S2A and B).
The concentration of VEGF was significantly lower in smokers compared to never-smokers and COPD ex-smokers (P<0.001 for both) and in COPD smokers compared to never-smokers (P<0.001) and COPD ex-smokers (P<0.01). Of the three TGF-β isotypes measured, only TGF-β2 proved to be significantly different. The levels were lower in smokers compared to never-smokers (P<0.01) (Figure S2C and D).
Smokers versus COPD smokers
Multivariate modeling comparing T-cell subsets and soluble analytes of smokers with normal lung function versus smokers with COPD was performed in order to discriminate disease-related factors separate from the effects of smoking. Supervised modeling using OPLS-DA® resulted in a significant separation between smokers and COPD smokers, however, with a low predictive value (R2=0.46, Q2=0.29, P[CV-ANOVA] =0.006).
Gender differences
In order to assess gender-associated differences, females and males were analyzed separately. Comparison of all T-cell subsets and soluble analytes revealed a strong discrimination between female smokers and female COPD smokers (R2=0.50, Q2=0.41, P[CV-ANOVA] =8×10−4). Eleven variables were driving the separation between the two groups (Figure 3A). A less robust model was found for the corresponding male cohort, comparing male COPD smokers and male smokers (R2=0.32, Q2=0.25, P[CV-ANOVA] =0.02) (Figure 3B). Univariate analysis confirmed that three of the eleven variables were significantly higher in female COPD smokers compared to female smokers: the CCR5 expression on CD8+ T cells in both blood (P<0.01) and BAL (P<0.05), and the percentage of CXCR3+ among CD8+ T cells (P<0.05) (Figure 4A–C).
Among eight suggested variables driving the separation between male smokers and male COPD smokers, the univariate analysis revealed significantly lower MFI values of CCR5 on CD8+ and CD4+ T cells in BAL from the COPD smokers compared to smokers (P<0.05 and P<0.01, respectively) (Figure 4D and E).
Of the significantly different variables within each of the two OPLS® models described earlier, the MFI of CCR5 on CD8+ T cells in BAL was found significant in both the female and the male models. Notably, the alterations in abundances due to disease were found to be opposite, with increased levels in female COPD patients and decreased levels in male COPD patients, as highlighted by shared and unique structure analysis (Figure 5).
Subsequent multivariate correlation analysis on a pathway basis identified significant correlations between pathways and clinical parameters, especially among female COPD smokers, where the Th2/Tc2 pathway was associated with HRCT measures of emphysema, that is, an increase relative volume of lung tissue with attenuation <−950 Hounsfield units (HU),24 (r=0.80, P=0.002). Furthermore, the Th1/Tc1 pathway was associated with macrophage numbers in BAL (r=0.79, P=0.006) and with bronchial epithelial goblet cell abundance in the airway epithelium sampled from bronchial brushings in female COPD smokers (r=0.80, P=0.02). Among male smokers, a correlation between the Th2/Tc2 pathway and levels of IgG in serum was found (r=0.62, P=0.003) (Figure 6A–D). No other correlations with clinical data were found.
Discussion
This study extends previous findings on gender-dependent immune processes in COPD by demonstrating disparate profiles of T-cell recruitment to the lower airways of females and males. In addition, links between biological attributes and Th1/Tc1 or Th2/Tc2 immune patterns in respective gender are described.
An elevated expression of CCR5 on CD4+ T cells and reduced percentage of CXCR3+CD4+ T cells was found in BAL from smokers, but not from COPD smokers, compared to never-smokers. This finding suggests a CCR5-mediated recruitment of CD4+ T cells. A Th1/Tc1 response to smoking and/or that in COPD has been described previously, with infiltration of CCR5- and CXCR3-expressing T cells and higher levels of CCL5 and CXCL9–11 being observed in both the upper and the lower airways.10,25 Our findings on decreased levels of the Th2-associated IL-4 and CXCL12 in the lungs of current smokers further support the theory on a Th1 bias of smoke-induced inflammation and COPD.
There are several differences in the clinical presentation and prognosis between females and males with COPD. Extensive studies have shown that females have more severe symptoms compared to males, despite a shorter smoking history and a similar degree of obstruction and emphysema.26 Several underlying molecular reasons for this could be proposed as previously reported while analyzing BAL-cell proteome from the same cohort.17
Despite several disease-associated variables being significantly altered in the female and male models, respectively, expression of CCR5 on CD8+ T cells in BAL was the only alteration identified in both the models. Intriguingly, females and males showed opposing shifts in CCR5 abundance, due to COPD, indicating that the CCR5 receptor may play a crucial role in CD8+ T-cell recruitment to the lungs in a female-associated COPD phenotype. A functional role for CCR5 has been demonstrated at sites of inflammation. Among others, the receptor has been shown to accelerate recruitment of antigen-specific memory CD8+ T cells to the lung in response to respiratory viral infections.15,27 Fukada et al28 observed an upregulation of CCR5 expression during antigen-specific CD8+ T-cell differentiation, coupled with an increased cytotoxic activity of these cells. The higher expression of CCR5 on CD8+ T cells in females may thus imply the presence of one or several specific antigens, autologous as well as foreign, that trigger T cells to a greater extent in the lungs of females. In fact, an autoimmune component in COPD has been reported.29,30 Moreover, autoimmune diseases are in general more common in females than males.31
Intriguingly, previous studies show a relationship between gender-related hormones and lung function.32 More respiratory symptoms and deteriorating lung function are reported for post-menopausal females, and a protective role for estrogen in the development of COPD has been proposed.26 Among others, it has been shown to stimulate the expansion of CD4+CD25+ T regulatory cells33 important for controlling inflammation. Here, all female COPD smokers and half of the female smokers were post-menopausal.17 However, the observed alterations related to COPD did not display any correlations with menopause (Figure S3), which is consistent with our findings from the BAL macrophage proteome of these subjects.17
Pathway-based correlation analyses revealed a correlation between the Th2/Tc2 pathway and an HRCT-indicated emphysema among female COPD smokers. Our results implicate that the Th2/Tc2 network is associated with lung tissue destruction in females. Both Th1/Tc1 and Th2/Tc2 cytokines are probable initiators of respective downstream pathways generating emphysema.34–36 An involvement of IL-4 and IL-13 has primarily been suggested in patients exhibiting features of both COPD and asthma.37 As the relative volume with attenuation <−950 HU was fairly small in the COPD patients in this study, it is possible that our findings indicate an effect of the Th2/Tc2 pathway on the early events of emphysema. However, whether this pathway could also be linked to protective mechanisms, for instance, through cross-inhibition of type 1 T cells, is yet to be explored.
Both goblet cell density and BAL macrophage numbers correlated with the Th1/Tc1 pathway in the female smokers with COPD. The interplay between macrophages and Th1 cytokines in COPD is well established. IFN-γ is the main cytokine responsible for the activation of macrophages, and the Th1/Tc1-associated chemokines are produced by macrophages.38
The present study provides evidence for a Th1-dominated immune process, particularly through the CCR5 chemokine receptor, in the lower airways of smokers with normal lung function. We further propose a multivariate model with key factors important in the T-cell recruitment to the lung separating female smokers with normal lung function from female smokers with COPD. Associations between chemokine receptors and cytokine markers of the suggested Th2/Tc2 pathway and measures of emphysema were shown among female smokers with COPD, as well as associations between the suggested Th1/Tc1 pathway and both macrophage numbers in BAL and bronchial epithelial goblet cell density, respectively. Our findings add further support to the hypothesis that gender differences in clinical manifestations of COPD result from different cellular and biological events in the lung. An increased knowledge on the role of immune cell subtypes and inflammatory mediators in smoke-induced inflammation and COPD is central for future development of gender-specific caretaking and treatment.
Acknowledgments
The authors thank research nurses Gunnel de Forest, Heléne Blomqvist, and Margitha Dahl, as well as Louise Berg and Yvonne Sundström (Karolinska Institutet, Stockholm) for kindly providing the Bio-Rad Bio-Plex instrument and for their helpful recommendations. This study was supported by grants from the Swedish Heart-Lung Foundation, the King Oscar II Jubilee Foundation, the Mats Kleberg Foundation, King Gustaf V’s and Queen Victoria’s Freemasons’ Foundation, the Hesselmans Foundation, Sandoz A/S, Swedish Governmental Agency for Innovation Systems (VINNOVA), the Swedish Foundation for Strategic Research (SSF), European Union (EU) Fp6 Marie Curie International Reintegration Grant (IRF), the Foundation of the Finnish Anti-Tuberculosis Association, the Swedish Research Council (VR), the Stockholm County Council (ALF project), and Karolinska Institutet.
Author contributions
HF was the principal investigator and was responsible for the integrity of the data and accuracy of data analysis. ÅMW and CMS initiated and conceived the project and provided funding and coordinated the research; HF, MY, MM, JW, ÅMW, and CMS contributed to study design; HF, MY, MM, RK, SN, BE, HM, JG, JW, ÅMW, and CMS contributed to data collection and analysis; HF, JW, ÅMW, and CMS contributed to drafting the manuscript. All the authors contributed to the interpretation of data and to the critical review of the manuscript, including approval of the final version.
Disclosure
The authors report no conflicts of interest in this work.
References
The National Board of Health and Welfare (Socialstyrelsen) Hälso-och sjukvårdsrapport 2009. Socialstyrelsen; Stockholm, Sweden. Available from: http://www.socialstyrelsen.se/publikationer2009/2009-126-72. Accessed October 6, 2016. | ||
Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. MMWR Surveill Summ. 2002;51(6):1–16. | ||
Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS Data Brief. 2011;63:1–8. | ||
Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J. 2003;21(6):1017–1023. | ||
Ben-Zaken Cohen S, Pare PD, Man SF, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women: examining sex differences in cigarette smoke metabolism. Am J Respir Crit Care Med. 2007;176(2):113–120. | ||
Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378(9795):1015–1026. | ||
Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. | ||
Enelow RI, Mohammed AZ, Stoler MH, et al. Structural and functional consequences of alveolar cell recognition by CD8(+) T lymphocytes in experimental lung disease. J Clin Invest. 1998;102(9):1653–1661. | ||
Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1666–1672. | ||
Freeman CM, Curtis JL, Chensue SW. CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am J Pathol. 2007;171(3):767–776. | ||
Barcelo B, Pons J, Fuster A, et al. Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol. 2006;145(3):474–479. | ||
Barczyk A, Pierzchala W, Kon OM, Cosio B, Adcock IM, Barnes PJ. Cytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117(6):1484–1492. | ||
Syrbe U, Siveke J, Hamann A. Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol. 1999;21(3):263–285. | ||
Onuffer JJ, Horuk R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol Sci. 2002;23(10):459–467. | ||
Palmer LA, Sale GE, Balogun JI, et al. Chemokine receptor CCR5 mediates alloimmune responses in graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(3):311–319. | ||
Forsslund H, Mikko M, Karimi R, et al. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest. 2014;145(4):711–722. | ||
Kohler M, Sandberg A, Kjellqvist S, et al. Gender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):743–751. | ||
Karimi R, Tornling G, Forsslund H, et al. Lung density on high resolution computer tomography (HRCT) reflects degree of inflammation in smokers. Respir Res. 2014;15:23. | ||
Lofdahl JM, Cederlund K, Nathell L, Eklund A, Skold CM. Bronchoalveolar lavage in COPD: fluid recovery correlates with the degree of emphysema. Eur Respir J. 2005;25(2):275–281. | ||
Karimi R, Tornling G, Grunewald J, Eklund A, Skold CM. Cell recovery in bronchoalveolar lavage fluid in smokers is dependent on cumulative smoking history. PLoS One. 2012;7(3):e34232. | ||
Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 6th ed. Edinburgh: Churchill Livingstone; 2008. | ||
Wong DM, Varesio L. Depletion of macrophages from heterogeneous cell populations by the use of carbonyl iron. Methods Enzymol. 1984;108:307–313. | ||
Wheelock AM, Wheelock CE. Trials and tribulations of ‘omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol Biosyst. 2013;9(11):2589–2596. | ||
Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–192. | ||
Saetta M, Mariani M, Panina-Bordignon P, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165(10):1404–1409. | ||
Ohar J, Fromer L, Donohue JF. Reconsidering sex-based stereotypes of COPD. Prim Care Respir J. 2011;20(4):370–378. | ||
Kohlmeier JE, Miller SC, Smith J, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–113. | ||
Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J Immunol. 2002;168(5):2225–2232. | ||
Packard TA, Li QZ, Cosgrove GP, Bowler RP, Cambier JC. COPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotype. Immunol Res. 2013;55(1–3):48–57. | ||
Daffa NI, Tighe PJ, Corne JM, Fairclough LC, Todd I. Natural and disease-specific autoantibodies in chronic obstructive pulmonary disease. Clin Exp Immunol. 2015;180(1):155–163. | ||
Selmi C, Brunetta E, Raimondo MG, Meroni PL. The X chromosome and the sex ratio of autoimmunity. Autoimmun Rev. 2012;11(6–7):A531–A537. | ||
Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11:24. | ||
Tai P, Wang J, Jin H, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214(2):456–464. | ||
Grumelli S, Corry DB, Song LZ, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1(1):e8. | ||
Shan M, Cheng HF, Song LZ, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1(4):4ra10. | ||
Wang Z, Zheng T, Zhu Z, et al. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192(11):1587–1600. | ||
Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106(9):1081–1093. | ||
Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118(11):3546–3556. |
Supplementary materials
Materials and methods
Study subjects and patients
To detect inflammatory processes related to COPD and not smoking, smokers with normal lung function and COPD patients were matched for smoking history in terms of pack-years. Patients treated with oral or inhaled corticosteroids, with a history of allergy or asthma, as stated in questionnaires, or with exacerbations during the last 3 months were not included. In vitro screenings for the presence of specific immunoglobulin E (IgE) antibodies (PHADIATOP®; Pharmacia, Uppsala, Sweden) were negative. Reversibility test was performed after inhalation of two doses of 0.25 mg terbutaline (BRICANYL® TURBUHALER®; AstraZeneca, Södertälje, Sweden). Serum IgG was analyzed according to routines at Karolinska University Laboratory, Department of Clinical Chemistry.
Flow cytometric analysis of T cells
Bronchoalveolar lavage (BAL) and blood cells were stained with antibodies against the surface molecules CD3 (Pacific Blue), CD4 (allophycocyanin-Cy7), CD8 (Amcyan), CXCR3 (allophycocyanin), CCR5 (phycoerythrin), CXCR4 (phycoerythrin-Cy5), and CD69 (fluorescein isothiocyanate) or with matched isotype controls (Becton Dickinson [BD], Mountain View, CA, USA) for 25 minutes in the dark (BAL cells at 4°C and blood at room temperature). The blood cells were thereafter incubated with fluorescence-activated cell sorting lysing solution (BD) for 8 minutes and both BAL, and blood cells were washed twice with cell wash (BD). T cells were acquired and analyzed using an 8-parameter flow cytometer FACSCanto (BD) and FACS Diva 6.1.2 software (BD). Because of difficulties in discriminating CCR5- and CXCR4-positive T cells from negative, the expression of these receptors was measured as the difference between median fluorescence intensity (MFI) and the fluorescence of the corresponding isotype control.
Multiplex analysis of inflammatory mediators
Frozen BAL supernatants were thawed at room temperature and concentrated using Amicon Ultra 15ML 3K centrifugal filters (Millipore Corporation, Bedford, MA, USA) prior to cytokine analysis. The assay was performed according to the manufacturer’s instructions, and bead MFI was detected with a Bio-Plex 200 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Multiplex data were analyzed using Bio-Plex Manager software, version 6.0 (Bio-Rad). All samples were run in duplicate. Data out of range or below the lowest limit of quantification (defined as 5× standard deviation of the background noise) or sample with high technical variance (defined as a coefficient of variance of replicates >50%) were excluded from all further analyses. Correction for batch effects was performed through mean-centering and scaling to univariate variance. Strong intra-group outliers, as identified by Dixon’s q-test (P<0.05), were excluded.
Statistical analyses
Unsupervised analysis by principal component analysis (PCA) was used for quality control and outlier identification, and Orthogonal Projections to Latent Structures Discriminate Analysis (OPLS-DA) for separation between groups. In contrast to PCA, OPLS is a supervised modeling approach designed to separate structured noise unrelated (orthogonal) to the predictive variance of interest (eg, between COPD patients and smokers), thereby increasing the ability to identify biomarkers driving the observed group separation.
Prior to analysis, data were log transformed, scaled to unit variance, and mean-centered to avoid weighting based on variable abundance in the analyses.
The results of the OPLS analysis are displayed in a scores plot displaying the separation of groups (predictive component) along the y-axis and within-group variation between individuals (orthogonal components) in the x-axis, as well as the corresponding loadings plot or variables’ importance plot, showing the relative importance of variables for separating the groups.
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.