Back to Journals » Vascular Health and Risk Management » Volume 18
Gender Differences in the Correlations Between Immune Cells and Organ Damage Indexes of Acute Myocardial Infarction Patients
Authors Song BY, Chen C, Xu WH, Cong BL, Guo ZY, Zhao ZH, Cui L, Zhang YH
Received 23 May 2022
Accepted for publication 12 October 2022
Published 2 December 2022 Volume 2022:18 Pages 839—850
DOI https://doi.org/10.2147/VHRM.S374157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Bai Yi Song1 *, Chen Chen2 *, Wen Hu Xu,1 Bai Lin Cong,3 Zheng Yi Guo,1 Zai Hao Zhao,4 Lan Cui,1 Yin Hua Zhang1,4,5
1Cardiovascular Medicine Department, Yanbian University Hospital, Yanji, 133000, People’s Republic of China; 2Department of Emergency Medicine, Yanbian University Hospital, Yanji, 133000, People’s Republic of China; 3Cardiovascular Medicine Department, Jilin City Central Hospital, Jilin, 132011, People’s Republic of China; 4Research Center, Yanbian University Hospital, Yanji, 133000, People’s Republic of China; 5Department of Physiology & Biomedical Sciences, Ischemic/Hypoxic Disease Institute, Seoul National University, College of Medicine, Seoul, 03080, Republic of Korea
*These authors contributed equally to this work
Correspondence: Yin Hua Zhang; Lan Cui, Yanbian University Hospital, Yanji, 133000, People’s Republic of China, Tel +86 433 2660129, Email [email protected]; [email protected]
Background: There are clear gender differences in the pathological process and outcome in acute myocardial infarction (AMI) patients but inflammatory responses remain clarified. Here, we aimed to analyse the correlations between inflammatory cells and organ injury parameters in AMI patients and compared between male and female groups.
Methods: A total of 603 AMI patients who underwent percutaneous coronary intervention (PCI) within 24 hours of the onset were analysed retrospectively. Basic information and hematological parameters detected 6 hours before the PCI were collected, neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) were calculated. Renal, liver function indicators, and myocardial enzymes were measured. Left ventricular ejection fraction (EF) and fractional shortening (FS) on days 5– 7 after PCI were obtained. Western blot was performed to detect iNOS, eNOS and nNOS expression in H9C2 rat cardiomyocytes treated with IL-6 with and without estrogen and testosterone.
Results: WBC, NEU, MON, MLR, CK, ALT and CREA of male patients were significantly higher than females, but FS was lower in females. NEU, MON and MLR were positively correlated with CK, CK-MB, AST, and ALT in males, whereas LYM were correlated with these parameters in female. NEU and NLR were inversely correlated with EF or FS only in female. Estrogen and testosterone reduced IL-6-induced iNOS protein expression in H9C2 cardiomyocytes, estrogen enhanced IL-6-induced nNOS protein expression.
Conclusion: NEU, MON, MLR in male AMI patients, and LYM in female patients were associated with organ injury parameters. Estrogen regulation of nitric oxide pathway may mediate the protective effects in female.
Keywords: acute myocardial infarction, gender differences, immune cells, renal function indicators, liver function indicators, nitric oxide synthases
Introduction
Myocardial infarction (MI) is the leading cause of cardiovascular mortality worldwide. MI has been associated with various risk factors, including high blood pressure, diabetes, hyperlipidemia, hyperuricemia, smoking, obesity and aging. Recent consensus supports gender differences in the incidence and outcome of MI patients.1 Differences in the susceptibility and inflammatory responses between genders may contribute to the variances, however, detailed mechanisms remain unidentified. Different sex hormone receptors (androgen and E2 receptors) in male and female are largely attributing to different responses to the similar pathological insults.2 In line with this notion, epidemiological analysis demonstrated that the onset of MI is significantly delayed in female before the menopause.3 In addition, female patients present less myocardial injury and attenuated maladaptive remodelling in the ischemic or peri-infarct area of the hearts.4 Consequently, female hearts show delayed transition to heart failure or cardiac dysfunction.3 Animal studies also confirmed mitigated myocardial injury, dysfunction, long-term outcome and death in female MI mice or rats,5 in agreement with clinical observations in gender-dependent differences.
Acute MI triggers inflammatory cascades, with immune cells and inflammatory mediators (cytokines, chemokines) functioning in a timely and coordinated fashion to initiate apoptosis, necrosis, proteolysis, fibrosis as well as myocardial recovery and remodelling.6–11 Neutrophils (NEU) are the first type of immune cells those are recruited to the infarct site to initiate oxidative activity and to release proteolytic enzymes. Monocytes (MON) and macrophages, the most abundant immune cells, appear in the infarct area for debris removal and immune reactions. Subsequently, lymphocytes (LYM) are recruited (3 days – 1 week) to exert immune regression and anti-inflammatory reparative processes, including the wound healing and scar formation.12 Between male and female, more NEU has been shown to be infiltrated in infarct area in male and become more active, releasing higher amounts of TNFα and IL-6.13,14 Consistently, animal studies showed less IL-1β, IL-1α, IL-6, and TNFα levels in the coronary artery of ischemic/reperfusion female rats.15 Furthermore, fewer NEU was followed by higher macrophage abundance in female,16 indicating that leukocyte profiling and their activities during and post-MI are more favourable in females.16 Recently, Blokland et al showed higher LYM counts and LYM to MON ratio (LMR) in female ST-elevation MI patients; higher LMR was associated with lower creatine Kinase-MB (CK-MB), troponin and one-year mortality rate.17 The systemic inflammatory responses also affect other organ damages, eg liver and kidney. An integration of immune responses and various organ damage may improve the understandings of these differences, which is important in optimising the management of sex-specific risk stratification and treatment for better clinical outcomes.
In this study, we aimed to distinguish peripheral immune cell counts of 603 acute MI (AMI) patients and compared their associations with heart, liver and kidney damage parameters between female and male. Our results showed clear gender differences in inflammatory responses and their correlations with organ damage and functions.
Materials and Methods
Participants
Statistical analysis was performed on the data of 603 patients with AMI and ST elevation in ECG before undergoing percutaneous coronary intervention (PCI). These patients were admitted to Yanbian University Hospital of Jilin Province, China (January 2016 to December 2019) for the first time (primary PCI). The average age of these patients was 60.65 ± 10.80 years old, including 450 men (74.6%) and 153 women (25.40%). Patients were divided into male patient groups and female patient groups. Patients with acute and chronic infectious diseases, metabolic diseases, such as diabetes, acute and chronic cholecystitis, nephritis, bronchitis, myocarditis, rheumatoid arthritis, gout, trauma, liver and kidney dysfunction, thyroid insufficiency or hyperthyroidism, heart failure, immune system diseases and cancer were excluded from the study. Basic information (including gender, age, height, weight) and laboratory parameters including white blood cell (WBC) counts, hemoglobin (HGB), platelets (PLT), NEU, LYM, MON, eosinophil, taken 6 hours after the onset of symptoms were used to calculate neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR). Renal function (blood urea nitrogen (BUN), creatinine (CREA)), liver function (aspartate aminotransferase (AST), alanine aminotransferase (ALT)) and myocardial enzymes (CK, CK-MB) from sera were obtained from the laboratory examination results of the same samples. Medical history (including past history), the results of echocardiography (Canon, ZHB20X2193) on days 5–7 after PCI (left ventricular ejection fraction (EF) and fractional shortening (FS)) were obtained from the chart records.
Due to the retrospective design, using anonymized data, no ethical approval was necessary for this study.
Cell Culture and Western Blot Experiment Using H9C2 Cell Line
The H9C2 cells, a cardiac cell strain, were purchased from the Shanghai Fuheng Biotechnology Co., Ltd. (product No. FH1004). The cells (3×105 cells per well in a 6-well culture plate) were cultured in medium (DMEM) supplemented with 10% fetal bovine serum, 1% double antibiotics (penicillin, streptomycin). Cells were grown in a humidified incubator consisting of 95% O2 and 5% CO2 at 37°C.
Immunoblotting experiments were performed to observe inducible nitric oxide synthase (iNOS), endothelial NOS (eNOS) and neuronal NOS (nNOS) protein expressions in H9C2 rat cardiomyocytes treated with IL-6 (500 nM, 6h) with and without sex hormones estrogen (E2, 10μM, 24h), testosterone (T, 10μM, 24h). Proteins were extracted using RIPA lysate buffer and PMSF. The lysates were boiled at 95°C for 5 minutes and fractionated through SDS-PAGE and transferred to 5% concentrated gel (mL: H2O 2.1, 30% Acrylamide 0.5, 1.0 M pH 6.8 Tris 0.38, 10% SDS 0.03, 10% APS 0.03, TEMED 0.003) or 8% separation gel (mL: H2O 4.6, 30% Acrylamide 2.7, 1.5 M pH 8.8 Tris 2.5, 10% SDS 0.1, 10% APS 0.1, TEMED 0.006). After the protein was transferred to the PVDF membrane, the membrane was blocked in a blocking solution at 25°C for 2 hours. Antibodies for iNOS (NOVUS, NB300-605), eNOS (BD Transduction Laboratories TM, 610296), nNOS (BD Transduction Laboratories TM, 610309) and Anti-β-Actin (Bioss, bs-0061R) were incubated at 4°C overnight, followed by secondary antibodies (goat anti-rabbit IgGHRP, Life Sciences, NC-AP132P; goat anti-mouse IgG/horseradish enzyme label, Nakasugi Jinqiao ORIGENE, ZB-2305 for 2h) and washing. The blots were developed using Fluorchem HD2, and the relative density was calculated by normalizing each blot with β-actin.
Statistical Analysis
SPSS 23.0 was used for analysis. Data were expressed as mean±SE (SEM). Continuous variables with deviations and skewed distributions were expressed as medians with interquartile range. The t-test was used for analysis between groups. Non-parametric tests and Pearson’s correlation were performed for the links between inflammation indicators, NLR and MLR with organ injury indicators and the cardiac function (EF, FS) of the male and female groups. The scatter plots were presented to visually display the correlations. All analyses concluded that a double-sided P value of less than 0.05 is statistically significant.
Results
Statistical Analysis of Laboratory Test Results in Male (M) and Female (F) Patients
603 AMI patients were divided into male (M, n = 450) and female (F, n = 153) groups. There were no age or BMI differences between two groups. All the patients received PCI for the first time and venous blood was collected on admission before PCI. The WBC, RBC, HGB, NEU and MON, MLR were significantly greater in M group compared to those in F group (Table 1). CK, ALT and CREA were significantly higher in M group and FS was smaller in F group (Table 1). These results showed gender differences in inflammatory responses and organ damages.
 |
Table 1 Descriptive Statistical Analysis of Male and Female Groups in Patients with AMI |
Correlation Analysis Between Immune Cells and Organ Injury Parameters in All Patients
Correlations between WBC, NEU, LYM, MON or NLR and MLR and heart, liver and kidney damage indexes were analysed. In all patients, WBC was positively correlated with CK (r = 0.270, P < 0.001), CK-MB (r = 0.249, P < 0.001), ALT (r = 0.109, P = 0.007) and AST (r = 0.268, P <0.001). Similarly, NEU was positively correlated with CK (r = 0.257, P <0.001), CK-MB (r = 0.241, P <0.001) and AST (r = 0.275, P <0.001). There was a tendency in correlation between NEU and ALT (r=0.076, P=0.06) (Figure 1A, Table 2). LYM showed no correlation with all parameters (Figure 1B, Table 2). However, MON was positively correlated with CK (r = 0.113, P = 0.005), CK-MB (r = 0.09, P = 0.025), AST (r = 0.109, P =0.007), ALT (r = 0.081, P =0.045) and CREA (r = 0.082, P =0.045) (Figure 1C, Table 2). Only NEU showed tendency to be negatively correlated with FS (r=−0.078, P=0.05) (Table 2). These results showed specificity of immune cells in organ damage or cardiac functional indexes.
 |
Table 2 Correlation Analysis of WBC, NEU, LYM, MON and Laboratory Indexes in Patients with AMI |
Correlation Analysis Between M and F Groups
WBC was positively correlated with CK and CK-MB in both M and F groups (CK in M group: r = 0.278, P <0.001; CK in F group: r = 0.218, P = 0.007; CK-MB in M group: r = 0.248, P <0.001, CK-MB in F group: r = 0.249, P = 0.002) (Table 3). WBC positively correlated with AST only in M group (r=0.124, p=0.009). WBC showed negative correlation with EF and FS only in F group (r=−0.159, P=0.05; r = −0.183, P = 0.024) (Table 3).
 |
Table 3 Correlation Analysis of WBC, NEU, LYM, MON and Laboratory Indexes in Male and Female Patients with AMI |
In M group, NEU was positively correlated with CK (r = 0.289, P <0.001), CK-MB (r = 0.266, P <0.001), AST (r =0.309, P <0.001) and ALT (r =0.108, P = 0.022). No correlation between NEU and organ damage parameters was observed in F group. However, NEU was negatively correlated with EF (r = −0.167, P = 0.039) and FS (r = −0.202, P = 0.013) (Table 3).
LYM was not related to any laboratory parameters in M group, except that LYM was negatively correlated with AST in M group (r=−0.121, P=0.01). In F group, LYM was positively correlated with CK (r = 0.310, P <0.001), CK-MB (r = 0.286, P <0.001), AST (r = 0.328, P < 0.001) and ALT (r = 0.319, P < 0.001). LYM did not show correlation with EF or FS in either group (Table 3).
MON showed significant positive correlation with CK (r = 0.120, P = 0.011) and AST (r = 0.103, P = 0.029) only in M group. MON showed tendency towards correlation with CK-MB (r=0.086, P=0.06) (Table 3). MON showed no correlation with any organ damage parameters or cardiac functional parameters in F group.
Next, we analysed the correlations between NLR or MLR and organ damage parameters in both groups. Results showed that NLR and MLR were positively correlated with heart and liver injury parameters in all patients (Figure 2A and Figure 2). In M group, NLR was positively correlated with CK (r = 0.111, P = 0.018) and AST (r = 0.133, P = 0.005). NLR showed tendency towards correlation with CK-MB (r=0.086, P=0.06) and with ALT (r=0.084, P=0.07). MLR was positively correlated with CK (r = 0.175, P <0.001), CK-MB (r = 0.135, P = 0.004), AST (r = 0.184, P < 0.001) and ALT (r = 0.112, P = 0.017) in M group (Table 4). No correlation was observed between NLR or MLR with organ damage parameters in F group apart from NLR with FS (r = −0.188, P = 0.020) (Table 4). These results confirmed the gender differences of AMI patients in associations between inflammatory parameters and organ damage at admission and cardiac functional indexes 5–7 days post- PCI.
 |
Table 4 Correlation Analysis of MLR, NLR and Laboratory Indexes in All Patients, Male and Female Patients with AMI |
Effects of Sex Hormones on IL-6-Stimulated iNOS, eNOS and nNOS Protein Expressions in H9C2 Cell Line
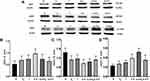
Inflammatory cytokines during MI induces iNOS protein expression, which are detrimental in cardiac function. However, constitutive NOS (eNOS and nNOS) can exert protective effects. Therefore, we tested whether iNOS, eNOS and nNOS protein expressions are affected in the presence of inflammatory cytokine IL-6, and whether these changes are affected by sex hormones, to gain insights into the changes of NOS in cardiomyocytes. Results showed that IL-6 significantly increased iNOS protein expression in H9C2 cells (P<0.05) and E2 or T co-treatment reduced iNOS expression (Figure 3A and Figure 3). eNOS protein expression was significantly reduced by E2 or IL-6 (P=0.032, P=0.009, Figure 3A and Figure 3).
In contrast, nNOS protein expression was significantly increased by IL-6 and further increase was observed with IL-6+E2 in H9C2 cells (P=0.037, P=0.035, Figure 3A and Figure 3). However, IL-6+T did not increase nNOS protein expression (P=0.096, Figure 3A and Figure 3).
Discussion
The present analysis showed clear gender differences in the associations between peripheral venous blood immune cells and organ damage parameters in AMI patients before PCI. In general, the stronger the inflammatory response, the greater the organ damage indexes, but inflammatory characteristics were different between male and female AMI patients. Male patients showed that NEU, MON, NLR or MLR were positively linked with cardiac and liver damage parameters, but female patients showed correlations with LYM. In addition, EF and FS 5–7 days after PCI were lower in female AMI patients and EF or FS were negatively correlated with the inflammatory indexes in this group. Furthermore, pro-inflammatory cytokine (IL-6) significantly increased nNOS protein expression in H9C2 cardiomyocyte cell lines and the increase was further upregulated by E2, indicating that female hormone may trigger protective mechanisms to reduce myocardial and organ damage in the presence of inflammatory cytokines, but nNOS-upregulation may exert negative inotropic effect in the heart. These results also draw attention to LYM in systemic and local inflammatory responses in female AMI patients.
In recent years, convincing evidence of the gender differences in AMI patients emerge for better understandings and management of acute coronary syndrome (ACS) and cardiovascular diseases. Inflammatory responses are of central regulatory mechanisms.18 Previous studies have shown higher inflammatory responses in young female AMI patients, due in part to more severe coronary microvascular dysfunction, delay in getting treatment and impaired coronary blood flow reserve.19,20 Indeed, female patients with myocardial ischemia frequently shows infarct sites in the microvascular coronary arteries21 and more comorbidities in ACS.22–24This is despite of the fact that E2 can protect the heart and improve myocardial damage following ischemic reperfusion.25,26 In premenopausal female AMI patients who undergo PCI, the interruption of the ovulation cycle, characterized by hypoestrogenemia of hypothalamic origin, appears to be related to coronary atherosclerotic heart disease.27 In the present study, the degree of ischemia was comparable between male and female, but female AMI patients showed less organ damages and the cardiac contraction was lower 5–7 days following PCI. It is known that old female patients (>70 years old) show worse outcome post-PCI. In this cohort, majority of female patients are postmenopausal (4 patients were premenopausal) but the age of female patients was comparable to male patients because we collected patients consecutively (2016–2019) who has complete clinical data and with clear exclusion criteria. This is counterintuitive to the consensus of the prevalence of older female AMI patients. A number of reasons may account for lower number of female patients and less aged female patients (<70 years old): 1) patients older than 70 years tend to decline PCI procedure for fear of adverse post-PCI outcome; 2) older female patients may have more comorbidities and are excluded from the cohort; 3) older female patients decline treatment due to financial difficulties. It should be noted that the ratio between male and female AMI patients around this age group represents scenarios witnessed in clinic. These data indicate that the inflammatory responses and cardiac recovery are different between male and female, which are consistent with those from previous studies.
After myocardial infarction, cell death triggers series of inflammatory responses, which play vital roles in repairing the infarcted myocardium, but inflammation is also related to the pathogenesis of poor ventricular remodeling.28 Early studies have shown that NEU exerts cytotoxic effects on viable myocardium in the infarct border zone, thereby prolonging ischemic damage,29 especially when NEU are followed by MON accumulation to the injured area, amplifying the inflammatory responses.30 The cytokines or chemokines produced from NEU and MON act synergistically in the infarcted myocardium, resulting in adverse outcome of the patients.30 Subsequently, the expression of chemokines in infarcted myocardium increases and mediates the accumulation of cytokines, eg IL-6, which trigger more leukocytes in peripheral venous during acute phase of MI. The increase of WBC and NEU in the acute phase of MI are important in predicting left ventricular contractile dysfunction.31 After myocardial infarction, MON accumulation in infarcted area leads to a significant increase in the number of LYM subsets in the infarcted myocardium, and LYM are involved in inflammatory processes and also contributes to the reduction of the inflammatory responses by secreting protective cytokines (such as IL-10).32,33 In the current study, NEU, MON and WBC counts are significantly higher in male AMI patients, and the amount of immune cells are positively linked with heart, liver and kidney damages. In contrast, LYM linked to heart and liver damage indexes in female. The reason for the immune cell discrepancy is not clear. The severity of ischemia or the timing of infarction may contribute to the activation of different immune cell types.34 However, the blood samples were collected within 6 hours after the onset of symptoms in both groups and the degree of infarct was similar between male and female patients suggesting genuine sex-dependent inflammatory responses following myocardial infarction. Importantly, reduced LYM has been associated with immunosenescence, which controls the pathogenesis of cardiovascular diseases, in particular, exacerbates the outcomes post-Covid-19 infection.35 LYM subtypes and their releasing cytokines may help to understand the clinical relevance of LYM in AMI; nevertheless, our results showed clear gender specificity of immune cell profiles and their correlations with tissue/organ damage parameters.
Recently, NLR and MLR are shown to be important indicators of inflammation reflecting the degree of systemic inflammation and NLR and/or MLR predicts increased morbidity and mortality in a wide range of cardiovascular diseases including AMI.36–39 Our results showed greater MLR and higher tendency of NLR in male. And the associations between NLR and MLR and organ damage indexes were different between male and females, further support the gender specificity during AMI. The major difference seems to be with LYM; indeed, LYM count decrease is shown to be associated with the stress response and they are negatively correlated with the prognosis of ACS.40,41
E2 can protect the heart and improve myocardial damage caused by ischemia and ischemia/reperfusion.42 One of the potential effects downstream of E2 receptors are nitric oxide (NO)-dependent signalling, including cGMP-mediated relaxation and intracellular Ca regulation.26,42 Conversely, disabling the ovarian function may diminish cardiac protective functions in females.43 Endothelial NO synthase (eNOS) are generally considered to be downstream of E2 receptor.40 However, neuronal isoforms of NOS (nNOS) are proved to be strong cardiovascular protectors in diseased hearts, including AMI and post ischemic/reperfusion.44,45 In addition, it is known that inflammatory and pro-inflammatory cytokines induce iNOS expression.46,47 High concentration of NO could be produced through iNOS activation, which combines with superoxide (O2-) to generate toxic peroxynitrite (ONOO−) during reperfusion, leading to ONOO− -induced cytotoxicity.48,49 Accordingly, we have investigated three NOS isoforms in response to inflammatory cytokine, IL-6, in the presence and absence of E2 or T. Our results showed that E2 and T reduced IL-6-induced iNOS expression in H9C2 cell line. Conversely, eNOS is reduced by IL-6, but neither E2 or T reversed the reduced expression of eNOS. Intriguingly, IL-6 increased nNOS protein expression, and IL-6 + E2 further increased nNOS expression, whereas T did not show such an effect. Therefore, E2 exerts protective effects by stimulating nNOS and reducing iNOS in cardiomyocytes under inflammatory insults with cytokines. nNOS may prevent Ca overload in cardiomyocytes by restricting Ca influx through L-type Ca channels and/or by enhancing Ca reuptake through SERCA into the sarcoplasmic reticulum.50,51 nNOS upregulation may also inhibit mitochondrial and cytosol ROS production post-MI, which further prevents myocardial damage during reperfusion. The effects of nNOS in response to different immune cell or immunocytokine stimulation and the mechanisms leading to myocardial protection need to be studied in more detail.
Using clinical information, our results showed convincing evidences of gender differences in inflammatory responses of AMI patients. The immune cells correlate differently with organ damage parameters between male and female AMI patients. Furthermore, we also provided in vitro experimental evidences indicating that E2 may protect cardiomyocytes through regulating nNOS and iNOS. The results are of clinical significance in directing therapeutic regime for better target AMI patients.
Data Sharing Statement
The data used to support the findings of this study are included within the article.
Acknowledgements
We thank all the participants who took part in this study and the assistants who helped with the participants. The study was provided by Yanbian University Hospital, Cardiology department and Central Laboratory Department. This work was financially supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (NRF-2019R1A2C1005720) and National Natural Science Foundation of China (NSFC 31660284, NSFC31860288). Korean Hypertension Society (2021), Jilin Province Education Department funding (JJKH20200529KJ).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Bai Lin Cong reports grants, personal fees, non-financial support from Department of Cardiovascular Medicine, Yanbian University Hospital, outside the submitted work. The authors declare that they have no other potential conflicts of interest in relation to this work.
References
1. Lu Y, Zhou S, Dreyer RP, et al. Sex differences in inflammatory markers and health status among young adults with acute myocardial infarction: results from the VIRGO (Variation in recovery: role of gender on outcomes of young acute myocardial infarction patients) study. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003470. doi:10.1161/CIRCOUTCOMES.116.003470
2. Puhl SL, Steffens S. Neutrophils in post-myocardial infarction inflammation: damage vs. resolution? Front Cardiovasc Med. 2019;6:25. doi:10.3389/fcvm.2019.00025
3. Benn M, Voss SS, Holmegard HN, et al. Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol. 2015;35(2):471–477. doi:10.1161/ATVBAHA.114.304821
4. Guerra S, Leri A, Wang X, et al. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;851:856–866. doi:10.1161/01.res.85.9.856
5. Wu JC, Nasseri BA, Bloch KD, et al. Influence of sex on ventricular remodeling after myocardial infarction in mice. J Am Soc Echocardiogr. 2003;16:1158–1162. doi:10.1067/S0894-7317(03)00648-5
6. Timmers L, Pasterkamp G, de Hoog VC, et al. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–283. doi:10.1093/cvr/cvs018
7. Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011y;8:292–300. doi:10.1038/nrcardio.2011.38
8. de Haan JJ, Smeets MB, Pasterkamp G, et al. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013;2013:206039. doi:10.1155/2013/206039
9. Ghigo A, Franco I, Morello F, et al. Myocyte signalling in leucocyte recruitment to the heart. Cardiovasc Res. 2014;102:270–280. doi:10.1093/cvr/cvu030
10. Mann DL, Kirschenbaum L. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108(9):1133–1145. doi:10.1161/CIRCRESAHA.110.226936
11. Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(3):a006049. doi:10.1101/cshperspect.a006049
12. Hofmann U, Beyersdorf N, Weirather J, et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi:10.1161/CIRCULATIONAHA.111.044164
13. DeLeon-Pennell KY, Mouton AJ, Ero OK, et al. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol. 2018;113(5):40. doi:10.1007/s00395-018-0699-5
14. Fang L, Gao XM, Moore XL, et al. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol. 2007;43(5):535–544. doi:10.1016/j.yjmcc.2007.06.011
15. Wang M, Baker L, Tsai BM, et al. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2005;288(2):E321–326. doi:10.1152/ajpendo.00278.2004
16. Cavasin MA, Tao Z, Menon S, et al. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75(18):2181–2192. doi:10.1016/j.lfs.2004.04.024
17. van Blokland IV, Groot HE, Hendriks T, et al. Sex differences in leukocyte profile in ST-elevation myocardial infarction patients. Sci Rep. 2020;10(1):6851. doi:10.1038/s41598-020-63185-3
18. Siennicka A, Jastrzebska M, Smialkowska K, et al. Gender differences in hemostatic and inflammatory factors in patients with acute coronary syndromes: a pilot study. J Physiol Pharmacol. 2018;69(1):91–98. doi:10.26402/jpp.2018.1.10
19. Taqueti VR, Shaw LJ, Cook NR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135(6):566–577. doi:10.1161/CIRCULATIONAHA.116.023266
20. Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ. 2018;363:k4247. doi:10.1136/bmj.k4247
21. Lerman A, Sopko G. Women and cardiovascular heart disease: clinical implications from the Women’s Ischemia Syndrome Evaluation (WISE) study. Are we smarter? J Am Coll Cardiol. 2006;47(3):S59–62. doi:10.1016/j.jacc.2004.10.083
22. Graham G. Acute coronary syndromes in women: recent treatment trends and outcomes. Clin Med Insights Cardiol. 2016;10:1–10. doi:10.4137/CMC.S37145
23. Khan E, Brieger D, Amerena J, et al. Differences in management and outcomes for men and women with ST-elevation myocardial infarction. Med J Aust. 2018;209(3):118–123. doi:10.5694/mja17.01109
24. Stehli J, Martin C, Brennan A, et al. Sex differences persist in time to presentation, revascularization, and mortality in myocardial infarction treated with percutaneous coronary intervention. J Am Heart Assoc. 2019;8(10):e012161. doi:10.1161/JAHA.119.012161
25. Zhang Z. The effect of androgens on the cardiac function and myocardial apoptosis of isolated rat heart ischemia/reperfusion. Xi’an: Fourth Military Medical University; 2007.
26. Collins P, Rosano GM, Jiang C, et al. Cardiovascular protection by oestrogen--a calcium antagonist effect? Lancet. 1993;341(8855):1264–1265. doi:10.1016/0140-6736(93)91158-i
27. Bairey Merz CN, Johnson BD, Sharaf BL, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41(3):413–419. doi:10.1016/s0735-1097(02)02763-8
28. Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. doi:10.1002/cphy.c150006
29. Entman ML, Youker K, Shoji T, et al. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90(4):1335–1345. doi:10.1172/JCI115999
30. Alard JE, Ortega-Gomez A, Wichapong K, et al. Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil HNP1 and platelet CCL5. Sci Transl Med. 2015;7(317):317ra196. doi:10.1126/scitranslmed.aad5330
31. Eskandarian R, Ghorbani R, Asgary Z. Relationship between leucocytosis and left ventricular ejection fraction in patients with acute myocardial infarction. Singapore Med J. 2013;54(1):40–43. doi:10.11622/smedj.2013010
32. Frangogiannis NG, Mendoza LH, Lindsey ML, et al. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165(5):2798–2808. doi:10.4049/jimmunol.165.5.2798
33. Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015;116(2):354–367. doi:10.1161/CIRCRESAHA.116.304072
34. Mansueto G, Costa D, Capasso E, et al. The dating of thrombus organization in cases of pulmonary embolism: an autopsy study. BMC Cardiovasc Disord. 2019;19(1):250. doi:10.1186/s12872-019-1219-8
35. Napoli C, Tritto I, Mansueto G, et al. Immunosenescence exacerbates the COVID-19. Arch Gerontol Geriatr. 2020;90:104174. doi:10.1016/j.archger.2020.104174
36. Balta S, Demirkol S, Unlu M, et al. Neutrophil to lymphocyte ratio may be predict of mortality in all conditions. Br J Cancer. 2013;109(12):3125–3126. doi:10.1038/bjc.2013.598
37. Altun B, Turkon H, Tasolar H, et al. The relationship between high-sensitive troponin T, neutrophil lymphocyte ratio and SYNTAX Score. Scand J Clin Lab Invest. 2014;74:108–115. doi:10.3109/00365513.2013.860619
38. Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: the NHANES-I epidemiologic follow-up study. Ann Epidemiol. 2005;15(4):266–271. doi:10.1016/j.annepidem.2004.08.009
39. Chen C, Cong BL, Wang M, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res. 2018;7(2):192–199. doi:10.1016/j.imr.2018.02.006
40. Heidt T, Courties G, Dutta P, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115(2):284–295. doi:10.1161/CIRCRESAHA.115.303567
41. Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi:10.1084/jem.20070885
42. da Silva JS, Montagnoli TL, Rocha BS, et al. Estrogen receptors: therapeutic perspectives for the treatment of cardiac dysfunction after myocardial infarction. Int J Mol Sci. 2021;22(2):525. doi:10.3390/ijms22020525
43. McLean MR, Bates GW, Sternfeld B, et al. Decreased ovarian function in premenopausal women is associated with increased central adiposity over ten years: the coronary artery risk development in young adult women’s study. Fertil Steril. 2012;98:S42.
44. Zhang YH, Jin CZ, Jang JH, et al. Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. J Physiol. 2014;592:3189–3200. doi:10.1113/jphysiol.2013.270306
45. Sun J, Picht E, Ginsburg KS, et al. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi:10.1161/01.RES.0000202707.79018.0a
46. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi:10.1056/NEJM199312303292706
47. Schulz R, Nava E, Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992;105:575–580. doi:10.1111/j.1476-5381.1992.tb09021.x
48. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142.
49. Ihnken K, Morita K, Buckberg GD, et al. Nitric-oxide-induced reoxygenation injury in the cyanotic immature heart is prevented by controlling oxygen content during initial reoxygenation. Angiology. 1997;48:189–202. doi:10.1177/000331979704800301
50. Zhang YH, Zhang MH, Sears CE, et al. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102:242–249. doi:10.1161/CIRCRESAHA.107.164798
51. Sears CE, Bryant SM, Ashley EA, et al. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–59. doi:10.1161/01.RES.0000064585.95749.6D
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.