Back to Journals » International Journal of General Medicine » Volume 14
Gender Differences in the Association Between Serum Uric Acid and Arteriosclerotic Cardiovascular Risk Among Chinese Type 2 Diabetes Mellitus Patients
Authors Yang H, Gao J, Li S, Xia H, Chen Z, Zhu S, Pan Z
Received 2 January 2021
Accepted for publication 12 February 2021
Published 1 March 2021 Volume 2021:14 Pages 687—695
DOI https://doi.org/10.2147/IJGM.S300196
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hua Yang,1,* Jian Gao,2,* Shuyu Li,1 Huiling Xia,1 Zhangyan Chen,1 Shanzhu Zhu,1 Zhigang Pan1
1Department of General Practice, Zhongshan Hospital of Fudan University, Shanghai, 200030, People’s Republic of China; 2Department of Nutrition, Zhongshan Hospital of Fudan University, Center of Clinical Epidemiology and Evidence-Based Medicine, Fudan University, Shanghai, 200030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shanzhu Zhu; Zhigang Pan
Department of General Practice, Zhongshan Hospital of Fudan University, No. 180, Road Fenglin, Shanghai, 200030, People’s Republic of China
Tel +86 13501770447; +86 13681971967
Email [email protected]; [email protected]
Background and Objectives: Serum uric acid (sUA) level has been reported to be associated with arteriosclerotic cardiovascular risk, yet remains poorly defined in Chinese type 2 diabetes patients. The purpose of the current study was to evaluate gender differences in the association between sUA level and arteriosclerotic cardiovascular risk in Chinese type 2 diabetes patients.
Methods: The cross-sectional study was conducted in six community health service centers in Shanghai, China from December 2014 to December 2016. A stratified random sampling method was used to recruit participants. From a total of 3977 type 2 diabetic patients, 2537 were included for the analysis of the association between sUA level and arteriosclerotic cardiovascular risk. Clinical and biochemical data were obtained from participants. Arteriosclerotic cardiovascular risk was evaluated by the ten-year risk profile for arteriosclerotic cardiovascular disease. The associations between sUA level and arteriosclerotic cardiovascular risk were assessed via multiple logistic regression.
Results: Of the 2537 participants, the average sUA level was 317± 77umol/L in men and 294± 73 umol/L in women, and 54.8% (1391/2537) of participants had high ten-year risk of arteriosclerotic cardiovascular disease (ASCVD), which was ≥ 20%. High ten-year risk of ASCVD odds ratio was increased by 1.596 (95% CI, 1.113– 2.289, p for trend 0.004) comparing fourth to first quartiles of sUA in women. However, no significant association was found between sUA and high ten-year risk of ASCVD in men.
Conclusion: This community-based study indicated that sUA levels were independently associated with high ten-year risk of ASCVD in women with type 2 diabetes mellitus, but not in men.
Keywords: serum uric acid, ten-year risk, arteriosclerotic cardiovascular disease, type 2 diabetes mellitus
Introduction
Among individuals with type 2 diabetes mellitus (T2DM), arteriosclerotic cardiovascular disease (ASCVD), defined as acute coronary syndromes (ACSs), a history of myocardial infarction (MI), stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease, are the leading causes of morbidity and mortality worldwide.1–3 In the nationwide 3B STUDY conducted in China, 14.6% and 10.1% of patients with T2DM had a history of cardiovascular disease (CVD) and cerebrovascular disease, respectively.4
Serum uric acid (sUA), the waste product of purine metabolism, has been reported as a mediator of pathological processes, including inflammation and endothelial dysfunction.5,6 Many studies indicated that hyperuricemia is associated with a number of diseases including hypertension, diabetes mellitus, dyslipidemia, obesity, metabolic syndrome, chronic kidney disease, and pulmonary function.7–10 Several studies have suggested that hyperuricemia is a risk factor for ASCVD in the general population,11–13 but others have been unable to confirm such a relationship.14,15 Furthermore, gender differences could be found in the relationship between sUA and ASCVD. In the Framingham Heart Study, levels of sUA were associated with an increased risk of cardiovascular death in women but not in men, and the association disappeared after adjustment for well-known cardiovascular risk factors.16 Ndrepepa et al demonstrated that hyperuricemia could predict an increased cardiovascular risk of mortality in both genders, with a stronger association in women.17 However, few studies have focused on the relationship between sUA and ASCVD in patients with T2DM.18
Therefore, the aim of the present study was to explore the association between sUA and ASCVD risk, especially the gender disparities between them, in a community-based population with T2DM in China.
Materials and Methods
The current study was cross-sectional and partly based on data from our previous studies.19 Briefly, a stratified random sampling procedure was conducted to recruit participants between December 2014 and December 2016. Three districts were purposefully selected from 16 districts of Shanghai. Next, we randomly selected two community health centers (CHCs) from each district, with the inclusion of six CHCs in total. Finally, we randomly recruited participants who were ≥18 years of age, diagnosed with T2DM for ≥3 months, included in the diabetes mellitus management system of China,20 and not pregnant from these six CHCs. Written informed consent was obtained from all the volunteers. The study protocol was approved by the Ethics Committee of Zhongshan Hospital of Fudan University (B2016-029) and conducted in accordance with the Declaration of Helsinki.
Demographic Characteristics and Clinical Measurements
The demographic characteristics and clinical measurements used in the present study have been previously described.19 Briefly, the demographic characteristics included age, gender, educational attainment, marital status, smoking status, family history of diabetes, duration of diabetes, current medical treatment, history of hypertension, coronary heart disease (CHD) and stroke. The physical examinations included bodyweight, height, blood pressure (measured on the spot three times after resting for more than 10 minutes and then the average was taken) and non-mydriatic fundus photography. The laboratory measurements included fasting blood glucose (FBG), hemoglobin A1c (HbA1c), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), blood urea nitrogen (BUN), creatinine (CRE), sUA, urinary albumin creatinine ratio (UACR), and urine analysis. Venous blood samples were taken under a fasting overnight condition of participants in the morning. All blood and urine samples were analyzed in the local laboratories meeting national standards.
ASCVD Risk Evaluation
Patient’s 10-year ASCVD risk was estimated using the gender-specific parameters from the ACC/AHA Pooled Cohort equations,21 which was based on age, systolic blood pressure, treatment of hypertension, TC and HDL-C levels, current smoking, and history of diabetes mellitus. The patients who were over 79-years-old or less than 40-years-old, or had the history of CHD or stroke, or LDL-C >190 mg/dL (>4.9 mmol/L) were excluded according to the ACC/AHA guideline. High 10-year risk of ASCVD was defined as ASCVD risk score ≥20%.21
Statistical Analysis
Statistical analysis was carried out using SPSS software, version 17.0 (SPSS Inc, Chicago USA), SAS software, version 9.2 (SAS Institute, Cary, NC).
Descriptive analyses were used to characterize the participant population by sociodemographic data, health status and clinical measurements. Categorical variables were presented as percentages. Continuous variables without normal distribution were presented as medians and those for normal distribution as means±standard deviations. Significant differences between the groups were calculated using Chi-square tests for the percentages and unpaired t-tests for the mean values. The parameters of sUA were divided into quartiles according to the linear scores. The associations between sUA level and ASCVD risk were assessed via multiple logistic regression. Multiple logistic regression models were fitted with ASCVD risk score ≥20% as dependent variables, and the quartiles of sUA as independent variables. Adjustments were made for confounding factors, including educational attainment, marital status, family history of diabetes mellitus, duration of diabetes mellitus, current medical treatment, and BMI, HbA1c, eGFR. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A two-tailed alpha with p<0.05 was considered statistically significant for all analyses.
Results
Characteristics of Participants According to ASCVD Risk Score
From a total of 3977 participants, 2537 participants, including 1068 men and 1469 women, were included in the current analysis of the association between sUA level and 10-year ASCVD risk, in which average sUA level was 317±77 umol/L in men and 294±73umol/L in women, and 54.8% (1391/2537) of participants had high ten-year risk of ASCVD (Figure 1).
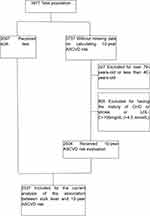 |
Figure 1 Inclusion/exclusion criteria for the study participants for assessment of the association between sUA level and 10-year ASCVD risk. |
Significant differences were observed between the participants with or without high 10-year risk of ASCVD. A higher proportion of older participants, male, current smokers, single or divorced or windowed, with higher educational attainment, longer duration of diabetes, without family history of diabetes mellitus, having comorbid hypertension, with higher systolic hypertension (SBP), HDL-C, BUN, CRE, sUA, abnormal UACR, and lower eGFR tended to have high 10-year risk of ASCVD (p<0.05) (Table 1).
 |
Table 1 Prevalence of High 10-Year Risk of ASCVD Among Type 2 Diabetes Patients Based on Clinical and Biochemical Data |
Association Between sUA Level and High Ten-Year Risk of ASCVD in Different Genders
Gender differences were found in the association between sUA level and high ten-year risk of ASCVD (p for interaction 0.0048) (Figure 2). The female T2DM participants with higher sUA levels were more likely to have high ten-year risk of ASCVD. Comparing fourth to first quartiles of sUA in women, the OR for high ten-year risk of ASCVD was 1.596 (95% CI, 1.113–2.289, p for trend 0.004). However, no significant association was found between sUA and high ten-year risk of ASCVD in men (Table 2).
 |
Table 2 Association Between sUA Level and High Ten-Year Risk of ASCVD in Different Genders |
 |
Figure 2 Gender differences in the association between sUA level and high ten-year risk of ASCVD. |
Discussion
In this study, sUA was independently associated with high ten-year risk of ASCVD in women with type 2 diabetes mellitus but not in men. Notably, such associations were independent of several confounding factors, such as educational attainment, marital status, family history of diabetes mellitus, duration of diabetes mellitus, current medical treatment, BMI, HbA1c and eGFR.
Previous studies on the associations of sUA with ASCVD have shown contradictory results. Silbernagel G et al had followed 3245 individuals referred for coronary angiography for all-cause mortality, cardiovascular mortality, and sudden cardiac death with a mean duration of 7.3 years and found that high sUA independently indicated increased risk for cardiovascular and sudden cardiac death in these subjects.22 In a prospective observational study that recruited 494 patients with diabetes mellitus and followed them for mean 12.8 months, sUA was confirmed as a predictor of cardiac events.23 In a review of 21 articles with data of 33,580 stroke and 1,100,888 participants, Tariq et al found that increasing sUA levels poses a higher risk for incidence of stroke.24 However, in a meta-analysis on sUA as a risk factor of all-cause mortality and cardiovascular events among type 2 diabetes mellitus population, sUA levels were found to be associated with a higher risk of all-cause mortality and stroke, but not with CHD.25 Sotoda et al found that sUA levels are associated with the degree of leg ischemia in patients with peripheral arterial disease (PAD).26 However, the gender differences in the association between sUA and arteriosclerotic cardiovascular risk among type 2 diabetes mellitus patients have been rarely studied.
In the current study, the ten-year risk of ASCVD score was used, which was not a replacement for clinical judgment, but a valid evaluation for the primary prevention of ASCVD.21 Furthermore, we found that sUA was independently associated with high ten-year risk of ASCVD in women with type 2 diabetes mellitus but not in men. Higher level of sUA is a cause of endothelial dysfunction, therefore increasing the oxidative stress, which leads to a decrease in nitric oxide bioavailability and vascular smooth muscle cell proliferation.27 The vast majority of ASCVD are caused by atherosclerosis, and diabetes mellitus is known to accelerate this process.28 However, the mechanism of interaction between sUA and ASCVD, and the role of diabetes mellitus in this relationship remain unknown.
This study had some limitations. Certain class of anti-diabetic drugs that has an impact on increasing sUA level was not examined, which may be a potential confounding factor. Furthermore, the study involved a cross-sectional design, and therefore, only association rather than causation could be evaluated. Longitudinal studies would be helpful to further understand the relationship between sUA and ASCVD among T2DM patients.
Conclusions
In summary, this community-based study indicated that sUA was independently associated with high ten-year risk of ASCVD in women with type 2 diabetes mellitus but not in men. More prospective research should be conducted to further elucidate the relationship between sUA levels and ASCVD since this association could have global clinical implications in the management of diabetes patients.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors are grateful to their colleagues and primary medical workers from Kangjian, Xujiahui, Huamu, Weifang, Gongyequ, Huangdu Community Health Care Centers who contributed to the field data collection. They also thank Professor Doris Young (Department of General Practice, University of Melbourne, Carlton, Melbourne, VIC, Australia) for her insightful comments on this study.
Funding
This study was supported by the Project of Shanghai Foundation for Senior Citizens (S15027), Construction Project of Key Discipline of Public Health in Shanghai (12GWZX1001), Science and Technology Project of Pudong New Area Commission of Health and Family Planning (PW2015C-25), Shanghai Municipal Commission of Health and Family Planning, Key Developing Disciplines (2015ZB0601).
Disclosure
Hua Yang and Jian Gao are co-first authors. The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med. 2011;171(5):404–410.
2. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502.
3. Sonne DP, Hemmingsen B. Standards of Medical Care in Diabetes-2017. Diabetes Care. 2017;40(Suppl.1):S1–S135.
4. Ji L, Hu D, Pan C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126(10):
5. Gustafsson D, Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013;14:164.
6. Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15(3):175–181.
7. Ali N, Miah R, Hasan M, et al. Association between serum uric acid and metabolic syndrome: a cross-sectional study in Bangladeshi adults. Sci Rep. 2020;10(1):7841.
8. Hong JW, Noh JH, Kim DJ. Association between serum uric acid and spirometric pulmonary function in Korean adults: the 2016 Korea National Health and Nutrition Examination Survey. PLoS One. 2020;15(10):e0240987.
9. Lee CL, Tsai SF. Association between mortality and serum uric acid levels in non-diabetes-related chronic kidney disease: an analysis of the national health and nutrition examination survey, USA, 1999–2010. Sci Rep. 2020;10(1):17585.
10. Ali N, Rahman S, Islam S, et al. The relationship between serum uric acid and lipid profile in Bangladeshi adults. BMC Cardiovasc Disord. 2019;19(1):42.
11. Mankovsky B, Kurashvili R. Is serum uric acid a risk factor for atherosclerotic cardiovascular disease?: a review of the clinical evidence. Part 1. Diabetes and metabolic syndrome. Clin Res Rev. 2010;4(3):176–184.
12. Kleber ME, Delgado G, Grammer TB, et al. Uric Acid and Cardiovascular Events: a Mendelian Randomization Study. J Am Soc Nephrol. 2015;26(11):2831–2838.
13. Dutta A, Henley W, Pilling LC, Wallace RB, Melzer D. Uric acid measurement improves prediction of cardiovascular mortality in later life. J Am Geriatr Soc. 2013;61(3):319–326.
14. Skak-Nielsen H, Torp-Pedersen C, Finer N, et al. Uric acid as a risk factor for cardiovascular disease and mortality in overweight/obese individuals. PLoS One. 2013;8(3):e59121.
15. Odden MC, Amadu AR, Smit E, Lo L, Peralta CA. Uric acid levels, kidney function, and cardiovascular mortality in US adults: national Health and Nutrition Examination Survey (NHANES) 1988–1994 and 1999-2002. Am J Kidney Dis. 2014;64(4):550–557.
16. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13.
17. Ndrepepa G, Cassese S, Braun S, et al. A gender-specific analysis of association between hyperuricaemia and cardiovascular events in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2013;23(12):1195–1201.
18. Panero F, Gruden G, Perotto M, et al. Uric acid is not an independent predictor of cardiovascular mortality in type 2 diabetes: a population-based study. Atherosclerosis. 2012;221(1):183–188.
19. Yang H, Gao J, Ren L, et al. Association between knowledge-attitude-practices and control of blood glucose, blood pressure, and blood lipids in patients with type 2 diabetes in shanghai, china: a cross-sectional study. J Diabetes Res. 2017;2017:3901392.
20. The Central People’s Government of the People’s Republic of China, “Notice of the Ministry of Health on printing and distributing the Regulations of the National Basic Public Health Service, [EB/OL]; 2011, http://www.gov.cn/zwgk/2011-05/24/content_1870181.htm.
21. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25Suppl 2):S49–73.
22. Silbernagel G, Hoffmann MM, Grammer TB, Boehm BO, Marz W. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr Metab Cardiovasc Dis. 2013;23(1):46–52.
23. Resl M, Clodi M, Neuhold S, et al. Serum uric acid is related to cardiovascular events and correlates with N-terminal pro-B-type natriuretic peptide and albuminuria in patients with diabetes mellitus. Diabet Med. 2012;29(6):721–725.
24. Tariq MA, Shamim SA, Rana KF, Saeed A, Malik BH. Serum uric acid - risk factor for acute ischemic stroke and poor outcomes. Cureus. 2019;11(10):e6007.
25. Shao Y, Shao H, Sawhney MS, Shi L. Serum uric acid as a risk factor of all-cause mortality and cardiovascular events among type 2 diabetes population: meta-analysis of correlational evidence. J Diabetes Complications. 2019;33(10):107409.
26. Sotoda Y, Hirooka S, Orita H, Wakabayashi I. Association of serum uric acid levels with leg ischemia in patients with peripheral arterial disease after treatment. J Atheroscler Thromb. 2017;24(7):725–734.
27. Zhao J, Chen H, Liu N, et al. Role of hyperhomocysteinemia and hyperuricemia in pathogenesis of atherosclerosis. J Stroke Cerebrovasc Dis. 2017;26(12):2695–2699.
28. Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108(13):1546–1551.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
