Back to Journals » Cancer Management and Research » Volume 14
Gender Differences in Quality of Life of Metastatic Lung Cancer Patients
Authors Koch M, Rasch F, Rothammer T, Müller K, Mohr A, Koller M, Schulz C
Received 28 March 2022
Accepted for publication 20 August 2022
Published 11 October 2022 Volume 2022:14 Pages 2971—2977
DOI https://doi.org/10.2147/CMAR.S368204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Myriam Koch,1 Frederike Rasch,1 Tobias Rothammer,1 Karolina Müller,2 Arno Mohr,3 Michael Koller,2 Christian Schulz1
1Department of Internal Medicine 2, University Hospital Regensburg, Regensburg, Germany; 2Center for Clinical Studies, University Hospital Regensburg, Regensburg, Germany; 3Center for Pneumology, Donaustauf Hospital, Donaustauf, Germany
Correspondence: Myriam Koch, Department of Internal Medicine 2, University Hospital Regensburg, Franz-Josef-Strauß Allee 11, Regensburg, D-93053, Germany, Tel +49 941 9440, Email [email protected]
Background: Gender aspects in lung cancer patients are a topic of growing interest. But little is known about gender aspects affecting the quality of life (QoL) for those with this life-threatening disease. The aim of the following study was to investigate how gender differences affect QoL in metastatic lung cancer patients.
Methods: In a prospective, multicenter study patients filled out the EORTC QLQ-C30 questionnaire and the recently updated lung cancer module QLQ-LC29 at an undefined point in time during first-line therapy. Gender differences were calculated for all QoL scores using ANCOVAs, which controlled for confounders.
Results: A total of 130 patients with metastatic lung cancer (UICC stage IV) (46 female and 84 male, mean age 66 years) were enrolled in this study by completing the questionnaires. The only significant gender difference in QoL was found regarding hair loss (mean women= 42.498, mean men=25.490, p-value= 0.010), although women received fewer chemotherapy treatments than men (women n=34, 74% and men n=68, 84%).
Conclusion: This study provides evidence that the typical cancer related gender difference effect on QoL, suggesting that men suffer less than women, cannot be found in metastatic tumor stages of lung cancer patients.
Keywords: metastatic lung cancer, gender differences, quality of life, EORTC QLQ-C30/QLQ-LC29
Introduction
As various studies illustrate, gender aspects in lung cancer patients are a topic of growing interest.1–3 Women are increasingly presenting at a younger age and with more advanced disease stages than men, due to the effects of smoking and other environmental factors.2 The most common type of lung cancer in female patients is adenocarcinoma.1,2 Furthermore, in patients suffering from non-small cell lung cancer (NSCLC) evidence points to a higher activity of combined chemo-immunotherapy in women than in men.4
Worldwide lung cancer is one of the most leading form of cancer and its incidence is rising in women.2,3,5–7 As new therapies with longer overall survival rates are quickly emerging, quality of life (QoL) is becoming a very important issue for those suffering from this life-threatening disease. In large-scale German, Dutch, Norwegian and Swedish studies, women have consistently shown a higher symptom burden as well as lower levels of QoL than men when assessed by the EORTC (European Organization for Research and Treatment of Cancer) QLQ-C30 core questionnaire, a well-known questionnaire in oncological trials used to assess QoL in cancer patients.8–14 In contrast, using the QLQ-C30 and the QLQ-LC29 questionnaires, an international, multicenter study on lung cancer patients at any tumor stage, could find no relevant gender difference regarding QoL.15 The QLQ-LC29 is a recently, due to the changes in diagnostic and therapeutic options in lung cancer treatment, updated lung cancer-specific questionnaire used to assess QoL.16,17
However, patients with advanced lung cancer suffer from numerous symptoms which impair QoL.6,18,19 The goal of the present project was to evaluate the EORTC QLQ-C30 combined with the QLQ-LC29 in a cohort of stage IV lung cancer patients and to determine gender aspects in areas that impair QoL.
Method
Study Design
The current report is based on a prospective, multicenter study to analyze the EORTC-QLQ-C30 and the EORTC QLQ-LC29, a newly designed module to assess the QoL of lung cancer patients. Recruitment for this study took place from October 2019 to February 2021 at the University Hospital Regensburg and the Hospital Barmherzige Brüder in Regensburg.
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was registered with the Deutsches Register Klinischer Studien – DRKS (https://drks.de/drks_web/setLocale_EN.do) (reference number DRKS00023355). Approval from the Ethics Committee of the University Regensburg was obtained (reference number 19-1418-101).
Patients
The following eligibility criteria applied: histologically proven non-small cell lung cancer (NCSLC) or small cell lung cancer (SCLC) in metastasized stage (UICC IV) during first-line therapy, 18 years of age or older, no other previous or recurrent tumor, ability to fill out a questionnaire and provide written informed consent. Patients were excluded from the study if any of the above criteria was not fulfilled.
Procedure
Upon being informed about the study and providing written consent, patients filled out the paper-and-pencil version of the EORTC QLQ-C30 and the recently updated lung cancer module QLQ-LC29.
Questionnaires
The EORTC QLQ-C30 (version 3.0) is a core questionnaire designed for use in international clinical trials of cancer patients of any tumor type. The questionnaire consists of 30 individual items and also contains multi- and single item scales, five multi-item function scales (social, role, physical, cognitive and emotional functioning), three multi-item symptom scales (nausea, pain, fatigue), five single items (diarrhea, constipation, dyspnea, appetite loss, insomnia) and one two-item scale to assess global QoL. Every item, except for the two global QoL items, is accompanied by four-item Likert scales with the response options labeled (1) “not at all”, (2) “a little”, (3) “quite a bit” and (4) “very much”. Whereas the two global QoL items are to be completed using a seven-item Likert scale (1=very bad to 7=very good). According to the EORTC scoring manual all scores are subject to linear transformation and are presented on scales ranging from 0 to 100. In the case of functional scores, 0 denotes the lowest and 100 the highest functioning; in the case of symptom scales, 0 denotes the lowest and 100 the highest symptom burden.20
The updated EORTC lung cancer module consists of 29 items, including five single item scales (coughing blood, pain in the chest, pain in the shoulder, bodily pain, problems with weight loss), and five multi-item symptom scales (coughing, shortness of breath, side effects, fear of progression, surgery-related symptoms).
Statistical Analyses
Basic descriptive statistics included counts, percentages, medians/interquartile ranges (IQR), and means and confidence intervals.
Gender differences in all QoL aspects were analyzed using univariate (t-test) and multivariable (analyses of covariance, ANCOVA) models. ANCOVAs adjusted for the following covariates: age, tumor type (NSCLC vs SCLC) and comorbidity (yes/no). Estimated marginal means with corresponding 95%-confidence intervals were presented as effect estimates. A p-value < 0.05 was considered to be the threshold of statistical significance. Due to the exploratory nature of all the analyses, corrections for alpha-error were not applied.
The analyses were performed using the software packages SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA) and SAS 9.4 (SAS Institute, Cary, NC, USA).
Minimal Important Difference
The minimal important difference (MID) is the smallest difference a patient recognizes as an important change in QoL without being of significant value.21 It helps to interpret QoL scores used in oncological trials.21 A Canadian study on patients with small-cell lung cancer and breast cancer suggested that changes in scores of 5–10 represent a small difference, a change of 10–20 represents a moderate difference and a score more than 20 represents a large difference by means of a subjective significance questionnaire and the QLQ-C30 questionnaire.22 In accordance with that study, we defined changes in scores ≥ 5 as representing a perceivable and clinically important difference with respect to cancer-related changes in QoL.
Results
Patient Characteristics
A total of 198 patients with lung cancer were recruited. For the present analysis 130 patients (46 female and 84 male) with metastatic lung cancer were enrolled (Table 1). Median age was 66 years. 112 patients suffered from comorbidities (86.2%, female n=37, male n=75). Chronic obstructive pulmonary disease (COPD) was the most frequent pulmonary disease (n=36, 69.2%) followed by hypoxemic respiratory failure (n=13, 25%). Non-small cell lung cancer was the predominant histological type (NSCLC n=98, 75.4% vs SCLC n= 32, 24.6%). Table 2 shows the administered therapies. 3 patients had still not received any therapy by the date of the survey.
 |
Table 1 Baseline Characteristics |
 |
Table 2 First-Line Therapy |
Gender Differences Regarding Functional and Symptom Scores
Unequivocal gender difference effects on QoL were neither seen by means on QLQ-C30 nor QLQ-LC29 assessments. Table 3 presents the estimated marginal means (EMM) of QoL for women and men and the respective p-values. The only significant gender difference in QoL was found regarding hair loss (mean women= 42.498, mean men=25.490, p-value= 0.010). Otherwise no other statistically significant differences were found.
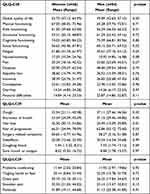 |
Table 3 EORTC QLQ-C30 and QLQ-LC29 Results |
Based on descriptive analytics, minimal important differences that were not statistically significant were found in global QoL (mean women=55.753, mean men=49.890, p-value= 0.200), role functioning (mean women=51.299, mean men=56.393, p-value=0.407), fatigue (mean women=51.855, mean men=45.605, p-value=0.240), nausea/vomiting (mean women=17.548, mean men=10.915, p-value=0.081), financial difficulties (mean women=14.836, mean men=23.866, p-value=0.110), coughing blood (mean women=1.444, mean men=7.428, p-value=0.093), chest pain (mean women=20.104, mean men=26.254, p-value=0.242) and shoulder pain (mean women=32.529, mean men=23.136, p-value=0.121).
Discussion
Gender differences in QoL are an important topic in many studies.8–14 An investigation of the QoL in the general German population using the EORTC QLQ-C30 found that men consistently reported better functioning and fewer symptoms than women.8 Population-based studies in Norway, Sweden and the Netherlands came to the same conclusion.11,13 A Swedish study on advanced NSCLC patients showed that female gender was associated with a worse QoL.6 However, an international, multicenter study on lung cancer patients at any tumor stage, could find no relevant gender difference regarding QoL.15 This study was conducted based on the hypothesis that gender differences in QoL exist mainly at the metastatic tumor stage.
In contrast, we observed only one statistically significant difference to the detriment of women: hair loss. In patients treated with chemotherapy, alopecia is a common symptom that decreases QoL.18,19 However, our female participants received fewer chemotherapy treatments than their male counterparts. This implies that hair loss has a greater impact on QoL in women, which is most likely due to hair having a higher priority in a woman’s appearance compared to men.
In the present study, no other well defined gender differences in QoL of advanced stage lung cancer patients could be found. One explanation for this could be that at the metastatic tumor stage, patients experience the same fears and symptoms regardless of gender, thus diminishing gender effects. One other English study on 987 lung cancer patients corroborates our results. It demonstrates that with clinically assessed decrease in performance status, gender differences regarding depression in lung cancer patients seem to disappear.23 This is mainly due to an increased risk of depression in male patients following worsening of performance status.
With respect to MIDs, which are differences that were not statistically significant but clinically relevant, our study group showed the largest of the overall small MIDs to be nausea/vomiting, financial difficulties, fatigue and shoulder pain, with women having a higher symptom burden concerning fatigue, shoulder pain and nausea/vomiting, this despite the women in our study group received fewer chemotherapy treatments than the men. This is of importance, since an earlier study on metastatic cancer patients showed that the main symptom cluster for women is comprised of nausea, fatigue, pain, drowsiness, and decreased appetite.24
According to a Canadian study, women with advanced cancer face significantly more nausea, a common side effect during chemotherapy compared to men.24 Another study on gender differences in cancer patients comes to the same conclusion regarding nausea.25 Fatigue, one of the most frequently reported symptoms in female lung cancer patients, can occur at any time and can impair QoL itself.26–29 Pain is also a frequently experienced symptom in patients with advanced cancer.25 Generally, women and men differ in their pain threshold and perception due to differences in sex-specific hormones and social expectations.25,30 But an American study showed no significant gender differences in pain for cancer patients.31 Financial difficulties is a common problem for male cancer patients, since they are often primarily responsible for family income.32 The results of the present study are in accordance with these considerations, most likely demonstrating that the loss of a perceived function has an impact on the cancer-related QoL.
However, MIDs can be interpreted as signs of gender differences. Because p-values are highly dependent on sample size, it cannot be excluded that the small sample size of the cohort presented could be responsible for the lack of significance.21 On the other hand, it is possible that gender differences in cancer-related quality of life do not exist at an advanced tumor stage, because existential fears dominate the agenda and have a similar impact on QoL in both male and female lung cancer patients. Thus, a study on gender differences in QoL in lung cancer patients at advanced tumor stages should be performed with a larger sample size. Moreover, to our knowledge the definition of MIDs is only described for the use of the QLQ-C30 and not for the QLQ-LC29. Furthermore, our study only provides limited picture of the QoL of patients with advanced lung cancer. Future research is needed to conduct a longer observation period of QoL from early stages to advanced lung cancer in order to determine whether gender differences disappear with increasing tumor stages. Moreover, the point in time in which the participants were asked, was not documented in this study, although the point in time has an influence on the QoL of cancer patients. During the first diagnosis, QoL may be very low due to existential feelings, whereas during treatment it depends on the regime how the cancer patients might feel. Six months after diagnosis, QoL may be better, since the burdensome treatment phase is finished for some patients.
Conclusion
This study adds to the literature in showing that the typical gender difference effect on QoL, suggesting that men suffer less than women, does not exist in metastatic tumor stages of lung cancer.
Abbreviations
COPD, Chronic obstructive pulmonary disease; EORTC, European Organization for Research and Treatment of Cancer; MID, minimal important difference; N, number; NSCLC, non-small cell lung cancer; QoL, Quality of life; SCLC, small cell lung cancer; UICC, Union for International Cancer Control.
Data Sharing Statement
Dr. Myriam Koch will respond to data sharing requests under the premise that an adequate research question is formulated. Original anonymized data will be made available up to one year after the publication of the paper.
Disclosure
Dr. Myriam Koch, Frederike Rasch, Tobias Rothammer, Karolina Müller, Dr. Arno Mohr and Prof. Dr. Christian Schulz have nothing to disclose for this work. Prof. Dr. Michael Koller reports a grant from the EORTC. (Reference number: Koller Lung 03/2016).
References
1. Sakurai H, Asamura H, Goya T, et al. Survival differences by gender for resected non-small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol. 2010;5(10):1594–1601. doi:10.1097/JTO.0b013e3181f1923b
2. Ulas A, Tokluoglu S, Kos M, et al. Lung cancer in women, a different disease: survival differences by sex in Turkey. Asian Pacific J Cancer Prev. 2015;16(2):815–822. doi:10.7314/APJCP.2015.16.2.815
3. Kim HR, Kim SY, Kim CH, et al. Sex-specific incidence of EGFR mutation and its association with age and obesity in lung adenocarcinomas: a retrospective analysis. J Cancer Res Clin Oncol. 2017;143(11):2283–2290. doi:10.1007/s00432-017-2473-8
4. Vavalà T, Catino A, Pizzutilo P, Longo V, Galetta D. Gender differences and immunotherapy outcome in advanced lung cancer. Int J Mol Sci. 2021;22(21):11942. doi:10.3390/ijms222111942
5. Rauma V, Salo J, Sintonen H, Rasanen J, Ilonen I. Patient features predicting long-term survival and health-related quality of life after radical surgery for non-small cell lung cancer. Thorac Cancer. 2016;7(3):333–339. doi:10.1111/1759-7714.12333
6. Larsson M, Ljung L, Johansson BB. Health-related quality of life in advanced non-small cell lung cancer: correlates and comparisons to normative data. Eur J Cancer Care (Engl). 2012;21(5):642–649. doi:10.1111/j.1365-2354.2012.01346.x
7. Khamboon T, Pakanta I. Intervention for symptom cluster management of fatigue, loss of appetite, and anxiety among patients with lung cancer undergoing chemotherapy. Asia Pacific J Oncol Nurs. 2021;8(3):267–275. doi:10.4103/2347-5625.311003
8. Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37(11):1345–1351. doi:10.1016/S0959-8049(00)00447-0
9. Hinz A, Singer S, Brahler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: results of a German investigation and a summarizing analysis of six European general population normative studies. Acta oncologica. 2014;53(7):958–965. doi:10.3109/0284186X.2013.879998
10. Waldmann A, Schubert D, Katalinic A. Normative data of the EORTC QLQ-C30 for the German population: a population-based survey. PLoS One. 2013;8(9):e74149. doi:10.1371/journal.pone.0074149
11. Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: the QLQ=C30 (+ 3). J Clin Oncol. 1998;16(3):1188–1196. doi:10.1200/JCO.1998.16.3.1188
12. Scott Neil W, Aaronson Neil K, Andrew B, et al.; on hehalf of the EORTC Quality of Life Group. EORTC QLQ-C30 reference values. 2008.
13. Derogar M, van der Schaaf M, Lagergren P. Reference values for the EORTC QLQ-C30 quality of life questionnaire in a random sample of the Swedish population. Acta oncologica. 2012;51(1):10–16. doi:10.3109/0284186X.2011.614636
14. van de Poll-Franse LV, Mols F, Gundy CM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667–675. doi:10.1016/j.ejca.2010.11.004
15. Koch M, Hjermstad MJ, Tomaszewski K, et al. Gender effects on quality of life and symptom burden in patients with lung cancer: results from a prospective, cross-cultural, multi-center study. J Thorac Dis. 2020;12(8):4253–4261. doi:10.21037/jtd-20-1054
16. Koller M, Hjermstad MJ, Tomaszewski KA, et al. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Ann Oncol. 2017;28(11):2874–2881. doi:10.1093/annonc/mdx453
17. Koller M, Warncke S, Hjermstad MJ, et al. Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: a systematic review of the literature 20 years after its development. Cancer. 2015;121(24):4300–4323. doi:10.1002/cncr.29682
18. Daroszewski C, Stasiewicz M, Jaźwińska-Tarnawska E, et al. Quality of life in patients with advanced non-small-cell lung cancer receiving palliative chemotherapy. Adv Exp Med Biol. 2019;1160:11–18.
19. de Oliveira PI, Pereira CA, Belasco AG, Bettencourt AR. Comparison of the quality of life among persons with lung cancer, before and after the chemotherapy treatment. Rev Lat Am Enfermagem. 2013;21(3):787–794. doi:10.1590/S0104-11692013000300019
20. Fayers PM, Bjordal K, Groenvold M, Curran D, Bottomley A; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual.
21. Maringwa JT, Quinten C, King M, et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer. 2011;19(11):1753–1760. doi:10.1007/s00520-010-1016-5
22. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi:10.1200/JCO.1998.16.1.139
23. Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18(4):893–903. doi:10.1200/JCO.2000.18.4.893
24. Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011;19(3):417–423. doi:10.1007/s00520-010-0865-2
25. Chow S, Ding K, Wan BA, et al. Gender differences in pain and patient reported outcomes: a secondary analysis of the NCIC CTG SC. 23 randomized trial. Ann Palliat Med. 2017;6(Suppl 2):S185–S194. doi:10.21037/apm.2017.08.12
26. Carnio S, Di Stefano RF, Novello S. Fatigue in lung cancer patients: symptom burden and management of challenges. Lung Cancer. 2016;7:73–82. doi:10.2147/LCTT.S85334
27. Horneber MFI, Dimeo F, Rüffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Deutsches Ärzteblatt. 2012;109(9):161–172.
28. Visovsky C, Schneider SM. Cancer-related fatigue. Online J Issues Nurs. 2003;8(3):8.
29. Sarna L. Correlates of symptom distress in women with lung cancer. Cancer Pract. 1993;1(1):21–28.
30. Pieretti S, Di Giannuario A, Di Giovannandrea R, et al. Gender differences in pain and its relief. Annali dell’Istituto superiore di sanita. 2016;52(2):184–189. doi:10.4415/ANN_16_02_09
31. Turk DC, Okifuji A. Does sex make a difference in the prescription of treatments and the adaptation to chronic pain by cancer and non-cancer patients? Pain. 1999;82(2):139–148. doi:10.1016/S0304-3959(99)00041-X
32. Syse A, Tretli S, Kravdal O. The impact of cancer on spouses’ labor earnings: a population-based study. Cancer. 2009;115(18 Suppl):4350–4361. doi:10.1002/cncr.24582
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
