Back to Journals » International Medical Case Reports Journal » Volume 11
Gastric metastasis of ovarian serous cystadenocarcinoma
Authors Yang S
Received 23 April 2018
Accepted for publication 3 July 2018
Published 5 September 2018 Volume 2018:11 Pages 201—204
DOI https://doi.org/10.2147/IMCRJ.S171985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Shiqiang Yang
Xintai Hospital Affiliated to Taishan Medical University, Tai’an City, People’s Republic of China
Background: Gastric metastasis from ovarian serous cystadenocarcinoma is extremely rare.
Case presentation: We herein report one case of a 45-year-old female with ovarian carcinoma who underwent cytoreductive surgery. Two years later, endoscopic ultrasonography-guided fine needle aspiration revealed gastric metastasis. The patient underwent laparoscopic resection of gastric metastases. She is currently in complete remission.
Conclusion: Gastric metastasis from ovarian cancer should not be ignored in the clinic.
Keywords: gastric neoplasm, ovarian neoplasm, metastasis
Introduction
Ovarian cancer is one of the common gynecologic tumors, and the mortality is the first among all kinds of female tumors.1 Metastasis of ovarian cancer to the stomach is extremely rare because ovarian cancer typically spreads along the peritoneum and throughout the pelvic and abdominal cavities.2 Most gastric metastases are reported to arise from breast cancer, lung cancer, and melanoma.3 The incidence of gastric metastasis in breast cancer and lung cancer is 3.6% and 1.3%, respectively.4 We herein describe the first case of gastric metastasis from ovarian carcinoma in our hospital. Gastrointestinal stromal tumor was suspected based on the imaging and endoscopic findings. The diagnosis of gastric metastasis of ovarian cancer was confirmed by biopsy and pathological investigation.
Case presentation
In October 2015, a 45-year-old female was admitted to our hospital with a 1-year history of pelvic mass. Computed tomography (CT) examination showed bilateral ovarian tumors. Her serum cancer antigen 125 (CA125) level was 11.22 kU/L (reference range: <35.0 kU/L). Serum carcinoembryonic antigen (CEA) level was 1.31 µg/mL (reference range: 0–6.5 µg/mL). She underwent a total hysterectomy with bilateral salpingo-oophorectomy and pelvic and para-aortic lymph node dissection with total omentectomy subsequently. Postoperative pathological examination showed bilateral ovarian serous cystadenocarcinoma. Immunohistochemical examination yielded the following results: CK (+++) (Figure 1A), Ki-67 (+++) (Figure 1B), P53 (+++) (Figure 1C), S-100 (−), and villin (−) (Figure 1D). The International Federation of Gynecology and Obstetrics (FIGO) stage was IIA. After cytoreductive surgery, the patient followed eight cycles of adjuvant chemotherapy with paclitaxel 175 mg/m2 over 3 hours plus carboplatin area under the curve (AUC) =5, every 21 days as one cycle. After the chemotherapy, the patient was followed up by means of reexamination every 3 months. Therapeutic evaluation was stable.
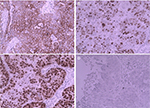  | Figure 1 Immunohistochemistry revealed (A) CK(+++); (B) Ki-67(+++); (C) P53(+++); (D) Villin(–). Note: Magnification ×200. |
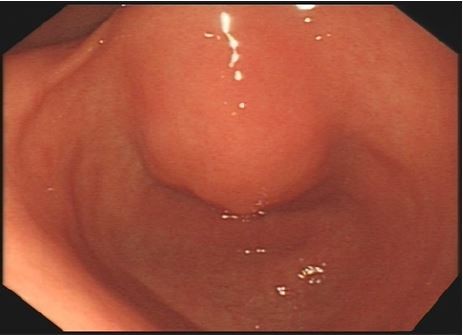
In November 2017, the patient was presented to our hospital again with abdominal pain. Subsequent abdominal enhanced CT examination showed the lesion occupying the lesser curvature of the gastric antrum, which was of size 3.6×2.8 cm (Figure 2). Gastroscopy showed chronic superficial gastritis and submucous eminent lesions in the lesser curvature of the gastric antrum (size 3.5×2.5 cm; Figure 3). Combined with the results of CT and gastroscopy, we diagnosed the patient with gastrointestinal stromal tumor (GIST). Then, we performed a fine needle aspiration (FNA) biopsy under endoscopic ultrasonography. The gastric metastasis was located in the intrinsic myometrium. H–E staining revealed the gastric metastasis of ovarian serous cystadenocarcinoma (Figure 4A and B). The serum CA125 level was 10.40 kU/L. The patient underwent pelvic enhanced CT examination and brain magnetic resonance imaging (MRI) examination, and no other metastases were found. Subsequently, laparoscopic resection of the gastric metastasis was accepted. Because the interval between the last chemotherapy was ~18 months, ie, the platinum-free interval (PFI) was >12 months,5 the patient underwent another eight cycles of adjuvant chemotherapy with carboplatin plus gemcitabine. The patient was reexamined after every two cycles of chemotherapy. The serum CA125 levels showed no elevation. She is currently in complete remission. Written informed consent has been provided by the patient to have the case details and any accompanying images published.
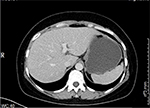  | Figure 2 Abdominal CT examination showed the occupying lesion of the lesser curvature of gastric antrum (size 3.6×2.8 cm). |
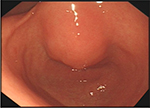  | Figure 3 Gastroscopy showed chronic superficial gastritis and submucous eminent lesions in the lesser curvature of gastric antrum (size 3.5×2.5 cm). |
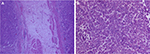  | Figure 4 Gastric metastasis of ovarian serous cystadenocarcinoma ×50 (A); ×100 (B). |
Discussion
Ovarian carcinomas are mainly composed of three pathological types: epithelial cancer, malignant germ cell tumor, and sex cord stromal tumor. Among the three types, the incidence of epithelial cancer is the highest. In epithelial cancer, serous cystadenocarcinoma is the most common, accounting for ~75%–80% of all cases. The early clinical symptoms of ovarian serous cystadenocarcinoma are not obvious. The most common symptom is abdominal mass. Implantation metastasis is the main path of ovarian cancer. Epithelial ovarian cancer usually spreads along the peritoneum to the entire pelvic cavity, or even the abdominal cavity, and often metastasizes to the intestinal serosa, mesentery, and greater omentum, followed by local spread and lymph node metastasis. Ovarian cancer with hematogenous metastasis is very rare and usually metastasizes to solid organs, such as the liver, brain, lung, and bone. The central nervous system, skin, and pericardium are rarely metastasized.6 Even if gastrointestinal metastasis occurs, it is usually transferred from the outer serous layer to the inner.
Gastric metastasis from ovarian serous cystadenocarcinoma is extremely rare in the clinic.7 Ovarian cancer comprises only 0.013%–1.6% of all gastric metastatic tumors.8 The most frequent locations of occult metastatic disease from ovarian cancer are the omentum, the uterus, the fallopian tubes, the lymph nodes, the abdominal peritoneum, and the pelvic peritoneum.9 Although the mechanism of gastric metastasis remains unclear, it can be explained by the stomach receiving a rich supply of blood; so, it should be considered a possible target organ of metastasis. Clinical manifestations of gastric metastasis are nonspecific and include epigastric pain, melena, anemia, nausea, and vomiting. The patient was presented for abdominal pain in this case. A GIST was suspected in the present case based on the abdominal CT and gastroscopy. Kang et al10 reported one case of ovarian carcinoma with gastric metastasis that mimicked GIST. Similarly, in this present case, we misdiagnosed that the gastric lesion was GIST. After endoscopic ultrasonography-guided fine needle aspiration and pathological examination, it was diagnosed as ovarian cancer with gastric metastasis.
In this case, the patient was diagnosed with gastric metastasis 2 years after cytoreductive surgery of ovarian cancer and chemotherapy. We performed laparoscopic resection of the gastric metastases. Combined with the PFI, we carried out subsequent systemic chemotherapy. The best long-term survival strategy for metastatic ovarian cancer is complete cytoreductive surgery. Chi et al11 have found that cytoreductive surgery for recurrent ovarian cancer was an independent prognostic factor. Additional systemic chemotherapy was administered when gastric metastasis was confirmed. Endoscopic ultrasonography with fine needle aspiration or biopsy is useful for the correct diagnosis of gastric submucosal lesions. The prognosis for patients with gastric metastasis is poor, and the median survival period is 170 days (range: 16–892 days) for all cases.12 Currently, the patient has achieved complete remission after surgery and chemotherapy. Now, the patient is reexamined every 3 months. We will continue to focus on the follow-up treatment of the patient.
Conclusion
Gastric metastasis of ovarian serous cystadenocarcinoma is a rare event. Clinicians should nevertheless be aware that, in patients with a submucosal tumor and a history of ovarian cancer, gastric lesions may be secondary metastases from the ovarian cancer. For gastric metastatic lesions, laparoscopic resection is recommended when possible.
Disclosure
The author reports no conflicts of interest in this work.
References
Choi M, Fuller CD, Thomas CR, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988-2001. Gynecol Oncol. 2008;109(2):203–209. | ||
Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surg Today. 2014;44(8):1392–1399. | ||
Obeidat F, Mismar A, Shomaf M, Yousef M, Fram K. Gastric perforation secondary to metastasis from ovarian cancer: Case report. Int J Surg Case Rep. 2013;4(6):541–543. | ||
Zhou JJ, Miao XY. Gastric metastasis from ovarian carcinoma: a case report and literature review. World J Gastroenterol. 2012;18(43):6341–6344. | ||
Tomao F, D’Incalci M, Biagioli E, Peccatori FA, Colombo N. Restoring platinum sensitivity in recurrent ovarian cancer by extending the platinum-free interval: Myth or reality? Cancer. 2017;123(18):3450–3459. | ||
Cormio G, Rossi C, Cazzolla A, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13(2):125–129. | ||
Jung HJ, Lee HY, Kim BW, et al. Gastric Metastasis from Ovarian Adenocarcinoma Presenting as a Submucosal Tumor without Ulceration. Gut Liver. 2009;3(3):211–214. | ||
Akce M, Bihlmeyer S, Catanzaro A. Multiple gastric metastases from ovarian carcinoma diagnosed by endoscopic ultrasound with fine needle aspiration. Case Rep Gastrointest Med. 2012;2012:610527–3. | ||
Powless CA, Bakkum-Gamez JN, Aletti GD, Cliby WA. Random peritoneal biopsies have limited value in staging of apparent early stage epithelial ovarian cancer after thorough exploration. Gynecol Oncol. 2009;115(1):86–89. | ||
Kang WD, Kim CH, Cho MK, et al. Primary epithelial ovarian carcinoma with gastric metastasis mimic gastrointestinal stromal tumor. Cancer Res Treat. 2008;40(2):93–96. | ||
Chi DS, Mccaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106(9):1933–1939. | ||
Kobayashi O, Murakami H, Yoshida T, et al. Clinical diagnosis of metastatic gastric tumors: clinicopathologic findings and prognosis of nine patients in a single cancer center. World J Surg. 2004;28(6):548–551. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
