Back to Journals » OncoTargets and Therapy » Volume 13
Gastric H+/K+-ATPase Expression in Normal Laryngeal Tissue and Laryngeal Carcinoma
Authors Bao YY, Jiang Q, Li ZW, Yu E, Zhou SH , Yao HT, Fan J, Yong WW
Received 11 August 2020
Accepted for publication 30 November 2020
Published 17 December 2020 Volume 2020:13 Pages 12919—12931
DOI https://doi.org/10.2147/OTT.S276233
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Prof. Dr. Takuya Aoki
Yang-Yang Bao,1 Qian Jiang,1 Zhen-Wei Li,1,2 Er Yu,1 Shui-Hong Zhou,1 Hong-Tian Yao,3 Jun Fan,4 Wei-Wei Yong5
1Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China; 2Department of Otolaryngology, The First People’s Hospital of Hangzhou City, Hangzhou, Zhejiang 310013, People’s Republic of China; 3Department of Pathology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China; 4State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China; 5Department of Pathology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China
Correspondence: Shui-Hong Zhou
Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China
Tel +86-13868060120
Email [email protected]
Background: Several studies have suggested that laryngopharyngeal reflux disease (LPRD) or gastroesophageal reflux disease (GERD) is an independent risk factor for laryngeal carcinoma. However, it remains unclear whether either condition affects the level of H+/K+-ATPase expression in laryngeal carcinoma.
Materials and Methods: Immunohistochemistry, real-time RT-PCR, and Western blotting were used to explore the distributions of proton pump (H+/K+-ATPase) α- and β-subunits in normal laryngeal tissue and laryngeal carcinoma.
Results: Messenger RNAs encoding both the α- and β-subunits were found in the normal epiglottic, ventricular fold, vocal fold, and arytenoid mucosae, as well as epiglottic cartilage. The distributions and expression levels of H+/K+-ATPase α-subunits in various laryngeal subregions did not significantly differ in IHC, RT-PCR, or Western blotting. However, Western blotting revealed a significant difference between the expression level of the β-subunit protein in the epiglottic cartilage and the levels in other sites. The expression levels of both subunits were significantly higher in carcinomatous than in paracarcinomatous tissue and normal laryngeal tissue. The mean follow-up duration was 66.2 months (range, 17– 162 months). In all, 4 patients died during follow-up, 4 were lost to follow-up, and 22 were alive and free of disease at the end of follow-up. Two patients developed lung metastases and six developed disease recurrences (at 2, 8, 14, 16, 36, and 41 months). The 3- and 5-year overall survival (OS) rates were 93.0% and 77.0%, respectively. Univariate analyses showed that the 5-year OSs were significantly associated with the T, N, and clinical stages but not with age, alcohol use, pathological differentiation, or the expression levels of the α- or β-subunits (as revealed by IHC, RT-PCR, or Western blotting). However, in multivariate regression analyses, the 5-year OSs were not significantly associated with any clinicopathological factor or the expression levels of either subunit.
Conclusion: H+/K+-ATPase is expressed in the normal larynx, including in the epiglottic cartilage and the mucosae of the epiglottis, ventricular fold, and arytenoid vocal fold. The expression levels of the H+/K+-ATPase α- and β-subunits in laryngeal carcinomas were higher than in normal laryngeal tissues.
Keywords: proton pump, H+/K+-ATPase, larynx, laryngeal carcinoma, laryngopharyngeal reflux disease
Corrigendum for this paper has been published
Introduction
Laryngopharyngeal reflux disease (LPRD) is a distinct variation of gastroesophageal reflux disease (GERD).1 The actual cause of LPRD remains unclear. It may be due to activated proton pump over-secreted gastric acid, which refluxes and leads to LPRD symptoms and pathology.2
The proton pump (H+/K+-ATPase) is composed of the α-subunit and β-subunit.2,3 The proton pump is primarily located in the parietal cells of the stomach, where it secretes hydrogen ions (protons) into the gastric lumen. H+/K+-ATPase is a member of the P2-type ATPase family, such as Na+/K+- and Ca2+-ATPases. The production of acid with exchange of K+ for H+ is an ATP-consuming process. The α-subunit plays a catalytic role, due to its binding site and hydrolysis of ATP. The α-subunit is composed of transmembrane helices, which form the ion transport pathway and the binding sites for proton pump inhibitors (PPI) and potassium-competitive acid blockers. The β-subunit is a non-catalytic glycoprotein, which consists of seven potential glycosylation sites that are responsible for stabilizing the α-subunit and assembling the entire enzyme, enabling proton pump function.2,3 The proton pump (H+/K+-ATPase) exists as gastric and non-gastric H+/K+-ATPase isoforms, which have structural and functional similarities.4,5
In 1995, the proton pump (H+/K+-ATPase) was first detected outside the stomach in the kidney. Since then the proton pump has been identified in the human lung mucous glands, rat heart, mouse thymus, rat prostate, and ciliary epithelium of the rabbit eye.6–10 Altman et al first detected the proton pump (H+/K+-ATPase) in the laryngeal submucosal gland of two human cadaveric larynges in 2003.11 Some studies also found H+/K+-ATPase distributed in the human larynx.2,12–14 Thus, these findings suggest that acid may be produced not only by the gastric parietal cells but also by H+/K+-ATPase cells in the human larynx. It may also explain how some patients without apparent LPRD may have a relatively satisfactory outcome from PPI pharmacotherapy.2 However, Herrmann et al reported that the only organ expressing significant levels of H+/K+-ATPase in humans is the stomach.15 Therefore, the question of whether H+/K+-ATPase is localized in the larynx needs further investigation.
Some studies have also found that GERD and/or its variant LPRD is related to laryngeal carcinoma. Although the prevalence of LPRD in patients with laryngeal carcinoma is as high as 67%, the role of GERD or LPRD in the development of laryngeal cancer is controversial. Several studies have suggested that GERD or LPRD is an independent risk for laryngeal carcinoma.16–20 Some experimental studies have reported that gastric refluxate, including acid, pepsin, and bile acids, has a direct role in laryngeal cell biology.19–21 However, whether H+/K+-ATPase is involved in this process has not been investigated. Some studies have shown that non-acidic reflux promotes the development of laryngeal carcinoma.22–24 Thus, the relationship between LPRD and laryngeal carcinoma requires further investigation.
In the present study, we studied the expression of the gastric proton pump (H+/K+-ATPase) α- and β-subunits in normal laryngeal tissues, particularly their distributions in supraglottic and glottic areas via immunohistochemistry (IHC), real-time RT-PCR, and Western blotting. We also studied the expression of the proton pump (H+/K+-ATPase) α- and β-subunits in laryngeal carcinoma and analyzed the relationships between expression of the α- and β-subunits and laryngeal carcinoma.
Materials and Methods
Specimens
Normal laryngeal tissues were excised from 57 subjects with laryngeal carcinoma or hypopharyngeal carcinoma (total 195 specimens: 137 specimens from epiglottic mucosa, 24 specimens from the ventricular fold mucosa, 14 from arytenoid mucosa, nine from epiglottic cartilage, and 11 from the vocal fold mucosa). Specimens from the subglottic area were not available. All patients underwent either open partial or total laryngectomy. Not all specimens underwent immunohistochemical staining, RT-PCR, and Western blotting assays because of a lack of sufficient tissue. In total, 181 and 195 specimens underwent α-subunit and β-subunit IHC: 168 specimens for α-subunit mRNA and 171 specimens for β-subunit mRNA using the real-time reverse transcription polymerase chain reaction (RT-PCR), 55 specimens for α- and β-subunit proteins by Western blotting. The specimens were taken from normal laryngeal mucosa or epiglottic cartilage far from the negative margin after open partial laryngectomy or total laryngectomy after tumor excision, and there were no abnormal changes confirmed by pathology.
Ninety specimens were taken from 30 patients with laryngeal carcinoma who underwent open partial or total laryngectomy; 60 paracarcinoma tissue samples (taken 0.5 cm from the negative margin after tumor excision) collected from 20 of 30 patients with laryngeal carcinoma. In all, 30 laryngeal carcinoma specimens and 17 paracarcinoma tissue samples, respectively, were subjected to α- and β-subunit IHC; 30 and 60 specimens, respectively, were subjected to α- and β-subunit RT-PCR and Western blotting analyses (Table 1).
 |
Table 1 Numbers of Specimens for Three Detection Methods |
All fresh samples were frozen immediately in liquid nitrogen following surgical removal and maintained until use. These specimens were banked and accessed retrospectively. The exclusion criteria were preoperative radiotherapy and/or chemotherapy and/or immunotherapy, a history of treatment with pump proton inhibitors (PPIs), and/or any metabolic or autoimmune disease.
Immunohistochemical Technique
The samples were fixed in formaldehyde immediately after thawing and embedded in paraffin. Five-micrometer-thick sections were cut, deparaffinized, and rehydrated. The sections were washed three times (3 min each) in phosphate-buffered saline (PBS; pH 7.4). Antigen retrieval was achieved using a high-temperature (100°C), high-pressure method for 15 min. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide for 10 min at room temperature. All sections were treated with normal non-immune serum for 10 min at room temperature. A drop of antibody solution containing anti-gastric H+/K+-ATPase α-subunit (1:285; D031–3, Clone 1H9) or β-subunit (1:285; D032–3, Clone 1B6; both from MBL International Corp., Woburn, MA, USA) was added to all sections, which were incubated overnight at 4°C; then the sections were washed three times (1 min each) in PBS at room temperature. Biotin-labeled secondary antibody was added to all sections, which then were incubated at room temperature for 10 min and washed three times (1 min each) in PBS. A drop of streptavidin–peroxidase solution was added to all sections at room temperature and incubated for 10 min. After three 1 min washes in PBS, a drop of chromogen diaminobenzidine was added, and the sections were observed under a microscope. After all sections had been washed with distilled water, they were stained with hematoxylin and eosin and dehydrated through a graded ethanol series for 4 min each.
To quantify the levels of the α- and β-subunits of H+/K+-ATPases, images were taken using an Axioskop 2 camera (Carl Zeiss, Jena, Germany) and analyzed using Image-Pro Plus ver. 6.0 software (National Institutes of Health, Bethesda, MD, USA). Cells were quantified via manual counting in four microscopic fields (×400 magnification). This was performed by two independent investigators in a blinded manner. Each was counted to five fields per tumor, and calculated the mean value of the top, middle, and basic sections.
Stomach tissue served as a positive control. PBS was added (instead of the primary antibody) as a negative control. Specimens were examined by two experienced pathologists (YHT and YWW) blinded to the nature of the slides (tumor vs. normal vs. paracarcinoma tissue).
Cells that were positive for the H+/K+-ATPase α- and β-subunits stained brown, and the staining intensities were scored as follows: 0, no expression; 1, weak expression; 2, moderate expression; and 3, strong expression. The cell staining rate was scored as <10%, 10–25%, 26–50%, and >50%. The total score was the staining rate plus the staining intensity score. Immunohistochemical expression of the H+/K+-ATPase α- and β-subunits was scored as the proportion of positive cells: (–, 0–1; +, 2; ++, 3–4; and +++; 5–6).
Detection of the H+/K+-ATPase α- and β-Subunits by Real-Time RT-PCR
H+/K+-ATPase α- and β-subunit mRNA expression was detected in the surgical specimens by real-time RT-PCR, in accordance with the manufacturer’s instructions. (1) RNA isolation: part of the fresh samples stored at –80°C were ground in liquid nitrogen and transferred to a 5 mL centrifuge tube containing 1 mL Trizol. The mixture was incubated for 5 min at room temperature and centrifuged for 10 min at 12,000 rad/min (4°C). The supernatant was transferred to another 1.5 mL centrifuge tube, and 200 µL chloroform was added; this was followed by a 10-min centrifugation at 12,000 rad/min (4°C). The supernatant was transferred to a new 1.5 mL centrifuge tube, 600 µL isopropanol was added and mixed evenly; this was followed by centrifugation for 15 min at 12,000 rad/min (4°C). The supernatant was discarded and 1 mL of 75% absolute ethanol (750 µL absolute ethanol + 250 µL DEPC-treated water) was added and centrifuged for 5 min at 12,000 rad/min (4°C). The supernatant was discarded and 1 mL absolute ethanol was added and centrifuged for 5 min at 12,000 rad/min (4°C). The supernatant was discarded and 40 µL DEPC-treated water was added to dissolve the RNA. (2) cDNA synthesis: DNA was removed from total RNA in a 10 µL reaction solution (1 µL DNase I, 1 µL 10× DNase I Buffer, ≤1 ng total RNA, and up to 10 µL DEPC-H2O). The reaction procedures were 37°C for 30 min, 1 µL EDTA, and 65°C for 10 min. Reverse transcriptase reaction: RNA was reverse-transcribed into cDNA in a 25 µL reaction volume consisting of 12 µL of RNA-Prime Mix, 5 µL of 5× RT Reaction Buffer, 1 µL of 25 mM dNTPs, 1 µL of 25 U/µL RNase Inhibitor, 1 µL of Oligo (dt), and up to 25 µL ddH2O (DNase-free). The reaction procedure conditions were 37°C for 60 min, 85°C for 5 min, 4°C for 5 min, and storage at –20°C. Real-time PCR reaction: the above product was amplified by real-time RT-PCR in a 25 µL amplification system: 12.5 µL of SYBR Green MIX, 0.5 µL of the upstream primer, 0.5 µL of the downstream primer, 9.5 µL of ddH2O, and up to 25 µL of the cDNA template. The reaction procedure conditions were 95°C for 10 min; (95°C, 15 s; 60°C, 45 s) × 40; 95°C for 15 s, 60°C for 1 min, 95°C for 15 s, 60°C for 15 s. The molecular weights of the α- and β-subunits were 94 and 70 kDa, respectively. H+/K+-ATPase: α-subunit forward primer: CTTTGCCATCCAGGCTAGTGA and reverse primer: CTTTGCCATCCAGGCTAGTGA and reverse primer: GGTGACGACAACCACAGCAAT; β-subunit forward primer: CCAGGTGGGTGTGGATCAG and reverse primer: GAGGCACAGGGCGAAGAG (https://www.mblintl.com/products/d031–3; https://www.mblintl.com/products/d032–3) and GAPDH forward GACAGTCAGCCGCATCTTCT and reverse primer: TTAAAAGCAGCCCTGGTGAC, respectively. The lengths of the PCR products were 88 bp (α-subunit), 208 bp (β-subunit), and 127 bp (GAPDH).
Detection of the H+/K+-ATPase α- and β-Subunit Proteins by Western Blotting
The α- and β-subunit proteins of the surgical specimens were detected by Western blotting, performed in accordance with the manufacturer’s instructions. The tissues were minced and incubated in radioimmunoprecipitation assay lysis solution. The tissues were centrifuged (12,000 rpm for 4 min, 4°C) and the supernatant was stored at –70°C. The amount of total protein was assayed using the BAC method (Beyotime Biological Technology Co. Ltd., Shanghai, China). (1) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis: the supernatant was added to the sample well and electrophoresed. The proteins were transferred to a nitrocellulose membrane. The membrane was incubated with the primary antibody overnight at 4°C. (2) Western blotting: The normal control sample received 5% blocking buffer (skimmed milk powder) and was washed with TBST (10 min × 3). The sample was incubated with the primary antibody (1:1000 α-subunit, mouse monoclonal antibody (mAb); 1:1000 β-subunit, mouse mAb (MBL International), GAPDH (abcam, ab8245, catalog no. 1500, Clone 6C5), overnight at 4°C, and was incubated with the secondary antibody at room temperature for 1 h. The immunocomplexes were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech, Uppsala, Sweden), and the images were scanned using the FluorChem E gel imager.
Statistical Analyses
Data were analyzed using SPSS statistical software for Windows (ver. 20.0; IBM Corp., Armonk, NY, USA). The associations between the expression levels of the H+/K+-ATPase α- and β-subunits, and clinicopathological parameters, were analyzed using the chi-square or Fisher’s exact test. Overall survivals (OSs) were the times from surgery to death from any cause and were plotted as Kaplan–Meier curves. Univariate survival analyses were performed using the Log-rank test, and multivariate analyses were performed via Cox proportional hazards regression analyses. A p-value < 0.05 was considered significant.
Results
Distribution and Expression of the Proton Pump (H+/K+-ATPase) in Normal Laryngeal Tissues
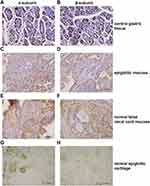
Of 181 normal laryngeal tissues, 45 (24.9%) were positive for the α-subunit (Figure 1, Table 2). Of 195 normal laryngeal tissues, 43 (22.1%) were positive for the β-subunit (Figure 1, Table 2). Both subunits tended to be more strongly expressed in the supraglottic area than in the glottic area (22.1% [43/195] vs. 8.3% [1/12]), (23.4% [43/184] vs. 0.0% [0/11])
 |
Table 2 The Expression of α-Subunit in Normal Laryngeal Tissues in Three Detection Methods |
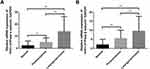
The expression levels of α- and β-subunit mRNA, detected via RT-PCR, were 2.314 ± 0.274 and 2.306 ± 0.268, respectively (Figure 2. Tables 2 and 3). The expression levels of mRNAs encoding the α- and β-subunits did not differ among the various sites (all p > 0.05).
 |
Table 3 The Expression of β-Subunit in Normal Laryngeal Tissues in Three Detection Methods |
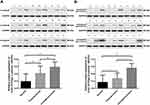
Expression of the α- and β-subunit proteins in normal laryngeal tissues was evaluated by Western blotting. The results showed that the α- and β-subunit protein expression levels were 0.193 ± 0.029 and 0.170 ± 0.270, respectively (Figure 3; Tables 2 and 3). The expression levels of the α- and β-subunit proteins did not differ among the various laryngeal mucosal tissues (all p > 0.05). A significant difference was detected between the expression of the β-subunit protein in the epiglottic cartilage and the other sites (p < 0.05).
Expression of the Proton Pump (H+/K+-ATPase) in Laryngeal Carcinoma Tissues
Patient Characteristics
All patients were men. Of the 30 patients with laryngeal carcinoma, 5 (16.7%) exhibited tumors in the supraglottic area, 24 (80.0%) in the glottic area, and 1 (3.3%) in the subglottic area. According to the 2012 International Union Against Cancer tumor, node, and metastasis (TNM) staging system, 5 (16.7%) patients exhibited stage T1N0M0, 18 (60.0%) exhibited stage T2N0M0, 3 (10.0%) exhibited stage T3N0M0, 2 (6.7%) exhibited stage T3N2M0, 1 (3.3%) exhibited stage T4N0M0, and 1 (3.3%) exhibited stage T4N1M0. The clinical stage distribution was as follows: stage I, n = 5 (16.7%); II, n = 18 (60.0%); III, n = 3 (10.0%); and IV, n = 4 (13.3%). The pathological results identified squamous cell carcinomas in all 30 patients, 4 of which exhibited poor differentiation and 26 of which exhibited moderate/high differentiation (Table 4).
 |
Table 4 The Relationship Between the Expression of α-Subunit or β-Subunits and Clinicopathological Features of Laryngeal Carcinoma |
Expression of the H+/K+-ATPase α- and β-Subunits
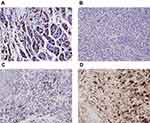
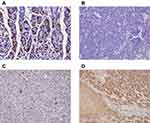
Normal gastric tissue was strongly positive on immunohistochemical staining for both the α- and β- subunits (Figures 4 and 5). Both subunits were expressed principally in the cytoplasm and plasma membrane. Of the 30 laryngeal carcinoma tissues, 22 (73.3%) were positive for the α-subunit. The expression level of this subunit was higher in carcinomatous tissue than in either paracarcinomatous tissue (11.8% [2/17], p <0.001) or normal tissue (24.9% [45/181], P < 0.001). Of the 30 laryngeal carcinoma tissues, 17 (56.7%) were positive for the β-subunit. The expression level of this subunit was higher in carcinomatous than either paracarcinomatous tissue (11.8% [2/17], p < 0.001) or normal tissue 22.1% [43/195], P < 0.001).
The expression of (H+/K+-ATPase) α- and β-subunit mRNA in laryngeal carcinoma tissues was evaluated by RT-PCR. The results showed that the expression levels of α-subunit mRNA in laryngeal carcinoma and paracarcinoma tissues were 13.626 ± 1.274 and 4.959 ± 0.448, respectively (Figure 2). The expression levels of β-subunit mRNA in laryngeal carcinoma and paracarcinoma tissues were 10.141 ± 0.907 and 5.842 ± 0.621, respectively (Figure 2). Expression of (H+/K+-ATPase), either the α- or β-subunit, was significantly higher in carcinoma tissues than in paracarcinoma tissues (p < 0.001 and p < 0.001, respectively) and normal laryngeal tissues (p < 0.001 and p < 0.001, respectively) (Figure 2).
The expression levels of the (H+/K+-ATPase) α- and β-subunit proteins in laryngeal carcinoma tissues were evaluated by Western blotting. The results show that the expression levels of the α-subunit protein in laryngeal carcinoma and paracarcinoma tissues were 0.587 ± 0.026 and 0.417 ± 0.046, respectively (Figure 3A). The expression levels of the β-subunit protein in laryngeal carcinoma and paracarcinoma tissues were 0.563 ± 0.023 and 0.275 ± 0.033, respectively (Figure 3). Expression of (H+/K+-ATPase) the α- or β-subunit was significantly higher in carcinoma tissues than that in paracarcinoma tissues (p < 0.001 and p < 0.001, respectively) and normal laryngeal tissues (p < 0.001 and p < 0.001, respectively) (Figure 3).
Relationships Between H+/K+-ATPase α- and β-Subunit Expression and the Clinicopathological Features of Laryngeal Carcinoma
We found no significant correlations between the expression levels of either subunit (on IHC, RT-PCR, or Western blotting) with any clinicopathological feature. The patient features evaluated included age, smoking and alcohol consumption histories, tumor site, TNM stage, clinical stage, and extent of pathological differentiation (all p > 0.05).
The mean follow-up duration was 66.2 months (range, 17–162 months). Four patients died during follow-up, 4 were lost to follow-up, and 22 were alive and free of disease at the end of follow-up. Two patients developed lung metastases and six developed disease recurrence (at 2, 8, 14, 16, 36, and 41 months). The 3- and 5-year overall survival (OS) rates were 93.0% and 77.0%, respectively. In univariate analyses, the 5-year survival rates of patients with early (T1–T2) and advanced (T3–T4) T-stage laryngeal carcinoma were 83.3% and 50.0%, respectively (χ2 = 6.756, p = 0.009). The 5-year OSs of patients with early (stage I–II) and advanced (stage III–IV) laryngeal carcinoma were 87.0% and 42.9%, respectively (χ2 = 11.78, p = 0.001). The 5-year OSs of patients without and with lymph node metastases were 81.5% and 33.3%, respectively (χ2 = 6.91, p = 0.009). The 5-year OSs were not significantly associated with age, alcohol use, pathological differentiation, or the expression levels of the α- or β-subunits (on IHC, RT-PCR, or Western blotting). In multivariate regression analyses, the 5-year OSs were not significantly associated with any clinicopathological factors or the expression levels of either subunit.
Discussion
The proton pump (H+/K+-ATPase) has been detected in different organs outside the stomach, such as the kidneys, lungs, heart, thymus, prostate, eye, and larynx.2,6–14 However, different results have been reported, depending on the detection method.6–15 Altman et al first detected distribution of the H+/K+-ATPase α- and β-subunits in the seromucinous glands of two human cadaveric larynges by immunohistochemical staining.11 Their subsequent study in 2005 reported positive staining rates for the α- and β-subunits of 96.3% and 85.2% in 27 specimens taken from 15 patients who underwent larynx surgery, respectively. The positive staining is mainly located in the seromucinous glands.2 They also observed α- and β-subunit positive staining in four human larynges through immunohistochemical and Western blotting analyses in 2011, although the positive signal was weak on Western blotting analyses.12 In 2015, Stevanović et al detected the β-subunit of the proton pump in 50 cadaver larynges and 11 larynges from laryngectomies performed on patients with laryngeal carcinoma.13 They found no expression in cadaver larynges; 36% of the surgical larynx specimens were positive in the seromucinous glands, which are located in the supraglottis. They first found the β-subunit in chondrocytes of all surgical specimens and most cadaver larynges.13 However, Herrmann et al detected significant levels of the α- and β- H+/K+-ATPase subunits in the stomach in a study regarding H+/K+-ATPase expression in different human tissues, including the larynx.15 The present study is the first to investigate the two H+/K+-ATPase subunits in normal laryngeal tissue and laryngeal carcinoma by IHC, real-time RT-PCR, and Western blotting. The distributions and expression levels of the H+/K+-ATPase α- and β-subunits in different subregions of the normal larynx did not differ significantly, regardless of the method of analyses. Notably, we detected the two subunits in the epiglottic cartilage, where the β-subunit protein was expressed at a higher level than at the other sites. This finding was similar to the results of Stevanović et al.13 Our findings and those of previous studies suggest that the α- and β-subunits of H+/K+-ATPase occur in normal laryngeal seromucinous glands and cartilage in the supraglottis. Another study demonstrated that the H+/K+-ATPase proton pump was positively expressed in two of 20 patients with LPRD symptoms.14 Pathological results were detected by DxpH and pH/MII in one patient, and normal results were detected by pH/MII in another patient. The two patients were positive for the PPI response, suggesting sporadic expression of H+/K+-ATPase in laryngeal submucosal glands. The possible reason for the lower expression of H+/K+-ATPase in the larynx in their study may have been missed submucosal glands containing the proton pump H+/K+-ATPase during random biopsies performed under endoscopic guidance.14 We speculate that the laryngeal cartilage could not be biopsied under this circumstance. Expression of the proton pump H+/K+-ATPase was much lower in laryngeal submucosal glands or laryngeal cartilages than in gastric parietal cells; however, the larynx is sensitive to changes in the acidic microenvironment25,26 via proton secretion or excretion by an “activated” H+/K+-ATPase proton pump in laryngeal submucosal glands stimulated by inflammation, infection, or gastroesophageal reflux,2,14,27,28 and/or by an “activated” H+/K+-ATPase proton pump in laryngeal cartilage stimulated by continuous mechanical load of the neck and laryngeal muscles.13 The main function of gastric H+/K+-ATPase is the secretion of gastric acid.29 However, the role of the proton pump H+/K+-ATPase outside gastric tissues, including the larynx, remains unclear. The function of acid secretion is damaged in the collecting duct of H+/K+-ATPase-deficient mice, suggesting that H+/K+-ATPase might play a role in luminal acidification of the collecting duct.30 H+/K+-ATPase is expressed in the cochlear outer wall in normal mice, where it is assumed to regulate endolymph pH by secreting H+ in exchange for K+.31–33 H+/K+-ATPase is a pathogenic factor of Meniere’s disease (MD)34 and a PPI might be an effective therapeutic option for patients with MD.35 Min et al demonstrated non-gastric H+/K+-ATPase (ng H, K-ATPase) expression in human sinonasal epithelial cells, and a human bronchial epithelial cell line was transformed with a hybrid adenovirus 12-simian virus 40 (BEAS-2B).36 ngH, K-ATPase exchanges extracellular K+ for intracellular H+; whereas activated ngH, K-ATPase might stimulate the expression of an inflammatory factor, such as interleukin (IL)-13. IL-13 can enhance H+/K+-exchange and PPIs can inhibit the exchange theoretically by inhibiting ngH, K-ATPase.36
On the other hand, few studies have investigated relationships between the H+/K+-ATPase proton pump and cancers. Goh et al detected gastric H+/K+-ATPase expression in breast cancer MDA-MB-468 cells and found that esomeprazole (a PPI) might decrease the adverse effects of doxorubicin and increase its anti-cancer effects.37 PPIs may have anti-gastric cancer activity by inhibiting H+/K+-ATPase38 and through silencing of ATP4B in the ATPase H+/K+ transporting β-subunit. However, only one study investigated the relationship between laryngeal carcinoma and H+/K+-ATPase in eight specimens from patients with laryngeal carcinomas detected via RT-PCR.39 However, the distributions of H+/K+ATPases among the various laryngeal regions were not explored, and no significant difference in expression among LPR, LSCC-tumor, and LSCC-adjacent tissues was evident because of the small sample size.39 The present study shows that expression of the α- and β-subunits of the H+/K+-ATPase proton pump increased gradually in normal laryngeal tissues, paracarcinoma tissues, and laryngeal carcinoma tissues using three different detection methods. Expression of the two subunits was significantly higher in laryngeal carcinoma tissues than in laryngeal or paracarcinoma tissues. Because of the small sample size, the expression levels of both subunits were not significantly associated with any clinicopathological factor and/or patient prognosis in the present study. Therefore, further prospective, multicenter large-scale studies are needed to determine the exact role played by H+/K+-ATPase in regulating laryngeal cancer, as well as the underlying molecular mechanisms.
The limitations of the study follow. First, we lacked data on GERD or LPRD status; the work was retrospective in nature. We plan to further investigate possible correlations between GERD or LPRD status and laryngeal and/or hypopharyngeal cancer, and possible correlations between H+/K+-ATPase levels and laryngeal cancer. Second, the function of H+/K+-ATPase in normal laryngeal tissue and its role in laryngeal carcinogenesis were not investigated in this study. Further studies should assess the function of H+/K+-ATPase in laryngeal seromucinous glands and cartilage by culturing normal laryngeal epithelial cells or laryngeal chondrocytes. Finally, we will establish in vivo animal models using knockout mice or H+/K+-ATPase-silenced tumor cells to clarify the pathological role played by H+/K+-ATPase in laryngeal carcinogenesis.
Conclusion
H+/K+-ATPase is detectable in normal laryngeal tissues, and the level increases in laryngeal carcinoma tissues.
Abbreviations
LPRD, laryngopharyngeal reflux disease; GERD, gastroesophageal reflux disease; PPI, pump proton inhibitors; IL, interleukin; ngH, K-ATPase, non-gastric H+/K+-ATPase.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Ethics Approval and Informed Consent
This study was approved by the Institutional Review Board of The First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, Zhejiang, China). The privacy of the patients involved was protected. Patients provided written informed consent. Study participants provided consent was obtained from the study participants prior to study commencement, for the publication of the data and any associated images. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
This work was supported by Science and Technology Department of Zhejiang Province, China (No. 2016C33144).
Author Contributions
Yang-Yang Bao designed the study and analyzed the data. Qian Jiang, Zhen-wei Li and Er Yu collected materials, aided during surgeries and took specimens. Shui-Hong Zhou designed the study, wrote and revised the article. Hong-Tian Yao and Wei-Wie Yong completed the immunohistochemistry and scoring. Jun fan completed the RT-PCR and Western blotting. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Lechien JR, Saussez S, Karkos PD. Laryngopharyngeal reflux disease: clinical presentation, diagnosis and therapeutic challenges in 2018. Curr Opin Otolaryngol Head Neck Surg. 2018;26(6):392–402. doi:10.1097/MOO.0000000000000486
2. Altman KW, Waltonen JD, Hammer ND, Radosevich JA, Haines GK
3. Abe K, Irie K, Nakanishi H, Suzuki H, Fujiyoshi Y. Crystal structures of the gastric proton pump. Nature. 2018;556(7700):214–218. doi:10.1038/s41586-018-0003-8
4. Jakab M, Hofer S, Ravasio A, et al. The putative role of the non-gastric H+/K+-ATPase ATP12A (ATP1AL1) as anti-apoptotic ion transporter: effect of the H+/K+ ATPase inhibitor SCH28080 on butyrate-stimulated myelomonocytic HL-60 cells. Cell Physiol Biochem. 2014;349(5):1507–1526. doi:10.1159/000366355
5. Streif D, Iglseder E, Hauser-Kronberger C, Fink KG, Jakab M, Ritter M. Expression of the non-gastric H+/K+ ATPase ATP12A in normal and pathological human prostate tissue. Cell Physiol Biochem. 2011;28(6):1287–1294. doi:10.1159/000335860
6. Altman KW, Waltonen JD, Tarjan G, Radosevich JA, Haines GK
7. Belisario DC, Rocafull MA, Del Castillo JR. Purification and characterization of the ouabain-sensitive H+/K+-ATPase from guinea-pig distal colon. Arch Biochem Biophys. 2010;496(1):21–32. doi:10.1016/j.abb.2010.01.014
8. Tang X, Yang X, Lai G, et al. Mechanism underlying hypokalemia induced by trimethyltin chloride: inhibition of H+/K+-ATPase in renal intercalated cells. Toxicology. 2010;271(1–2):45–50. doi:10.1016/j.tox.2010.02.013
9. Beisvag V, Falck G, Loennechen JP, et al. Identification and regulation of the gastric H+/K+-ATPase in the rat heart. Acta Physiol Scand. 2003;179(3):251–262. doi:10.1046/j.0001-6772.2003.01191.x
10. Alderuccio F, Gleeson PA, Berzins SP, Martin M, Van Driel IR, Toh BH. Expression of the gastric H/K-ATPase alpha-subunit in the thymus may explain the dominant role of the beta-subunit in the pathogenesis of autoimmune gastritis. Autoimmunity. 1997;25(3):167–175. doi:10.3109/08916939709008023
11. Altman KW, Haines GK
12. Altman KW, Kinoshita Y, Tan M, Burstein D, Radosevich JA. Western blot confirmation of the H+/K+-ATPase proton pump in the human larynx and submandibular gland. Otolaryngol Head Neck Surg. 2011;145(5):783–788. doi:10.1177/0194599811415589
13. Stevanović S, Radić R, Kačarević ŽP, et al. Proton pump (H+/K+-ATPase) expression in human larynx. Auris Nasus Larynx. 2015;42(6):458–462. doi:10.1016/j.anl.2015.04.013
14. Becker V, Drabner R, Graf S, et al. New aspects in the pathomechanism and diagnosis of the laryngopharyngeal reflux-clinical impact of laryngeal proton pumps and pharyngeal pH metry in extraesophageal gastroesophageal reflux disease. World J Gastroenterol. 2015;21(3):982–987. doi:10.3748/wjg.v21.i3.982
15. Herrmann M, Selige J, Raffael S, Sachs G, Brambilla A, Klein T. Systematic expression profiling of the gastric H+/K+ ATPase in human tissue. Scand J Gastroenterol. 2007;42(11):1275–1288. doi:10.1080/00365520701405579
16. El-Serag HB, Hepworth EJ, Lee P, Sonnenberg A. Gastroesophageal reflux disease is a risk factor for laryngeal and pharyngeal cancer. Am J Gastroenterol. 2001;96(7):2013–2018. doi:10.1111/j.1572-0241.2001.03934.x
17. Dagli S, Dagli U, Kurtaran H, Alkim C, Sahin B. Laryngopharyngeal reflux in laryngeal cancer. Turk J Gastroenterol. 2004;15(2):77–81.
18. Tae K, Jin BJ, Ji YB, Jeong JH, Cho SH. The role of laryngopharyngeal reflux as a risk factor in laryngeal cancer: a preliminary report. Clin Exp Otorhinolaryngol. 2011;4(2):101–104. doi:10.3342/ceo.2011.4.2.101
19. Zhang D, Zhou J, Chen B, Zhou L, Tao L. Gastroesophageal reflux and carcinoma of larynx or pharynx: a meta-analysis. Acta Otolaryngol. 2014;134(10):982–989. doi:10.3109/00016489.2014.927592
20. Sereg-Bahar M, Jerin A, Hocevar-Boltezar I. Higher levels of total pepsin and bile acids in the saliva as a possible risk factor for early laryngeal cancer. Radiol Oncol. 2015;49(1):59–64. doi:10.2478/raon-2014-0020
21. Sasaki CT, Vageli DP. miR-21, miR-155, miR-192, and miR-375 deregulations related to NF-kappaB activation in gastroduodenal fluid-induced early preneoplastic lesions of laryngeal mucosa in vivo. Neoplasia. 2016;18(6):329–338. doi:10.1016/j.neo.2016.04.007
22. Busch EL, Zevallos JP, Olshan AF. Gastroesophageal reflux disease and odds of head and neck squamous cell carcinoma in North Carolina. Laryngoscope. 2016;126(5):1091–1096. doi:10.1002/lary.25716
23. De Corso E, Baroni S, Agostino S, et al. Bile acids and total bilirubin detection in saliva of patients submitted to gastric surgery and in particular to subtotal billroth II resection. Ann Surg. 2007;245(6):880–885. doi:10.1097/01.sla.0000255574.22821.a1
24. Coca-Pelaz A, Rodrigo JP, Takes RP, et al. Relationship between reflux and laryngeal cancer. Head Neck. 2013;35(12):1814–1818. doi:10.1002/hed.23208
25. Cumpston EC, Blumin JH, Bock JM. Dual pH with multichannel intraluminal impedance testing in the evaluation of subjective laryngopharyngeal reflux symptoms. Otolaryngol Head Neck Surg. 2016;155(6):1014–1020. doi:10.1177/0194599816665819
26. Ummarino D, Vandermeulen L, Roosens B, Urbain D, Hauser B, Vandenplas Y. Gastroesophageal reflux evaluation in patients affected by chronic cough: restech versus multichannel intraluminal impedance/pH metry. Laryngoscope. 2013;123(4):980–984. doi:10.1002/lary.23738
27. Roussa E, Thevenod F. Distribution of V-ATPase in rat salivary glands. Eur J Morphol. 1998;36(suppl):147–152.
28. Roussa E, Thevenod F, Sabolic I, et al. Immunolocalization of vacuolar-type H-ATPase in rat submandibular gland and adaptive changes induced by acid-base disturbances. J Histochem Cytochem. 1998;46(1):91–100. doi:10.1177/002215549804600112
29. Sakai H, Fujii T, Takeguchi N. Proton-potassium (H(+)/K(+)) ATPases: properties and roles in health and diseases. Met Ions Life Sci. 2016;16:459–483.
30. Lynch IJ, Rudin A, Xia SL, et al. Impaired acid secretion in cortical collecting duct intercalated cells from H-K-ATPase-deficient mice: role of HK alpha isoforms. Am J Physiol Renal Physiol. 2008;294(3):F621–F627. doi:10.1152/ajprenal.00412.2007
31. Shibata T, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y. Gastric type H+, K+-ATPase in the cochlear lateral wall is critically involved in formation of the endocochlear potential. Am J Physiol Cell Physiol. 2006;291(5):C1038–C1048. doi:10.1152/ajpcell.00266.2006
32. Takumida M, Takumida H, Anniko M. Gastric-type H(+), K(+)-ATPase in mouse vestibular end organs. Acta Otolaryngol. 2017;137(5):455–459. doi:10.1080/00016489.2016.1245865
33. Miyazaki H, Wangemann P, Marcus DC. The gastric H, K-ATPase in stria vascularis contributes to pH regulation of cochlear endolymph but not to K secretion. BMC Physiol. 2016;17(1):1. doi:10.1186/s12899-016-0024-1
34. Pirodda A, Brandolini C, Raimondi MC, Ferri GG, Modugno GC, Borghi C. Meniere’s disease: update of etiopathogenetic theories and proposal of a possible model of explanation. Acta Clin Belg. 2010;65(3):170–175. doi:10.1179/acb.2010.036
35. Pirodda A, Modugno GC, Manzari L, et al. Meniere’s disease and the use of proton pump inhibitors. Swiss Med Wkly. 2010;140:w13104.
36. Min JY, Ocampo CJ, Stevens WW, et al. Proton pump inhibitors decrease eotaxin-3/CCL26 expression in patients with chronic rhinosinusitis with nasal polyps: possible role of the nongastric H, K-ATPase. J Allergy Clin Immunol. 2017;139(1):130–141. doi:10.1016/j.jaci.2016.07.020
37. Goh W, Sleptsova-Freidrich I, Petrovic N. Use of proton pump inhibitors as adjunct treatment for triple-negative breast cancers. An introductory study. J Pharm Pharm Sci. 2014;17:439–446. doi:10.18433/J34608
38. Lin S, Lin B, Wang X, et al. Silencing of ATP4B of ATPase H+/K+ transporting beta subunit by intragenic epigenetic alteration in human gastric cancer cells. Oncol Res. 2017;25(3):317–329. doi:10.3727/096504016X14734735156265
39. McCormick CA, Samuels TL, Battle MA, et al. H+/K+ATPase expression in the larynx of laryngopharyngeal reflux and laryngeal cancer patients. Laryngoscope. 2020. doi:10.1002/lary.28643
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.