Back to Journals » Journal of Inflammation Research » Volume 14
GARP and GARP-Treated tDC Prevented the Formation of Atherosclerotic Plaques in ApoE−/- Mice
Authors Cai Y , Zeng Q, Liu Y, Zhu R, Yu K, Xu W, Wang Y, Ding Y, Yu J, Pan C, Peng Y, Mao Y, Cheng P, Huang L , Mao X, Zhong Y
Received 8 April 2021
Accepted for publication 1 July 2021
Published 22 July 2021 Volume 2021:14 Pages 3465—3479
DOI https://doi.org/10.2147/JIR.S308963
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yifan Cai,1 Qiutang Zeng,1 Yuzhou Liu,2 Ruirui Zhu,1 Kunwu Yu,1 Wenbin Xu,1 Yue Wang,1 Yan Ding,1 Jian Yu,1 Chengliang Pan,1 Yudong Peng,1 Yi Mao,1 Peng Cheng,1 Lun Huang,1 Xiaobo Mao,1 Yucheng Zhong1
1Department of Cardiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China
Correspondence: Yucheng Zhong
Laboratory of Cardiovascular Immunology, Institute of Cardiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jie-Fang Avenue, Wuhan, 430022, People’s Republic of China
Tel +86-27-85726423
Fax +86-27-85726425
Email [email protected]
Purpose: This study aims to clarify the specific mechanism by which GARP affects the atherosclerotic plaques in ApoE−/- mice and the effect of GARP-tDC on atherosclerosis.
Methods: The mice were randomly divided into three groups: the control group, the GARP-overexpressed group and the GARP-inhibited group. After 12 weeks, all the mice were euthanized, and the specimens were collected. In vitro, experiments were conducted to observe the effect of GARP on DC phenotype and the changes of the proportion of CD4+CD25+Foxp3+ Treg cells when GARP-tDCs were co-cultured with CD4+ T cells. Furthermore, adoptive transmission of GARP-tDCs was used to observe the effect on atherosclerotic plaque in mice.
Results: The GARP-overexpressed group enhanced the biological activity of Foxp3+ CD4+CD25+ Tregs and resulted in increased expression of LAP in T cells. In addition, the GARP-overexpressed group significantly suppressed the function of Th1 and Th17, and decreased the secretion of INF-γ and IL-17A. Thus, GARP had a protective effect on atherosclerosis. In vitro, we found that GARP-tDC had a tolerance-inducing phenotype, and GARP-tDC also had the ability to induce tolerance when co-cultured with CD4+ T cells. More importantly, adoptive transmission of GARP-tDCs reduced the size of atherosclerotic plaques.
Conclusion: GARP and the GARP-tDC play protective roles in atherosclerosis. The protective effect of GARP on atherosclerosis is achieved by increasing CD4+CD25+Foxp3+ Treg cells and inhibiting the production of IFN-γ and IL-17A.
Keywords: GARP, Foxp3, GARP-tDC, atherosclerosis, LAP
Introduction
Atherosclerosis (AS) and its clinical complications can be considered the leading cause of death worldwide.1 Nowadays, more and more evidences supported that AS was a chronic inflammatory disease of large and medium-sized arteries involving innate and adaptive immune responses, and with that came a lot of antibody-based immunotherapy.2,3 Therefore, in-depth study of the mechanisms of immune and inflammatory response of AS and exploration of new targets for intervention has a profound medical and social values.
Natural CD4+CD25+ regulatory T lymphocytes (nTregs) repertoire is a group of unique regulatory T cell subsets with suppressive function, which plays an important role in inhibiting the excessive immune response and maintaining immunologic self-tolerance.4 Many researches had documented that nTregs showed a powerfully protective role in the progression of atherosclerosis by adoptive transfer through the interaction between CD40 and CD40L.5 Our previous study also found that nasal administration of oxLDL could reduce the size of atherosclerotic plaques in ApoE−/- mice by inducing the production of CD4+LAP+and CD4+CD25+Foxp3+ regulatory T cells.6
Recently, a research found that a new transmembrane protein-glycoprotein A repetitions predominant (GARP) existed in the surface of active nTregs. GARP is composed of 662 amino acid residues, including two exons.7,8 Unutmaz et al indicated that GARP is uniquely expressed on the surface of human activated nTregs and could not be detected in the fresh CD4+ T cells. In addition, GARP could not be induced in Tresp by CD3 and CD28 stimulation.9 As a result, GARP has been identified as a new specific marker of activated human Treg cells.10 So, whether GARP can affect the function of nTregs? Wang and his co-workers found that the ectopic expression of GARP in naïve CD4+ T cells effectively inhibited the proliferation of CD4+ T cells and secretion of cytokines; furthermore, overexpression of GARP in naïve CD4+ T cells promoted the expression of Foxp3 and CD4+ T cells were endowed the inhibitory function. In contrast, GARP was not affected when Foxp3 was downregulated in GARP-overexpressing cells, while silencing GARP in Foxp3-overexpressing cells reduced their suppressive activities.11 Therefore, we hypothesized that GARP could affect atherosclerosis. Our study has found that atorvastatin improves the atherosclerotic inflammatory response by up-regulating the expression of GARP,12 but no studies have demonstrated the direct effect of GARP on atherosclerosis at the gene level.
Tolerogenic DCs may induce peripheral tolerance by either directly down-regulating effector T cells or indirectly inducing the production of regulatory T cells (iTregs).13 Our previous studies found that DCs treated with IL-37 plus TnI in vitro can induce tDCs, and tDCs has the ability to induce tolerance, thus reducing ventricular remodeling after myocardial infarction. Moreover, DCs treated with IL-37 plus TnI are more “tolerant” than DCs treated with IL-37 alone or TnI alone.14 What’s more, it has also been shown that by inducing regulatory T cells and immature dendritic cells with tolerability, atherosclerosis in mice can be reduced using oral administration of vitamin D3.15 However, there have been no studies using GARP to treat DCs, whether GARP treatment can induce tolerogenic DCs and whether their adoptive transfer can have a possible effect on atherosclerosis is unknown.
Therefore, our study will attempt to investigate the effects of overexpressing GARP and GARP-tDCs on atherosclerotic plaque formation in ApoE−/- mice, and to search for possible mechanisms.
Materials and Methods
Atherosclerosis Mice Model
Male ApoE−/- mice (C57BL/6 background), 8 weeks old, were continuously fed a high-fat western diet (0.25% cholesterol and 15% cocoa butter) and housed under standard conditions (12-h light/12-h dark cycle, 22~25 °C room temperature). The mice were randomly divided into three groups: the control group (5×107 lentivirus-scrambled sequence intravenous injected per week); the GARP-overexpressed group [GARP (+), 5×107 lentivirus-GARP-overexpressed sequence intravenous injected per week]; and the GARP-inhibited group [GARP (-), 5×107 lentivirus-GARP-siRNA sequence intravenous injected per week], while each group had 6 mice (n=6). The lentivirus-GARP-overexpressed sequence and lentivirus-GARP-siRNA sequence were designed and synthesized by GENECHEM CO., LTD. Shanghai, China. After 12 weeks, all the mice were euthanized, and the specimens, including the peripheral blood, spleen, heart and aorta, were collected for further studies. The Ethical Committee of Huazhong University of Science and Technology granted ethical approval, and all experiments were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (Science and Technology Department of Hubei Province, China).
The body weights of the three groups at 8 and 24 weeks were shown in Table 1, and the level of the total cholesterol, triglyceride, LDL cholesterol and HDL cholesterol of the mice at 24 weeks was shown in Table 2.
 |
Table 1 Body Weight in Each Group (g, n=6) |
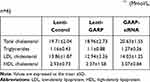 |
Table 2 Lipid Profile of Plasma in 24 Weeks ApoE−/- (Mmol/L, n=6) |
Atherosclerotic Lesion Analysis
The heart and aorta were flushed with PBS and then stained by 4% paraformaldehyde for 2 hours. The heart was transected and the end part was embedded in OCT compound, sliced in 8-μm thickness, mounted on slides, and stained with Oil red O. The middle part of the three aortic valves was used for analysis of atherosclerotic lipid plaques. Masson trichrome staining was achieved by Masson Trichrome Stain Kit (Sigma-Aldrich Inc., St. Louis, MO, USA). Lesion areas were measured by Image-pro plus 6.0.
Lipid plaques on the surface of aorta were also analyzed. The whole aorta was dissected out from ascending aorta, nailed on a black wax pan, and then stained with Oil red O. The total areas and the atherosclerotic lesions of each aorta were also measured by Image-pro plus 6.0.
Cell Isolation and in vitro Culture
Spleen was isolated from ApoE−/- mice. The splenic cells were obtained by milling the spleen and filtering the cells through a 40 mm filter. Lymphocytes were isolated from total splenic cells by density-gradient centrifugation using 1.083 g/mL Lymphocyte separation solution (MP Biomedicals Co., Ltd., USA). Splenic lymphocytes were cultured in 24-well plates at 37°C in an atmosphere of 5% CO2 in RPMI 1640 medium (HYCLONE, Co., Ltd., USA), supplemented with 10% fetal bovine serum (Gibco-BRL, Grand Island, NY, USA), 1% penicillin-streptomycin and 1% glutamine. CD4+ T cells were isolated from spleens by immunomagnetic beads.
In order to isolate the DC, the 6-week-old male C57BL/6J mice were sacrificed and immersed in 75% alcohol for 5 minutes. The tibia and femur were separated in the ultra-clean table. Then the medullary cavity was exposed with scissors, and repeatedly washed with PBS. The rinse was centrifuged at 1500rpm for 7 minutes after being transferred into the 10mLEP tube. After discarding supernatant, 3mL RBC lysis solution was added. After 2 min, the reaction was terminated by addition of 6 mL of PBS, centrifuged at 1500rpm for 7 minutes again, and then washed twice with PBS. Cells were resuspended in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/mL streptomycin, and 100 U/mL penicillin, and cultured in an incubator at 37° C with 5.0% CO2 after induction with GM-CSF and IL-4. On day 3, non-adherent cells were removed and added with GM-CSF and IL-4 at the same concentration. On the 6th day, half volume of culture medium was changed under the same culture conditions. After 8 days of culture, immature DCs (imDCs) were obtained.
CD4+ T Cells Were Co-Cultured with Different Types of DCs Respectively in vitro
CD11c+ imDCs were obtained by immunomagnetic beads. CD11c+ imDCs were divided into four groups: (1) no DC: Added with an equal volume of PBS;(2) imDCs: only DCs, without any additional treatment; (3) GARP-tDCs: treated with GARP and LPS for 4 hours; (4) mature DCs(mDCs): treated with LPS for 24 hours. Then the isolated CD4+ T cells were co-cultured with DCs in each group.
Flow Cytometry
Splenic lymphocytes were collected for flow cytometry. Prepared lymphocytes were incubated for 30 mins at 4°C in the dark for surface staining, with FITC-labeled anti-CD4 antibodies (eBioscience), PerCP-Cy5-5-labeled anti-LAP antibodies (eBioscience) or antigen presenting cell(APC)-labeled anti-GARP antibodies (eBioscience). For intracellular staining of Foxp3, lymphocytes were fixed with 4% paraformaldehyde after surface staining for CD4 and GARP and then permeabilized. Permeabilized lymphocytes were incubated with PE-labeled anti-Foxp3 antibodies (eBioscience) for 1 hours at 4°C in the dark.
For the detection of Th1, Th2 and Th17, splenic lymphocytes were stimulated by PMA (100ng/mL)/lonomycin (750ng/mL) for 4 hours in vitro. After stained with FITC-CD4, these lymphocytes were fixed and then permeabilized. The permeabilized lymphocytes were incubated with PE-labeled anti-INF-γ, IL-4 or IL-17 antibodies (eBioscience) for 30 mins at 4°C in the dark. Then, cells were washed 2~3 times and analyzed by a BD FACScan. All the results were analyzed by Flowjo 7.6.3.
Tregs Functional Suppression Assays
CD4+CD25+ regulatory T cells (2×105) sorted from each group were co-cultured with syngeneic CD4+CD25− effector T cells at different suppressor-responder ratios (1:4, 1:2 and 1:1). All groups were in triplicate. After 72 hours, the inhibited percentage of proliferation was determined by the following formula: 1-(median [3H] thymidine uptake of Tregs: median [3H] thymidine uptake of CD4+CD25− co-culture/ CD4+CD25− cells).
RT-PCR
The total RNAs were isolated from splenic lymphocytes using RNAiso Plus (TakaRa, Japan). Equal mRNAs were synthesized into cDNA by using PrimeScript® RT reagent kit (TakaRa, Japan). The specific primers for RT-PCR were synthesized by Newtsingke (Peking, China). The cycling program included 2 mins initial pre-incubation at 95°C followed by 40 cycles of 95°C for 10 seconds, 60°C for 20 seconds and 70°C for 1 second. The mRNA expression was quantified by an ABI PRISM 7900 Sequence Detector system (AB Applied Biosystems). Primers used for RT-PCR were listed in Table 1 of Supplement 1.
Western Blot
The protein was collected from splenic lymphocytes from each group by using lysing buffer for 30 mins on ice. The lymphocytes were then centrifuged at 12,000 g for 15 min at 4°C. Protein concentration in the supernatants was measured by BCA Protein Assay Kit (Beyotime Institute of Biotechnology). Then, 20μg protein was separated by SDS-PAGE and transferred into polyvinylidene fluoride microporous membranes. The membrane was blocked for 1 hour in room temperature, then incubated with primary antibodies for Smad3 (Santa Cruz’s) or β-actin (EPITOMICS) overnight at 4 °C, finally incubated with HRP conjugated secondary antibody (KPL Co., Ltd.) at room temperature for 30 mins. The blots were quantified by ECL Western Blotting Detection Reagents (Bio-Rad Laboratories, Hercules, CA).
Enzyme-Linked Immunosorbent Assay (ELISA)
After washing with PBS containing 2% fetal bovine serum, the isolated spleen cells were cultured in RPMI1640 medium for 2h at 37°C. Non-adherent cells were collected and added with 2μL /mL ConA, cultured on a 24-well plate with 1×106 cells per well. The supernatant was collected three days later and the levels of TGF-β, interleukin-10 (IL-10), interferon-γ, interleukin-4, and interleukin-17 were quantitatively detected using an ELISA kit (eBioscience, CA, USA) according to the manufacturer’s instructions. All samples were assayed at least in triplicate.
Immunohistochemistry
The 5μm-thick aortic valves in each group were collected for immunohistochemical assay, including CD3 (1:200; Abcam). The analysis was performed by using Image Pro Plus 6.0.
Statistics
All statistical analyses were performed with SPSS 19.0. One-way analysis of variance(ANOVA) was used to compare the mean of measurement data between different groups, and chi-square test was used to compare the enumeration data between different groups. Spearman analysis was used to investigate the association between the number of LAP+, Foxp3+ and GARP+ cells in all groups. All values are expressed as mean ± SEM, and p <0.05 were regarded as significant.
Results
The Overexpression of GARP Up-Regulated the Proportion of CD4+Foxp3+ Tregs in ApoE−/- Splenic Lymphocytes and Enhanced Immunosuppressive Function of Tregs in vivo
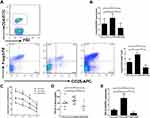
We detected the proportion of CD4+CD25+Foxp3+ regulatory T cells in splenic lymphocytes by flow cytometry, and observed a significant elevation of the proportion of CD4+CD25+Foxp3+ regulatory T cells in GARP-overexpressed group compared with GARP-inhibited group and control group (p<0.05, as Figure 1A), the mRNA expression of Foxp3 in GARP-overexpressed group also increased significantly (p<0.05, as Figure 1B).
We also found that in the same Tregs/CD4+CD25− T cells ratio, the suppressor function of regulatory T cells on the proliferation of CD4+CD25− T cells were significantly enhanced in GARP-overexpressed group compared with the control group (p<0.05, as Figure 1C). However, the suppressor function of regulatory T cells was decreased in GARP-inhibited group compared with the control group and the GARP-overexpressed group (p<0.05, as Figure 1C). All three groups showed that with the increase of the proportion of effector T cells, the inhibitory function of regulatory T cells on the proliferation of CD4+CD25− T cells gradually decreased.
Because transforming growth factor -β(TGF-β) plays an important role in the suppressive properties of Tregs, we used ELISA to measure the plasma levels of TGF-β in each group. The levels of TGF-β1 gene expression were analysed by real-time polymerase chain reaction. The mRNA expression and secretion of mature TGF-β1 increased significantly in GARP-overexpressed group compared with the control group and the GARP-inhibited group (p<0.05, as Figure 1D and E).
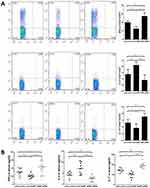
The Overexpression of GARP Inhibits the Frequency of Th1, Th17 in ApoE−/- Splenic Lymphocytes and Increases the Frequency of Th2
In order to assess the effect of overexpression of GARP on the frequency of Th cells, we detected the proportion of Th1, Th17 and Th2 cells in splenic lymphocytes by flow cytometry, and observed a significant decrease of Th1 and Th17 and an increase of Th2 in GARP-overexpressed group compared with the control group and the GARP-inhibited group (p<0.05, as Figure 2A). Similar results were observed on the level of IFN-γ, IL-4 and IL-17 (p<0.05, as Figure 2B).
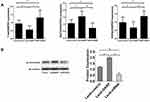
The relative mRNA expression levels of Th1-related T-bet, Th2-related GATA3, and Th17-related RORγt were also detected and compared. It was found that compared with the control group, the mRNA expression levels of T-bet and RORγt in the GARP-overexpressed group were significantly decreased, while the mRNA expression level of GATA3 was significantly increased. On the contrary, compared with the control group, the mRNA expression levels of T-bet and RORγt was significantly increased while the mRNA expression of GATA3 was significantly decreased in the GARP-inhibited group (P <0.05, as Figure 3A).
Phosphorylation of Smad2/3 is significantly associated with Th17 /Treg balance, so the expression of the phosphorylation of Smad3 in splenic CD4+ T cells stimulated by anti-CD3/CD28 was detected by Western blot, and it was found that the overexpression of GARP stimulated the phosphorylation of Smad3 (P <0.05, as Figure 3B).
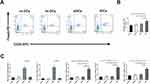
The Overexpression of GARP Inhibits Development of Atherosclerotic Plaques in ApoE−/- Mice
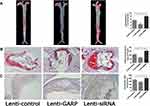
The aorta was stained with Oil red O to compare the atherosclerotic lesion sizes between these groups. The percentage of atherosclerotic lesion sizes in the entire aorta were significantly smaller in the GARP-overexpressed group than the control group (p<0.05, as Figure 4A), while GARP-siRNA significantly increased the size of atherosclerotic lesion compared with the control and the GARP-overexpressed group (p<0.05, as Figure 4A).
Proximal aortic sections were also examined. The atherosclerotic lesions were significantly reduced in the GARP-overexpressed group compared with the control group (p<0.05, as Figure 4B), while GARP-siRNA obviously elevated the size of atherosclerotic lesion compared with the control and the GARP-overexpressed group (p<0.05, as Figure 4B).
The stability of atherosclerotic plaques as well as infiltration of T lymphocytes in atherosclerotic plaques were analyzed by immunohistochemistry of CD3. Infiltration of T lymphocytes was significantly decreased in the GARP-overexpressed group than in the control group (p<0.05, as Figure 4C), while GARP-siRNA obviously elevated the infiltration of T lymphocytes compared with the control and GARP-overexpressed group (p<0.05, as Figure 4C).
The Overexpression of GARP Up-Regulated the Proportion of CD4+CD25+LAP+ in ApoE−/- Splenic Lymphocytes, and the Numbers of LAP+ Cells and Foxp3+ Cells Have a Positive Correlation with the Number of GARP+ Cells
We detected the proportion of CD4+CD25+LAP+ T cells in splenic lymphocytes after just modeling and 24 hours of modeling, and found that the expression of LAP in CD4+CD25+ T cells in GARP-overexpressed T cells was significantly higher than that in the control group and the GARP inhibited group, and the expression of LAP in CD4+CD25+ T cells in GARP-inhibited group was lower than that in the control group (P <0.05; Figure 5A).
Next, GARP+, LAP + and Foxp3 + cells in ApoE−/- splenic lymphocytes of the three groups were counted respectively. It was found that the number of GARP+, LAP + and Foxp3+ cells in the GARP-overexpression group was significantly higher than that of the other two groups. On the contrary, the number of GARP-inhibited group was lower than that of the control group (P <0.05, Figure 5B–D). Therefore, we attempted to conduct a Spearman analysis to find the association between LAP+, Foxp3+ and GARP+ cells in all groups, and found that the numbers of LAP+ cells and Foxp3+ cells have a positive correlation with the number of GARP+ cells, respectively (r=0.88,P < 0.001, Figure 5E; r =0.93,P < 0.001, Figure 5F).
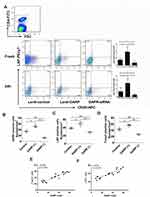
GARP-tDCs Had the Ability to Induce Tolerance When Co-Cultured with CD4+ T Cells
In order to study the effect of GARP-tDCs on Treg cells, CD4+ T cells were co-cultured with different types of DCs respectively in vitro. Compared with noDC, imDC and mDC, GARP-tDC significantly increased the percentage of CD4+CD25+Foxp3+ Treg cells in the CD4+ cells (P <0.05, Figure 6A and B), and the mRNA level of Foxp3 in the GARP-tDC group was significantly higher than that in the other three groups.
In addition, GARP-tDC significantly inhibited IFN-γ and IL-17A mRNA levels compared with the mDC group, with no significant difference compared with the no DC and the imDC group. However, compared with the other three groups, mRNA levels of IL-10 and TGF-β were significantly enhanced in the GARP-tDC group (P <0.05, Figure 6C). These results all indicated that GARP-tDC had the ability to induce tolerance when co-cultured with CD4+ T cells.
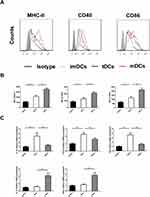
GARP-tDCs Had the Tolerogenic Phenotype
DC maturation is characterized by upregulation of antigen-presenting molecules such as MHC II and CD80/CD86. The DC was stained with isotype control antibody, anti-MHC-II antibody, CD40 and CD86 antibodies, and analyzed by FACS (P <0.05, Figure 7A), and then the mean fluorescence intensity (MFI) was quantified. Compared with the mDC and the GARP-tDC group, MHC-II, CD40 and CD86 of the imDC group showed low MFI. MFI of CD40, MHC-II and CD86 was significantly reduced in the GARP-tDC group compared with the mDC group (P <0.05, Figure 7B).
RT-PCR showed that the mRNA levels of IL-10, TGF-β and indoleamine 2, 3-dioxidase (IDO) were significantly higher in the GARP-tDC group than in the imDC and the mDC group, and the mRNA levels of IL-12 and IFN-γ were significantly lower in the GARP-tDC group than in the mDC group, with no significant difference with the imDC group (P <0.05, Figure 7C). In summary, GARP-tDCs had the tolerogenic phenotype.
tDCs Ameliorate Atherosclerosis in ApoE−/- Mice
To evaluate the effect of GARP-tDCs on atherosclerosis, 6-week-old mice were given a high-fat diet for 10 weeks to induce plaque formation in aortic root, and then mDCs (Figure 8A), GARP-tDCs (Figure 8B) and imDCs (Figure 8C) were administered to atherosclerotic mice by adoptive transmission. Sections from the aortic root were stained with oil red O and hematoxylin to observe the plaques. Adoptive transfer of GARP-tDCs significantly reduced atherosclerotic plaque size compared with mDCs (Figure 8D). Compared with the imDC and the GARP-tDC group, the plaque size of the mDC group was significantly increased. These results suggest that adoptive transfer of GARP-tDCs can significantly improve atherosclerosis, while adoptive transfer of mDCs can aggravate atherosclerosis. Image Pro was used to quantify the size of atherosclerotic plaque.
Discussion
GARP is a cell surface receptor on Treg cells, hepatic stellate cells, platelets, and some cancer cells, and is a potential type I cell-surface transmembrane receptor of TGF-β.16 It has been shown that knockdown of GARP or treatment with blocking antibodies significantly reduces the immunosuppressive capacity of regulatory T cells.17 Studies on GARP on asthma, tumor, colitis and other diseases are common,18 but studies on the effects of GARP on cardiovascular diseases are rare, especially the studies on the relationship between GARP and atherosclerosis. Our study is the first to propose that the overexpression of GARP could inhibit the formation of atherosclerotic plaques, which shows that the overexpression of GARP decreases inflammatory cells and inflammatory factors, and increases the number of anti-inflammatory cells and enhances their functions. Crucially, we first proposed the GARP-treated DCs, and found that the treated DCs had the tolerogenic phenotype and the ability to induce tolerance when co-cultured with CD4+ T cells, and the atherosclerotic plaques of mice after adoptive transfer of GARP-tDCs were significantly reduced. In a conclusion, the data suggests that GARP and GARP-tDCs therapy can alleviate atherosclerotic plaque areas in mice, and GARP is a potential therapeutic target for atherosclerosis.
Atherosclerosis is a pathological process involving multiple factors and so far the precise mechanism of AS is unclear. It has been known that activation of immune cells and inflammatory cytokines has a key role in the process, particularly T cell-mediated pathogenic immune responses, such as CD4+ T lymphocytes.19 The CD4+ T lymphocytes in the atherosclerosis lesion are major consisted of Th1 and Th17 cells.20 Natural Tregs, as an important subset of T lymphocytes, could regulate peripheral immune homeostasis by balancing the Th1, Th2 and Th17. So considerable reports confirmed that the suppressive function and frequency of nTregs had connection with the development of atherosclerosis lesion.21 Such as our previous studies suggested that immunologic tolerance induced by nasal administration of HSP60 was achieved by increasing TGF-β-dependent CD4+LAP+ and CD4+CD25+Foxp3+ Tregs, which can reduce atherosclerosis,22 while intranasal oxLDL administration also alleviates atherosclerosis by inducing the CD4+LAP+ and CD4+CD25+Foxp3+ regulatory T cells, and inhibiting the effector T cell response in ApoE−/- mice.6 GARP is critical for the expression of potential TGF-β on the surface of activated FOXP3+ regulatory T cells,23 and TGF-β is an important molecule for FOXP3+ Treg cells to perform their inhibitory function, suggesting that GARP can promote the inhibitory function of Treg cells. Therefore, we speculated that GARP had an effect on AS.
In this study, it was found that the overexpression of GARP up-regulated the proportion of CD4+Foxp3+ Tregs and CD4+CD25+LAP+ T cells in ApoE−/- splenic lymphocytes, and the number of LAP+ cells and Foxp3+ cells were positively correlated with GARP+ cells, indicating that the effect of GARP on nTregs was realized by up-regulating the expression of LAP on nTregs. This is consistent with previous studies, which have shown that GARP and LAP co-locate on the surface of activated Tregs, and the expression of GARP is highly correlated with the expression of LAP on T lymphocytes.24 It is well known that after non-covalently binding with LAP, TGF-β is secreted or expressed on membranes in many cell types as a non-bioactive form, and the potential TGF-β obtains biological activity after release of LAP.25 Membrane protein GARP can act as a receptor for latent TGF-β and produce active TGF-β by binding directly to the LAP of latent TGF-β.26 The function of Treg cells is partly dependent on TGF-β. In this study, it was found that the secretion of mature TGF-β in the GARP-overexpression group was significantly increased, indicating that the overexpression of GARP upregulated the immunosuppression effect of Treg in vivo, and we further demonstrated this by co-culture of Tregs/CD4+CD25− T cells. Studies have shown that active TGF-β1, released by the LAP-TGF-β complex, can bind with TGF-β receptor with high affinity, and it can phosphorylate Smad2 and Smad3 transcription factors interacting with Smad4, thereby activating the Smad signaling pathway to increase Foxp3 expression and nTregs proliferation.27 Our findings further confirmed that overexpression of GARP can stimulate the phosphorylation of Smad3.
In our study, it was found that the overexpression of GARP effectively restored the number and inhibitory function of nTregs, and the restored Tregs significantly inhibited the excessive proliferation of Th1 and Th17 in ApoE−/- mice, and the inhibition of inflammation may explain the reduction of atherosclerotic plaque formation in the GARP-overexpression group. We also found that Th2 cells in the GARP overexpressed group were significantly higher than those in the control group and the GARP inhibited group. In fact, the role of Th2 cells in atherosclerosis remains controversial. Based on intima-media thickness of the common carotid artery, patients with high Th2 cell count had lower thickness than those with low Th2 cell count,28 and Th2 cells have been shown to play a role in preventing the development of lipids at an early stage in murine models of atherosclerosis. Cytokines IL-5 and IL-13 are associated with the Th2 cells, which have been shown has a protective effect of atherosclerosis.29 However, in LDL receptor knockout mice, IL-4 deficiency does not affect the progression of atherosclerotic lesions,30 but it has been reported that the formation of atherosclerotic lesions is reduced in this condition.31 The results of this study tend to favor the protective effect of Th2 cells on atherosclerosis.
The role of dendritic cells in atherosclerosis is like a “double-edged sword”. Mature DC can promote the differentiation of naive T cells into effector T cells, thus promoting inflammation and aggravating atherosclerosis. Immature DCs can induce regulatory T cells by directly contacting with immature T cells or by secreting anti-inflammatory cytokines, thereby inhibiting differentiation of immature T cells and causing incompetence of CD4+ T cells and CD8+ T cells.32 The DC was immature in the peripheral tissues, and after draining to the lymph nodes, it matured after being stimulated by various pathogens and inflammatory stimuli. DC maturation process leads to the increase of the antigen presentation process, making the costimulatory molecules such as CD80 and CD86 up-regulated. MHC-II as an important molecular presenting antigen to DC, mainly presented extracellular antigen, will also be raised in the maturation process. Studies have shown that apoB100 shock can induce tolerogenic dendritic cells, thus weakening the response of T cells to apoB100, and promoting Tregs induction, thereby delaying the progression of atherosclerosis.33 Another study showed that the transfer of LDL-induced dendritic cells to hypercholesterolemic mice accelerated atherosclerosis,34 which suggests that we can modulate atherosclerosis by changing the phenotype of DC. In this study, the MFI of CD40, MHC-II and CD86 was significantly decreased in the GARP-tDC group, indicating that GARP hindered the maturation process of DCs, and GARP could also induce the generation of tolerogenic dendritic cells. Further adoptive transmission of GARP-tDCs to ApoE−/- mice showed that atherosclerotic plaques were reduced and the condition was improved. This suggests that GARP can protect atherosclerosis by inducing the production of tolerogenic dendritic cells.
IL-10 and TGF-β are typical inhibitory cytokines, while IDO produced by DC degrades tryptophan into kynurenic acid, thus playing a key role in immune cell-mediated suppression and tolerance.35 When APCs express IDO, the immunosuppressive effect of IDO may directly affect APCs or indirectly affect local T cells. In this study, it was found that the mRNA levels of IL-10, TGF-β and IDO in the GARP-tDC group were significantly increased, which also proved that GARP-tDCs showed a tolerogenic phenotype.
Our study found that when GARP-tDCs were co-cultured with CD4+ T cells, GARP-tDCs significantly increased the percentage of CD4+CD25+Foxp3+ Treg cells in the CD4+ cell population, and the mRNA level of Foxp3 in the GARP-tDC group was significantly higher than that in the other three groups. GARP-tDCs also significantly inhibit the mRNA level of IFN-γ and IL-17A. IL-17A is also known as IL-17, and its deficiency has been shown to reduce vascular inflammation and atherosclerosis,36 while IFN-γ has been found to contribute to the instability of atherosclerosis by activating multiple pathways such as JAK/STAT.37 Thus we guessed that the adoptive transmission of GARP-tDCs can improve atherosclerosis by increasing CD4+CD25+Foxp3+ Treg cells and inhibiting IFN-γ and IL-17A.
Conclusion
Anti-inflammatory is an effective treatment for atherosclerosis, among which induction of atherosclerotic protective dendritic cells may be a new treatment to prevent atherosclerosis. This study demonstrated for the first time that overexpression of GARP can enhance the frequency and immunosuppressive function of CD4+CD25+ Foxp3+ Tregs, and adoptive transfer of GARP-tDCs can improve atherosclerosis in mice, but the specific mechanism remains to be further studied. The application of GARP in cardiovascular diseases also needs to be further explored, so to further improve the inflammation theory of AS and provide a new theoretical and experimental basis for targeted immunotherapy of AS.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (NO: 81270354 and NO: 81300213).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Luo J, Wang X, Jiang X, et al. Rutaecarpine derivative R3 attenuates atherosclerosis via inhibiting NLRP3 inflammasome-related inflammation and modulating cholesterol transport. FASEB J. 2020;34(1):1398–1411. doi:10.1096/fj.201900903RRR
2. Narula J, Arbustini E. Inflammation, superadded inflammation, and out-of-proportion inflammation in atherosclerosis. JAMA Cardiol. 2018;3(10):912–914. doi:10.1001/jamacardio.2018.2760
3. Ait-Oufella H, Libby P, Tedgui A. Antibody-based immunotherapy targeting cytokines and atherothrombotic cardiovascular diseases. Arch Cardiovasc Dis. 2020;113(1):5–8. doi:10.1016/j.acvd.2019.11.001
4. Hasib L, Lundberg AK, Zachrisson H, Ernerudh J, Jonasson L. Functional and homeostatic defects of regulatory T cells in patients with coronary artery disease. J Intern Med. 2016;279(1):63–77. doi:10.1111/joim.12398
5. Mor A, Planer D, Luboshits G, et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(4):893–900. doi:10.1161/01.ATV.0000259365.31469.89
6. Zhong Y, Wang X, Ji Q, et al. CD4+LAP + and CD4 +CD25 +Foxp3 + regulatory T cells induced by nasal oxidized low-density lipoprotein suppress effector T cells response and attenuate atherosclerosis in ApoE-/- mice. J Clin Immunol. 2012;32(5):1104–1117. doi:10.1007/s10875-012-9699-7
7. Shevach EM. Garp as a therapeutic target for modulation of T regulatory cell function. Expert Opin Ther Targets. 2017;21(2):191–200. doi:10.1080/14728222.2017.1275568
8. Sun L, Jin H, Li H. GARP: a surface molecule of regulatory T cells that is involved in the regulatory function and TGF-β releasing. Oncotarget. 2016;7(27):42826–42836. doi:10.18632/oncotarget.8753
9. Probst-Kepper M, Balling R, Buer J. FOXP3: required but not sufficient. the role of GARP (LRRC32) as a safeguard of the regulatory phenotype. Curr Mol Med. 2010;10(6):533–539.
10. Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(32):13439–13444. doi:10.1073/pnas.0901965106
11. Probst-Kepper M, Geffers R, Kröger A, et al. GARP: a key receptor controlling FOXP3 in human regulatory T cells. J Cell Mol Med. 2009;13(9b):3343–3357. doi:10.1111/j.1582-4934.2009.00782.x
12. Zhao X, Liu Y, Zhong Y, et al. Atorvastatin improves inflammatory response in atherosclerosis by upregulating the expression of GARP. Mediators Inflamm. 2015;2015:841472. doi:10.1155/2015/841472
13. Boks MA, Kager-Groenland JR, van Ham SM, Ten Brinke A. IL-10/IFNγ co-expressing CD4(+) T cells induced by IL-10 DC display a regulatory gene profile and downmodulate T cell responses. Clin Immunol. 2016;162:91–99. doi:10.1016/j.clim.2015.11.011
14. Zhu R, Sun H, Yu K, et al. Interleukin-37 and dendritic cells treated with interleukin-37 plus troponin i ameliorate cardiac remodeling after myocardial infarction. J Am Heart Assoc. 2016;5(12):12. doi:10.1161/JAHA.116.004406
15. Takeda M, Yamashita T, Sasaki N, et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010;30(12):2495–2503. doi:10.1161/ATVBAHA.110.215459
16. Metelli A, Salem M, Wallace CH, et al. Immunoregulatory functions and the therapeutic implications of GARP-TGF-β in inflammation and cancer. J Hematol Oncol. 2018;11(1):24. doi:10.1186/s13045-018-0570-z
17. Fridrich S, Hahn SA, Linzmaier M, et al. How soluble GARP enhances TGFβ activation. PLoS One. 2016;11(4):e0153290. doi:10.1371/journal.pone.0153290
18. Pillai S. The (inner) world according to GARP: genetic susceptibility and regulatory T cells. Sci Immunol. 2020;5(50):eabe0976. doi:10.1126/sciimmunol.abe0976
19. Wang R, Nascimento BR, Neuenschwander FC. Atherosclerosis and inflammation: still a long way to go. Arq Bras Cardiol. 2020;114(4):699–700. doi:10.36660/abc.20200219
20. Gowdak LHW. Atherosclerosis, inflammation, and genetics - and you thought it was just LDL-cholesterol. Arq Bras Cardiol. 2020;114(2):273–274.
21. Sharma M, Schlegel MP, Afonso MS, et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ Res. 2020;127(3):335–353. doi:10.1161/CIRCRESAHA.119.316461
22. Li H, Ding Y, Yi G, Zeng Q, Yang W. Establishment of nasal tolerance to heat shock protein-60 alleviates atherosclerosis by inducing TGF-β-dependent regulatory T cells. J Huazhong Univ Sci Technol Med Sci. 2012;32(1):24–30. doi:10.1007/s11596-012-0004-z
23. Stockis J, Dedobbeleer O, Lucas S. Role of GARP in the activation of latent TGF-β1. Mol Biosyst. 2017;13(10):1925–1935. doi:10.1039/C7MB00251C
24. Battaglia M, Roncarolo MG. The Tregs’ world according to GARP. Eur J Immunol. 2009;39(12):3296–3300. doi:10.1002/eji.200940117
25. Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol. 2009;39(12):3315–3322. doi:10.1002/eji.200939684
26. Liénart S, Merceron R, Vanderaa C, et al. Structural basis of latent TGF-β1 presentation and activation by GARP on human regulatory T cells. Science. 2018;362(6417):952–956. doi:10.1126/science.aau2909
27. Feinberg MW, Jain MK. Role of transforming growth factor-beta1/Smads in regulating vascular inflammation and atherogenesis. Panminerva Med. 2005;47(3):169–186.
28. Tracy RP, Doyle MF, Olson NC, et al. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of atherosclerosis. J Am Heart Assoc. 2013;2(3):e000117. doi:10.1161/JAHA.113.000117
29. Silveira A, McLeod O, Strawbridge RJ, et al. Plasma IL-5 concentration and subclinical carotid atherosclerosis. Atherosclerosis. 2015;239(1):125–130. doi:10.1016/j.atherosclerosis.2014.12.046
30. Binder CJ, Hartvigsen K, Chang MK, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114(3):427–437. doi:10.1172/JCI200420479
31. King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor-/- mice. Arterioscler Thromb Vasc Biol. 2002;22(3):456–461. doi:10.1161/hq0302.104905
32. Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Dendritic cells: a double-edge sword in atherosclerotic inflammation. Curr Pharm Des. 2015;21(9):1118–1123. doi:10.2174/1381612820666141013162528
33. Hermansson A, Johansson DK, Ketelhuth DFJ, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123(10):1083–1091. doi:10.1161/CIRCULATIONAHA.110.973222
34. Frodermann V, van Puijvelde GH, Wierts L, et al. Oxidized low-density lipoprotein-induced apoptotic dendritic cells as a novel therapy for atherosclerosis. J Immunol. 2015;194(5):2208–2218. doi:10.4049/jimmunol.1401843
35. Mellor AL, Lemos H, Huang L. Indoleamine 2,3-dioxygenase and tolerance: where are we now? Front Immunol. 2017;8:1360. doi:10.3389/fimmu.2017.01360
36. Usui F, Kimura H, Ohshiro T, et al. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem Biophys Res Commun. 2012;420(1):72–77. doi:10.1016/j.bbrc.2012.02.117
37. Lu X. The impact of IL-17 in atherosclerosis. Curr Med Chem. 2017;24(21):2345–2358. doi:10.2174/0929867324666170419150614
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.