Back to Journals » Cancer Management and Research » Volume 12
Gambogic Acid Inhibits the Progression of Gastric Cancer via circRNA_ASAP2/miR-33a-5p/CDK7 Axis
Authors Lin D, Lin X, He T, Xie G
Received 28 June 2020
Accepted for publication 27 August 2020
Published 28 September 2020 Volume 2020:12 Pages 9221—9233
DOI https://doi.org/10.2147/CMAR.S269768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Dan Lin,1 Xiaoyang Lin,2 Tianlin He,1 Guoqun Xie1
1Department of Oncology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Integrated TCM and Western Medicine, The First People’s Hospital of Wenling, Wenling, Zhejiang, People’s Republic of China
Correspondence: Dan Lin Department of Oncology
Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, No. 128 Henggao Road, Gaojing Town, Baoshan District, Shanghai 200439, People’s Republic of China
Tel +86 15801988721
Fax +86 21-65161782-1663
Email [email protected]
Background: Gastric cancer (GC) is a major cancer-related mortality disease. Gambogic acid (GA) has been investigated to inhibit cancer progression. In the present study, the molecular mechanism of GA in regulating GC progression was studied.
Methods: The expression levels of circular RNA ASAP2 (circ_ASAP2), miR-33a-5p and cyclin-dependent kinases 7 (CDK7) were detected by quantitative real-time polymerase reaction (qRT-PCR). CDK7 protein level was evaluated by Western blot. Cell colony formation assay, 3-(4,5-Dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, transwell assay and flow cytometry analysis were employed to reveal the functional effects among circ_ASAP2, miR-33a-5p and CDK7 on GA-induced GC progression. Mechanistically, the binding relationship between miR-33a-5p and circ_ASAP2 or CDK7 was predicted with starBase v3.0 online database and verified by dual-luciferase reporter assay. In vivo tumor formation assay was used to explain the impacts of GA treatment on GC growth in vivo.
Results: Circ_ASAP2 and CDK7 expression were downregulated in GA-induced GC cells compared with GC cells. MiR-33a-5p expression was upregulated in GA-induced GC cells relative to GC cells. The protein expression level of CDK7 was lower in GA-induced GC cells than that in GC cells. Further, circ_ASAP2 overexpression decreased GA-induced inhibition effects on cell proliferation, migration and invasion and GA-induced promotion effect on cell apoptosis in both AGS and HGC-27 cells, whereas this phenomenon was reversed by miR-33a-5p. In addition, circ_ASAP2 functioned as a sponge of miR-33a-5p and miR-33a-5p was associated with CDK7. Furthermore, GA treatment inhibited GC growth in vivo.
Conclusion: Circ_ASAP2 overexpression promoted cell proliferation, migration and invasion, whereas inhibited cell apoptosis by upregulating CDK7 expression through binding to miR-33a-5p in GA-induced GC cells. This study provided a theoretical basis in GC treatment with GA.
Keywords: gambogic acid, circ_ASAP2, miR-33a-5p, CDK7, gastric cancer
Introduction
Gastric cancer (GC) ranks fifth among cancers worldwide.1 According to the data analyzed in 2014, 410,400 cases were newly diagnosed and 293,800 cases were related to death in China.2 Although surgical resection and chemo-radiotherapy have been applied to treat GC, the morbidity and mortality rates of GC are also high. Gambogic acid (GA), gotten from Garcinia hanburyi, is the major ingredient of gamboge.3 Numerous studies indicated that GA acted as the suppressor of cancers, including GC.3,4 For example, Wang et al indicated that GA could increase docetaxel sensitivity in GC cells.5 Zou et al revealed that GA induced cell apoptosis together with chemotherapeutic agents in GC cells.6 However, the regulatory mechanism of GA in GC progression has not been fully addressed.
Circular RNA (circRNA) is a noncoding RNA with closed loop.7 An increasing number of studies have indicated that circRNA acted as a promoter or suppressor in cancer progression.8,9 For instance, Yang et al revealed that circ_0049027 (circ_HuR) inhibited cell migration and invasion in vitro and in vivo;8 Wu et al also explained that circ-ZNF609 contributed to the proliferation of colorectal cancer cells by sponging microRNA-150 (miR-150).10 Additionally, circRNA was involved in GC progression. Wang et al disclosed that enforced circ_0027599 expression suppressed cell proliferation and metastasis in GC.11 Other like circ_101882 was unveiled to promote cell migration and invasion in GC.12 Nevertheless, the effects and mechanism of circ_ASAP2 in GA-mediated GC process are unknown.
MiRNAs are a kind of transcripts with 18–25 nucleotide in length. And miRNAs regulate gene expression at post-translational level.13 Some studies revealed that miR-33a-5p regulated metastasis and apoptosis in various cancers.14,15 However, there are few studies on the regulation of GC mediated by miR-33a-5p. Cyclin-dependent kinases 7 (CDK7) mainly takes part in Pol II transcription process by acting as enzymatic cofactors.16 CDK7 was expressed in various cancers and its downregulation was investigated to inhibit cell proliferation.17 Some studies indicated that THZ1,18 SNS-03219 and QS118920 could inhibit cancer progression by repressing CDK7 expression. These data meant that CDK7 may act as a tumor suppressor in GC process.
In this study, circ_ASAP2 expression was detected by qRT-PCR. The effects among circ_ASAP2, miR-33a-5p and CDK7 on GA-induced GC progression were determined by cell colony formation assay, MTT assay, transwell assay and flow cytometry analysis. Meanwhile, dual-luciferase reporter assay was employed to identify the target relationship between miR-33a-5p and circ_ASAP2 or CDK7. Furthermore, the effects of GA treatment on GC growth in vivo were explained by in vivo tumor formation assay.
Materials and Methods
Clinical Tissues Sample and Cell Culture
All procedures in this study were approved by the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine. The written informed consents were signed before surgery. Fifty pairs of GC tissues and paracancerous healthy tissues were obtained from Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine. These samples were instantly kept in −80°C for further study.
Sciencell (Carlsbad, CA, USA) provided the human gastric cancer cell lines AGS and HGC-27 and human normal gastric epithelial cell line GES-1. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Thermo Fisher, Waltham, MA, USA) and 1% penicillin-streptomycin. GES-1, AGS and HGC-27 cells were cultured at 37°C in an incubator with 5% CO2.
Cell Transfection
Small interfering RNA against CDK7 (si-CDK7), the overexpression vector of circ_ASAP2 (circ_ASAP2), miR-33a-5p mimic (miR-33a-5p), miR-33a-5p inhibitor (anti-miR-33a-5p) and control groups, including si-NC, Vector, miR-NC, and anti-miR-NC, were purchased from Ribobio Co., Ltd. (Guangzhou, China). Cell transfection was carried out using Lipofectamine 3000 (Thermo Fisher). AGS and HGC-27 cells were cultivated for 16 h. Plasmids, miR-33a-5p or miR-33a-5p inhibitor was transfected into GC cells and GES-1 cells with control groups. Cells were continued to culture and collected at indicated time. The sequences related to this study were si-CDK7 CCAACCAAATTGTCGCCAT, si-NC CCAAACTTACTGCGACCAT, miR-33a-5p mimics 5ʹ-GUGCAUUGUAGUUGCAUUGCA-3ʹ and miR-33a-5p inhibitor 5ʹ-TGCAATGCAACTACAATGCAC-3ʹ.
Colony Formation Assay
AGS and HGC-27 were cultured in 6-well plates for 2 weeks. And proliferating colonies were stained using 1% crystal violet. The colony numbers were calculated and photographed. A colony was defined when its numbers more than 50.
3-(4,5-Dimethylthazol-2-Yl)-2,5-Diphenyltetrazolium Bromide Assay (MTT Assay)
Cell viability was detected by MTT assay. Briefly, cells were cultivated into 96-well plate for 24 h. 20 μL MTT solution was added into the plate and continued to cultivate for 4 h after cells were treated with different treatments. Dimethyl sulfoxide was added to dissolve formazan crystals. The optical density of absorbance was detected at 490 nm by a microplate reader (Synergy H4 Hybrid Reader, BioTek, Winooski, USA).
Transwell Assay
The migratory and invasive abilities of cells were determined by transwell assay without or with Matrigel, respectively. Cells were seeded in upper chambers supplied with FBS-free medium. Then, medium with 10% FBS was added in the low chambers. The transwell chamber was taken from a 24-well plate after cells were cultured for 24 h. Medium was discarded and cells were washed twice. Then, cells were incubated with methanol and crystal violet, respectively. Cell migration and invasion were observed by a microscope at a 100 magnification.
Flow Cytometry Analysis
Apoptosis detection kit (Qcbio Science, Shanghai, China) was employed to determine cell apoptosis. The cells at logarithmic period were harvested and washed with phosphate-buffered saline buffer (PBS). Then, cells were re-suspended with 100 μL binding buffer and cells were incubated with 5 μL Annexin-FITC. After that, cells were incubated with 10 μL propidium iodide (PI) for 15 min. Results were analyzed with a FACSort flow cytometer.
Quantitative Real-Time Polymerase Reaction (qRT-PCR)
GC tissues and cells were lysed with TRIzol reagent (TaKaRa, Dalian, China). Then, RNA was extracted and cDNA was amplified with a reagent kit (TaKaRa). To quantity the amount of circRNA/miRNA/mRNA, PTC-220 Machine was employed with an SYBR Green SuperMix kit (Roche, Basel, Switzerland). GAPDH and U6 were chosen as references. The forward and reverse primers were: circ_ASAP2 5ʹ-CCTGACCTGCATCGAGTGTT-3ʹ and 5ʹ-GTAAGTTCTGTCATCAGCAGCTC-3ʹ; ASAP2 5ʹ-CCCATGAGGACTACAAGGCG-3ʹ and 5ʹ-CATTTTCCACGTGAGCCAGC-3ʹ; miR-33a-5p 5ʹ-GGTGCATTGTAGTTGCATTGC-3ʹ and 5ʹ-GTGCAGGGTCCGAGGTATTC-3ʹ; CDK7 5ʹ-GGCACACCAACTGAGGAACA-3ʹ and 5ʹ-AGTCGTCTCCTGCTGCACTG-3ʹ. GAPDH 5ʹ-CCATGGGGAAGGTGAAGGTC-3ʹ and 5ʹ-TGGAATTTGCCATGGGTGGA-3ʹ; U6 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ.
RNase R Digestion and Actinomycin D Treatment
Total RNA from cells was treated with RNase R (Amresco, Solon, OH, USA) at 37°C for 30 min, followed qRT-PCR was employed to detect circ_ASAP2 or ASAP2 expression. In addition, cells were treated with Actinomycin D (Amresco) for 0, 8, 16 and 24 h after cells were seeded. QRT-PCR was applied to measure circ_ASAP2 and ASAP2 expression.
Dual-Luciferase Reporter Assay
The binding relationship between miR-33a-5p and circ_ASAP2 or CDK7 was identified by dual-luciferase reporter assay. The wild-type (wt) sequences of circ_ASAP2 and CDK7 3ʹUTR containing the binding sequences of miR-33a-5p were amplified and inserted into pGL3-basic vector (Genecreate, Wuhan, China), and named as circ_ASAP2-wt and CDK7-wt. Mutant (mut) circ_ASAP2 and CDK7 3ʹUTR harboring the target sequences of miR-33a-5p were synthesized and cloned into pGL3 vector (Genecreate), and named as circ_ASAP2-mut and CDK7-mut. After that, cells were transfected using Lipofectamine 3000. Cells were continued to culture for 48 h. Luciferase activities were detected with a luciferase reporter system (Promega, Madison, WI, USA). Ranilla luciferase activity was utilized as a reference.
Western Blot
Sample was lysed with RIPA buffer (Beyotime, Jiangsu, China) with proteinase K inhibitor. Protein sample was loaded on 10% SDS-PAGE gel. After that, bands were transferred into PVDF membranes. Bands were incubated in 5% non-fat milk. Subsequently, the membranes were washed and incubated with primary antibodies. The membranes were washed and incubated with second antibody (peroxidase-conjugated IgG). The antigen-antibody complexes were visualized by enhanced chemiluminescence (KeyGen, Nanjing, China). GAPDH was used as an internal control. The primary antibodies were anti-CDK7 (1:1000; Abcam, Cambridge, MA, UK) and anti-GAPDH (1:2500; Abcam).
In vivo Tumor Formation Assay
GC cells (5×106) were injected into the flank of 6-week-old nude mice (Shanghai SLAC Animal Center, Shanghai, China). After 10 days, GA was abdominally injected into mice every 3 days. Tumor volume was measured every 3 days after injection. After 22 days, nude mice were euthanized and tumors were excised. The growth curve of tumor was drawn and tumor weight was measured. All produces were provided by the Animal Care and Use Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine. Guide for the Care and Use of Laboratory Animals was strictly followed.
Statistical Analysis
All data derived from three duplicate tests. GraphPad Prism version 5.0 and SPSS 19.0 software were carried out to analyze data. The difference between the two groups was analyzed by two-tailed Student’s t-tests. The difference between more than two groups was determined by one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
GA Inhibits Cell Proliferation, Migration and Invasion, and Induces Cell Apoptosis in AGS and HGC-27 Cells
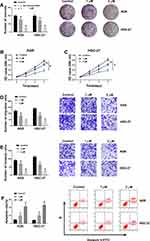
In order to explore the effects of GA on the GC cell progression, GC cells treated with 1 μM or 2 μM GA were detected by MTT assay, colony formation assay, transwell assay and flow cytometry analysis. As a result, colony formation assay and MTT assay demonstrated that GA treatment repressed cell proliferation and viability in a dose-dependent manner compared with control groups in AGS and HGC-27 cells (Figure 1A–C). Transwell assay revealed that the migration and invasion of AGS and HGC-27 cells also were suppressed after GA exposure, and this inhibition effect was positively correlated with GA concentration (Figure 1D and E). Similarly, GA treatment induced cell apoptosis in AGS and HGC-27 cells (Figure 1F). These results suggested that GA functioned as a tumor suppressor in GC development.
GA Treatment Downregulates circ_ASAP2 Expression in GC Cells
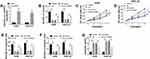
The effect of GA on circ_ASAP2 expression was determined in this part. Circ_ASAP2 expression was firstly detected by qRT-PCR in GC cells treated with 2 μM of GA, and results showed that circ_ASAP2 expression level was higher in AGS and HGC-27 cells than that in normal gastric cells, and this effect was restored after GA treatment (Figure 2A). Subsequently, results showed circ_ASAP2 expression had no obvious change after RNase R treatment, whereas ASAP2 expression was obviously downregulated (Figure 2B and C). And data also indicated that there was no apparent various in circ_ASAP2 expression after Actinomycin D exposure, but that significantly decreased ASAP2 expression (Figure 2D and E). Thus, RNase R digestion and Actinomycin D treatment assays showed that circ_ASAP2 was more stable than ASAP2. Furthermore, circ_ASAP2 expression was analyzed in GC tissues, and qRT-PCR result revealed that circ_ASAP2 expression level was higher in GC tissues than in normal gastric tissues (Figure 2F). All these data suggested that GA could downregulate circ_ASAP2 expression level in GC cells.
Circ_ASAP2 Partially Attenuates the Effects of GA Treatment on GC Progression
For the sake of understanding the impacts between circ_ASAP2 and GA on GC biological behavior, the overexpression vector of circ_ASAP2 was firstly constructed. QRT-PCR results showed circ_ASAP2 expression was greatly increased after circ_ASAP2 transfection in AGS and HGC-27 cells (Figure 3A). Subsequently, the effects between circ-ASAP2 and GA on cell proliferation, migration, invasion and apoptosis were evaluated in both AGS and HGC-27 cells. Colony formation assay showed that GA treatment inhibited the colony-forming ability of GC cells, whereas this phenomenon was partially reversed by circ_ASAP2 overexpression (Figure 3B). MTT assay revealed that GC cell viability was repressed by GA and circ_ASAP2 overexpression decreased this inhibition effect (Figure 3C and D). Transwell assay showed that GA impeded the migration and invasion of GC cells; however, this effect was partly abolished by circ_ASAP2 overexpression (Figure 3E and F). In addition, flow cytometry analysis explained that GA treatment induced GC cell apoptosis, which was restored after circ_ASAP2 overexpression (Figure 3G). All these data suggested that GA inhibited GC progression by regulating circ_ASAP2 expression.
Circ_ASAP2 Acts as a Sponge of miR-33a-5p
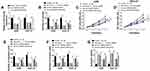
In order to understand the regulatory mechanism of circ_ASAP2 in GC progression, the target gene of circ_ASAP2 was predicted. Starbase v3.0 online database showed circ_ASAP2 contained the binding sites of miR-33a-5p (Figure 4A). Dual-luciferase reporter assay showed that the luciferase activity of circ_ASAP2-wt+miR-33a-5p group was dramatically decreased in both AGS and HGC-27 cells, whereas the luciferase activity of circ_ASAP2-mut+miR-33a-5p group was not obviously changed (Figure 4B and C). Meanwhile, qRT-PCR analysis showed that miR-33a-5p expression was downregulated by circ_ASAP2 (Figure 4D). Subsequently, qRT-PCR was applied to detect miR-33a-5p expression in GES-1 cells, GC cells and GA-induced GC cells. Results revealed that the expression level of miR-33a-5p was evidently downregulated in GC cells compared with that in GES-1 cells, whereas that was attenuated by GA treatment (Figure 4E). Data also showed that miR-33a-5p expression was downregulated in GC tissues compared with paracancerous normal tissues (Figure 4F). Besides, the relationship between miR-33a-5p and circ_ASAP2 was determined by Pearson correlation analysis, and results showed that miR-33a-5p was negatively related to circ_ASAP2 (Figure 4G). All these data suggested that circ_ASAP2 was a sponge of miR-33a-5p.
Circ_ASAP2 Sponges miR-33a-5p to Regulate the Progression of GA-Induced GC Cells
Based on the above data, we continued to explore the functional effects between circ-ASAP2 and miR-33a-5p on the progression of GA-induced GC cells. The effects between circ_ASAP2 and miR-33a-5p on miR-33a-5p expression were firstly detected. QRT-PCR analysis showed that circ_ASAP2 downregulated miR-33a-5p expression, and this phenomenon was impaired after miR-33a-5p transfection (Figure 5A). After that, the effects among GA, circ_ASAP2 and miR-33a-5p on GC progression were determined. Colony formation assay showed that circ_ASAP2 decreased the inhibition effect of GA on the colony-forming ability of AGS and HGC-27 cells, whereas this phenomenon was abolished by miR-33a-5p (Figure 5B). MTT assay also demonstrated that circ_ASAP2 abolished the GA-induced repression effect on cell viability and this effect was reversed after miR-33a-5p transfection in both AGS and HGC-27 cells (Figure 5C and D). Transwell assay investigated that GA-mediated inhibition effects on cell migration and invasion were impaired by circ_ASAP2 in both AGS and HGC-27 cells; however, this effect also was attenuated by miR-33a-5p (Figure 5E and F). Furthermore, GA-induced cell apoptosis was blocked by circ_ASAP2 in both AGS and HGC-27 cells, which was relieved by miR-33a-5p (Figure 5G). From these data, we could come to the conclusion that circ_ASAP2 promoted the progression of GA-induced GC cells by binding to miR-33a-5p.
Circ_ASAP2 Sponges miR-33a-5p to Upregulate CDK7 Expression in GC Cells
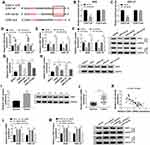
In order to understand the regulatory mechanism between circ_ASAP2 and miR-33a-5p in GA-induced GC cells, the target gene of miR-33a-5p was predicted by starBase v3.0 online database. Result showed that CDK7 3ʹUTR contained the target sites of miR-33a-5p (Figure 6A). Dual-luciferase reporter assay revealed miR-33a-5p greatly downregulated the luciferase activities of CDK7-wt+miR-33a-5p group, but not the luciferase activities of CDK7-mut+miR-33a-5p group (Figure 6B and C). After that, the transfection efficiency of miR-33a-5p and anti-miR-33a-5p was detected in both AGS and HGC-27 cells. QRT-PCR analysis revealed that miR-33a-5p expression was dramatically upregulated by miR-33a-5p mimic and downregulated by anti-miR-33a-5p in both AGS and HGC-27 cells (Figure 6D). Then, the impact of miR-33a-5p mimic or anti-miR-33a-5p on CDK7 expression was detected, and results showed that the mRNA and protein levels of CDK7 were inhibited by miR-33a-5p mimics and were upregulated by anti-miR-33a-5p in both AGS and HGC-27 cells (Figure 6E and F). These results showed that miR-33a-5p targeted CDK7.
Further, the effect of GA treatment on CDK7 expression was determined. QRT-PCR and Western blot results suggested that the mRNA and protein levels of CDK7 were higher in AGS and HGC-27 cells relative to GES-1 cells, whereas those were hindered under GA exposure in GC cells (Figure 6G and H). Subsequently, the protein and mRNA expression of CDK7 were determined in GC tissues. Results revealed that both protein and mRNA levels of CDK7 were higher in GC tissues than in adjacent normal tissues (Figure 6I and J). Meanwhile, CDK7 was negatively related to miR-33a-5p (Figure 6K). Furthermore, the effects between circ_ASAP2 and miR-33a-5p on CDK7 expression were analyzed by qRT-PCR and Western blot. Results showed both mRNA and protein levels of CDK7 were increased by circ_ASAP2, whereas this phenomenon was reversed by miR-33a-5p (Figure 6L and M). These results showed circ_ASAP2 sponged miR-33a-5p to regulate CDK7 expression.
MiR-33a-5p Inhibition Promotes the Progression of GA-CDK7
In order to explore the effects between miR-33a-5p and CDK7 in GA-induced GC progression, we firstly detected the effects between miR-33a-5p inhibition and CDK7 knockdown on CDK7 expression level. QRT-PCR and Western blot results showed that the mRNA and protein expression of CDK7 were upregulated by anti-miR-33a-5p in both AGS and HGC-27 cells, which was decreased by CDK7 silencing (Figure 7A and B). After that, colony formation assay investigated that miR-33a-5p deletion impaired the inhibition effect of GA treatment on cell proliferation in both AGS and HGC-27 cells, whereas this effect was blocked by CDK7 knockdown (Figure 7C). MTT assay also demonstrated that miR-33a-5p inhibition reversed the repressive effect of GA treatment on cell viability in both AGS and HGC-27 cells, which was decreased by CDK7 deletion (Figure 7D and E). Transwell assay revealed that anti-miR-33a-5p partially attenuated the inhibition effect of GA exposure on cell migration and invasion in both AGS and HGC-27 cells; however, this effect was relieved after CDK7 knockdown (Figure 7F and G). Furthermore, the promotion effect of GA exposure on apoptosis was inhibited by anti-miR-33a-5p in GC cells, which was partly abolished by CDK7 silencing (Figure 7H). These results demonstrated that miR-33a-5p inhibition attenuated the effects of GA treatment on GC development by regulating CDK7 expression.
GA Treatment Represses GC Growth in vivo
In order to investigate the effects of GA treatment on GC growth in vivo, in vivo tumor formation assay was employed. The effects of GA treatment on tumor volume and weight were firstly detected. Results showed tumor volume and weight were obviously decreased (Figure 8A and B). In addition, the expression of circ_ASAP2, miR-33a-5p and CDK7 was detected in tissue treated with GA in vivo. QRT-PCR results revealed GA exposure repressed circ_ASAP2 expression (Figure 8C) and upregulated miR-33a-5p expression level (Figure 8D). Both mRNA and protein levels of CDK7 were significantly downregulated by GA treatment (Figure 8E and F). These results showed GA repressed GC growth by regulating circ_ASAP2, miR-33a-5p and CDK7 in vivo.
Discussion
GC poses a great threat to human health in recent years. Although great efforts have been done, the morbidity and mortality of GC are also high. GA exhibits a series of antitumor behavior in various cancers.21,22 However, the studies on the mechanism of GA regulating GC development by circRNA/miRNA/mRNA axis are few. In the present study, GA was found to inhibit GC progression by regulating circ_ASAP2/miR-33a-5p/CDK7 axis.
It has been indicated that GA plays a vital part in anti-tumor. Li et al revealed that GA inhibited cell proliferation, migration and invasion in malignant melanoma.23 Zou et al explained that the combined usage of GA with chemotherapy drugs induced cell apoptosis in a synergistic manner.6 Zhao et al evaluated that GA promoted cell apoptosis by inhibiting bc1-2 or increasing bax protein expression in GC cells.24 Similarly, our results showed that GA repressed the proliferation, migration and invasion, whereas promoted apoptosis of GC cells.
CircRNA has been enrolled in GC process. For example, circ_0009910 silencing repressed cell proliferation, migration and invasion in GC.25 Circ_0066444 overexpression promoted cell proliferation and metastasis in GC.26 Besides, circRNAs were related to drug sensitivity in cancer process.27,28 In this study, circ_ASAP2 was the first to be revealed in regulating GC development mediated by GA. In this study, circ_ASAP2 was found to be overexpressed in GA-induced GC cells compared with GC cells. In addition, circ_ASAP2 attenuated the inhibition effects of GA treatment on GC cell proliferation, migration, invasion, and the promotion effect of GA treatment on cell apoptosis, which implied that circ_ASAP2 could promote GC progression. Previous studies have revealed that circRNA functioned as a competing endogenous RNA (ceRNA) to sponge miRNA, and thereby mediated cancer progression.29 Thus, in order to seek associated miRNA with circ_ASAP2, starBase v3.0 online database was employed. And our results showed that circ_ASAP2 was a sponge of miR-33a-5p. MiR-33a-5p was revealed to suppress cell proliferation, migration and invasion, and induced cell apoptosis in lung cancer.14 Yan et al revealed that miR-33a-5p repressed cell proliferation and migratory abilities in colorectal cancer.30 Besides, miR-33a-5p was demonstrated to suppress cell proliferation and metastasis in hepatocellular carcinoma.31 In consistent with their finding, our results explained miR-33a-5p overexpression abolished the effects of circ_ASAP2 on GC progression, and this meant that miR-33a-5p inhibited GC progression.
CDK7 plays a vital role in cancer progression and is an attractive target for studying anticancer drugs.32 CDK7 silencing repressed cell proliferation and promoted cell apoptosis in GC.33 Wang et al explained that CDK7 inhibition hindered cell proliferation and promoted cell apoptotic rate in GC.34 Coincidently, in the present study, CDK7 was identified as a target of miR-33a-5p. Our studies further showed that CDK7 knockdown attenuated the promotion impacts of anti-miR-33a-5p on GC cell proliferation, metastasis, and the inhibition effect of that on GC cells apoptosis. In other words, CDK7 silencing hindered cell proliferation and metastasis and promoted cell apoptosis in GC, which was consistent with previous studies.
In summary, GA repressed cell proliferation, migration and invasion, whereas induced cell apoptosis in GC. In addition, circ_ASAP2 and CDK7 expression were downregulated, and miR-33a-5p expression was upregulated in GA-induced GC cells. Functionally, circ_ASAP2 overexpression reversed the inhibition effects of GA treatment on GC cells proliferation and metastasis, and the promotion effect of that on GC cell apoptosis, whereas this phenomenon was relieved by miR-33a-5p mimic. Mechanistically, circ_ASAP2 upregulated CDK7 expression by sponging miR-33a-5p. Furthermore, GA was investigated to decrease the volume and weight of GC tumors in vivo. These data will enrich our understanding of GA regulating GC process and provide a theoretical basis for GC treatment with GA.
Abbreviations
GC, Gastric cancer; GA, Gambogic acid; CDK7, cyclin-dependent kinases 7; qRT-PCR, quantitative real-time polymerase reaction; PNMA1, Paraneoplastic Ma1; PBS, phosphate-buffered saline; ANOVA, one-way analysis of variance.
Ethics Approval and Consent Participate
Written informed consent was obtained from patients with approval by the Institutional Review Board in Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Zhang X, Li M, Chen S, et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology. 2018;155(2):347–354.e349. doi:10.1053/j.gastro.2018.04.026
2. Yang L, Zheng R, Wang N, et al. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30(3):291–298. doi:10.21147/j.issn.1000-9604.2018.03.01
3. Zhang D, Chu Y, Qian H, et al. Antitumor activity of thermosensitive hydrogels packaging gambogic acid nanoparticles and tumor-penetrating peptide iRGD against gastric cancer. Int J Nanomedicine. 2020;15:735–747. doi:10.2147/IJN.S231448
4. Huang J, Zhu X, Wang H, et al. Role of gambogic acid and NaI(131) in A549/DDP cells. Oncol Lett. 2017;13(1):37–44. doi:10.3892/ol.2016.5435
5. Wang T, Wei J, Qian X, Ding Y, Yu L, Liu B. Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Lett. 2008;262(2):214–222. doi:10.1016/j.canlet.2007.12.004
6. Zou ZY, Wei J, Li XL, et al. Enhancement of anticancer efficacy of chemotherapeutics by gambogic acid against gastric cancer cells. Cancer Biother Radiopharm. 2012;27(5):299–306. doi:10.1089/cbr.2010.0943
7. Li M, Liu Y, Liu J, et al. Circ_0006332 promotes growth and progression of bladder cancer by modulating MYBL2 expression via miR-143. Aging. 2019;11(22):10626–10643. doi:10.18632/aging.102481
8. Yang F, Hu A, Li D, et al. Circ-HuR suppresses HuR expression and gastric cancer progression by inhibiting CNBP transactivation. Mol Cancer. 2019;18(1):158. doi:10.1186/s12943-019-1094-z
9. Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi:10.1038/521S1a
10. Wu L, Xia J, Yang J, et al. Circ-ZNF609 promotes migration of colorectal cancer by inhibiting Gli1 expression via microRNA-150. J BUON. 2018;23(5):1343–1349.
11. Wang L, Shen J, Jiang Y. Circ_0027599/PHDLA1 suppresses gastric cancer progression by sponging miR-101-3p.1. Cell Biosci. 2018;8:58. doi:10.1186/s13578-018-0252-0
12. Yin GH, Gao FC, Tian J, Zhang WB. Hsa_circ_101882 promotes migration and invasion of gastric cancer cells by regulating EMT. J Clin Lab Anal. 2019;33(9):e23002. doi:10.1002/jcla.23002
13. Hua Y, Duan S, Murmann AE, et al. miRConnect: identifying effector genes of miRNAs and miRNA families in cancer cells. PLoS One. 2011;6(10):e26521. doi:10.1371/journal.pone.0026521
14. Wang Y, Zhao W, Zhang S. STAT3-induced upregulation of circCCDC66 facilitates the progression of non-small cell lung cancer by targeting miR-33a-5p/KPNA4 axis. Biomed Pharmacother. 2020;126:110019. doi:10.1016/j.biopha.2020.110019
15. Wu S, Ai H, Zhang K, Yun H, Xie F. Long non-coding RNA EGOT promotes the malignant phenotypes of hepatocellular carcinoma cells and increases the expression of HMGA2 via down-regulating miR-33a-5p. Onco Targets Ther. 2019;12:11623–11635. doi:10.2147/ott.S218308
16. Fisher RP. Cdk7: a kinase at the core of transcription and in the crosshairs of cancer drug discovery. Transcription. 2019;10(2):47–56. doi:10.1080/21541264.2018.1553483
17. Diab S, Yu M, Wang S. CDK7 inhibitors in cancer therapy: the sweet smell of success? J Med Chem. 2020;63(14):7458–7474. doi:10.1021/acs.jmedchem.9b01985
18. Li -B-B, Wang B, Zhu C-M, et al. Cyclin-dependent kinase 7 inhibitor THZ1 in cancer therapy. Chronic Dis Transl Med. 2019;5(3):155–169. doi:10.1016/j.cdtm.2019.08.006
19. Zhang J, Liu S, Ye Q, Pan J. Transcriptional inhibition by CDK7/9 inhibitor SNS-032 abrogates oncogene addiction and reduces liver metastasis in uveal melanoma. Mol Cancer. 2019;18(1):140. doi:10.1186/s12943-019-1070-7
20. Choi YJ, Kim DH, Yoon DH, et al. Efficacy of the novel CDK7 inhibitor QS1189 in mantle cell lymphoma. Sci Rep. 2019;9(1):7193. doi:10.1038/s41598-019-43760-z
21. Ishaq M, Khan MA, Sharma K, Sharma G, Dutta RK, Majumdar S. Gambogic acid induced oxidative stress dependent caspase activation regulates both apoptosis and autophagy by targeting various key molecules (NF-kappaB, Beclin-1, p62 and NBR1) in human bladder cancer cells. Biochim Biophys Acta. 2014;1840(12):3374–3384. doi:10.1016/j.bbagen.2014.08.019
22. Wang S, Wang L, Chen M, Wang Y. Gambogic acid sensitizes resistant breast cancer cells to doxorubicin through inhibiting P-glycoprotein and suppressing survivin expression. Chem Biol Interact. 2015;235:76–84. doi:10.1016/j.cbi.2015.03.017
23. Li CY, Wang Q, Wang XM, Li GX, Shen S, Wei XL. Gambogic acid exhibits anti-metastatic activity on malignant melanoma mainly through inhibition of PI3K/Akt and ERK signaling pathways. Eur J Pharmacol. 2019;864:172719. doi:10.1016/j.ejphar.2019.172719
24. Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull. 2004;27(7):998–1003. doi:10.1248/bpb.27.998
25. Liu M, Liu KD, Zhang L, et al. Circ_0009910 regulates growth and metastasis and is associated with poor prognosis in gastric cancer. Eur Rev Med Pharmacol Sci. 2018;22(23):8248–8256. doi:10.26355/eurrev_201812_16519
26. Rong D, Dong C, Fu K, Wang H, Tang W, Cao H. Upregulation of circ_0066444 promotes the proliferation, invasion, and migration of gastric cancer cells. Onco Targets Ther. 2018;11:2753–2761. doi:10.2147/OTT.S156516
27. Huang X, Li Z, Zhang Q, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18(1):71. doi:10.1186/s12943-019-0969-3
28. Xu QY, Xie MJ, Huang J, Wang ZW. Effect of circ MTHFD2 on resistance to pemetrexed in gastric cancer through regulating expression of miR-124. Eur Rev Med Pharmacol Sci. 2019;23(23):10290–10299. doi:10.26355/eurrev_201912_19667
29. Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol. 2016;238:42–51. doi:10.1016/j.jbiotec.2016.09.011
30. Yan Y, Zhang D, Lei T, et al. MicroRNA-33a-5p suppresses colorectal cancer cell growth by inhibiting MTHFD2. Clin Exp Pharmacol Physiol. 2019;46(10):928–936. doi:10.1111/1440-1681.13125
31. Liu P, Chen B, Gu Y, Liu Q. PNMA1, regulated by miR-33a-5p, promotes proliferation and EMT in hepatocellular carcinoma by activating the Wnt/beta-catenin pathway. Biomed Pharmacother. 2018;108:492–499. doi:10.1016/j.biopha.2018.09.059
32. Larochelle S, Merrick KA, Terret M-E, et al. Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells. Mol Cell. 2007;25(6):839–850. doi:10.1016/j.molcel.2007.02.003
33. Huang J-R, Qin W-M, Wang K, et al. Cyclin-dependent kinase 7 inhibitor THZ2 inhibits the growth of human gastric cancer in vitro and in vivo. Am J Transl Res. 2018;10(11):3664–3676.
34. Wang BY, Liu QY, Cao J, Chen JW, Liu ZS. Selective CDK7 inhibition with BS-181 suppresses cell proliferation and induces cell cycle arrest and apoptosis in gastric cancer. Drug Des Devel Ther. 2016;10:1181–1189. doi:10.2147/DDDT.S86317
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.