Back to Journals » Cancer Management and Research » Volume 10
G-CSF associates with neurogenesis and predicts prognosis and sensitivity to chemotherapy in pancreatic ductal adenocarcinoma
Authors Zhang L, Tao L , Guo L, Zhan J, Yuan C, Ma Z, Jiang B, Xiu D
Received 10 February 2018
Accepted for publication 28 May 2018
Published 17 August 2018 Volume 2018:10 Pages 2767—2775
DOI https://doi.org/10.2147/CMAR.S165226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Lingfu Zhang,1 Lianyuan Tao,1 Limei Guo,2 Jun Zhan,3 Chunhui Yuan,1 Zhaolai Ma,1 Bin Jiang,1 Dianrong Xiu1
1Department of General Surgery, Peking University Third Hospital, Beijing, People’s Republic of China; 2Department of Pathology, Peking University Third Hospital, Beijing, People’s Republic of China; 3Laboratory of Molecular Cell Biology and Tumor Biology, Department of Anatomy, Histology and Embryology, Peking University Health Science Center, Beijing, People’s Republic of China
Background: Recent studies demonstrated that granulocyte colony-stimulating factor (G-CSF), regularly used for the prevention of neutropenia, is engaged in cancer progression. However, the role of G-CSF in pancreatic ductal adenocarcinoma (PDAC) is not clear. The aim of the present study was to investigate the expression and prognostic value of G-CSF in patients with PDAC.
Materials and methods: The localization and expression of G-CSF in PDAC were examined by immunohistochemistry (IHC). The analysis of the levels of G-CSF in plasma was evaluated using ELISA kit. The correlation between G-CSF expression and patients’ survival was assessed by Kaplan–Meier analysis.
Results: In IHC specimens, G-CSF was discovered predominantly in the cell cytoplasm and expressed in most of PDAC, while in plasma, the systemic level of G-CSF is no different between normal patients and pancreatic cancer patients. In 100 PDAC cases with IHC, patients with grades 2 and 3 were defined as the high expression group (41 patients, 41%), and those with grades 0 and 1 as the low expression group (59 patients, 59%). Significant correlation was noted between high G-CSF expression and neural invasion (P = 0.042) or early recurrence (P < 0.001). G-CSF appeared to be an independent adverse prognostic factor (hazard ratio = 1.774, 95% confidence interval 1.150–2.737, P = 0.010) in addition to N stage (P = 0.002). Specifically, adjuvant chemotherapy consisting of gemcitabine prolongs survival of patients with high G-CSF expression (median survival time 14 months vs 7.5 months). Morphologically, high G-CSF expression cells demonstrate the association with neurogenesis.
Conclusion: High expression of G-CSF is a prognostic marker and an indicator to chemotherapy response in PDAC.
Keywords: pancreatic ductal adenocarcinoma, granulocyte colony-stimulating factor, adjuvant chemotherapy, neural invasion, neurogenesis, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies, and is a leading cause of cancer-related mortality.1,2 Most of PDAC patients are diagnosed at an advanced stage and its 5-year survival rate is only 6%.1 With the development of chemotherapy, the prognosis of PDAC has improved, but it also increases the rates of peripheral neuropathy and myelosuppression.3 So, the simultaneous administration of granulocyte colony-stimulating factor (G-CSF) was adopted for the prevention of severe neutropenia and febrile neutropenia (FN) in PDAC patients treated with chemotherapy in some institution.4
G-CSF is known to mobilize hematopoietic progenitor stem cells from the bone marrow and stimulate the survival, proliferation, differentiation and function of neutrophil precursors and mature neutrophils, but its role as a neurotrophic factor is much less understood. However, recently the role of G-CSF in cancer progression via the promotion of nerve outgrowth has been reported in prostate cancer,5 and the relationship between high expression of G-CSF and poor prognosis has been demonstrated in cervical cancer and breast cancer.6,7 What is more, the prognosis of patients with G-CSF-producing pancreatic cancer is poor,8,9 and the response to different chemotherapy regimens are varied in these patients with G-CSF-producing pancreatic cancer.8,10
So, elucidating the role of G-CSF in pancreatic cancer progression and the association between high expression of G-CSF and prognosis in human PDAC are important in deciding whether it is rational to adopt simultaneous administration of G-CSF for the prevention of severe neutropenia and FN in PDAC patients and which type of chemotherapy for patients with G-CSF-producing pancreatic cancer is reasonable.
Our previous study showed that parasympathetic neurogenesis is an independent adverse factor in PDAC prognosis.11 The study of Dobrenis et al5 demonstrates that G-CSF has an effect on nerve outgrowth and promotes prostate cancer development. These results promote us to consider the role of G-CSF in pancreatic cancer, and so the aim of this study was to explore the relationships between G-CSF and clinicopathological factors of PDAC and the potentiation of G-CSF on parasympathetic neurogenesis and pancreatic cancer development.
Materials and methods
Patients and tissue/plasma samples
The study was approved by the Clinical Ethics Committee of Peking University Third Hospital (approval number 2015092). The Clinical Ethics Committee deemed that patient consent for use of the tissue samples was not required, as the paraffin-embedded tissues from the pathology department were analyzed anonymously. The data of 189 consecutive patients with suspected PDAC were retrospectively reviewed. They were treated with intended radical resection for resectable lesions between 2008 and 2014. After excluding patients who did not have a diagnosis of PDAC, 100 patients (52.9%) with the pathology of PDAC and available pathologic specimens from patients with benign periampullary lesions were enrolled in this study. In addition, 5 specimens of normal pancreatic tissues were used for the assessment and control of G-CSF expression. The plasmas of PDAC patients (104 cases) and control healthy individuals (10 cases) were prospectively collected from 2015 to 2016.
The clinicopathological parameters and the outcomes of these patients were recorded. They were followed up until death or April 15, 2016, with a median follow-up of 15 months (range: 2–62 months). Clinical and pathological factors were evaluated, including demographic information, TNM stage, G grade, and presence of vessel invasion and neural invasion. The clinical stages of all patients were classified according to the American Joint Committee on Cancer (AJCC) 7th Edition.12
Adjuvant chemotherapy
Patients with confirmed pathology of PDAC received adjuvant chemotherapy depending on their preference. The procedure of adjuvant chemotherapy was conducted according to the NCCN guideline. Gemcitabine was the first choice in our centers.
Immunohistochemistry
All tumor specimens employed for this study were reviewed by at least two pathologists for final diagnosis. Archived formalin-fixed paraffin-embedded PDAC specimen sections were dealt with as described previously.11 After deparaffinization in xylene, the sections were rehydrated in graded alcohols. Endogenous peroxidase was quenched with 3% hydrogen peroxide in methanol at room temperature (25°C). The sections were placed in a 95°C solution of 0.01 M sodium citrate buffer (pH 6.0) for antigen retrieval. A rabbit anti-G-CSF polyclonal antibody (1:1000, ab9691; Abcam) was used for the detection of G-CSF. The primary antibody was applied overnight at 4°C. PV9000 two-step plus a poly-horseradish peroxidase anti-mouse/rabbit immunoglobulin G detection system (Zhongshan Jinqiao, Jiaxing, People’s Republic of China) was then applied. Detection was performed with the Dako Envision System (Dako, Glostrup, Denmark), followed by chromogen detection with diaminobenzidine (DAB). Hematoxylin was used for counterstaining. Negative controls were performed by omitting the use of the primary antibody.
Semi-quantitative scoring
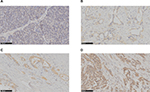
Semi-quantitative scoring of antibody staining on the slides was examined and confirmed by two pathologists, and scores were graded without prior knowledge of clinical information. These scores were highly consistent with a correlation coefficient of 0.92. Staining intensity and percentage of tumor cells stained were utilized in evaluating G-CSF staining on the tumor specimen section. Cytoplasmic G-CSF staining on tumor tissue slides was assessed using the following criteria: grade 0 = absent (Figure 1A), grade 1 = weak (Figure 1B), grade 2 = moderate (Figure 1C) and grade 3 = high (Figure 1D). “Scores 0 and 1” were defined as low expression and “scores 2 and 3” as high expression. Reevaluations were conducted simultaneously in cases with discrepancies by the two pathologists until a consensus was reached. The value of kappa was 0.775. If there was a discrepancy between staining intensity and percentage of tumor cells, the higher grade was chosen to represent the grade.
Analysis of systemic level of G-CSF
The plasmas of PDAC patients and healthy control individuals were collected and stored at −80°C for later analysis of the levels of G-CSF using ELISA kit (MultiSciences Biotech, Hangzhou, People’s Republic of China) according to the manufacturer’s instructions.
Follow-up
Patients were followed up in the outpatient clinic with physical examinations and abdominal–pelvic CT every 3–6 months for the first 3 years. Overall survival was defined as the interval between the first radical operation and the date of death (all-cause mortality). Disease-free survival was defined as the interval between the first radical operation and the date of the first evidence of recurrence (local, regional or metastatic). Follow-up time was defined as the time from the first radical operation to the date of the last follow-up. Follow-up information was obtained from outpatient clinic records, hospital records and telephone interviews. Follow-up and survival times were recorded in months.
Statistical analysis
Study data were collected on standard forms. The continuous variables not meeting the normal distribution were expressed as median (p25, p75), and the Mann-Whitney U test was used to analyze the differences between groups. The categorical variables were described by frequencies and proportions and tested by the chi-square test or Fisher’s exact test. Differences of G-CSF expression among various subgroups were identified through the Kruskal–Wallis rank sum test. Student’s t-test was used for paired studies. The Kaplan–Meier method was performed to represent survival curves, and the log-rank test was applied to verify significant differences in survival time. Association of overall survival with G-CSF expression was examined by Cox regression model using backward stepwise (likelihood ratio) method, and adjusted by T stage, N stage, AJCC stage, R status and neural invasion. Results were considered statistically significant when P < 0.05 (two-sided) using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient demographics
The characteristics of clinical data of PDAC patients enrolled for immunohistochemistry (IHC) evaluation are summarized in Table 1. The study cohort of 100 PDAC patients with intended radical resection had a median age of 63.5 years (range: 23–86 years). Among them, 61 were male and 39 were female. The majority of tumors were located at the head or neck of the pancreas (73%). Eighty-seven (87%) of the tumors were pT3, 50 patients (50%) had lymph node metastases and 19 patients (19%) had distant metastasis including 14 with paraaortic lymph node metastasis, 3 local peritoneal metastasis and 2 local liver metastasis. In total, 41 (41%) patients were treated with adjuvant chemotherapy. Among the cohort, 54 patients showed recurrence within 6 months, of whom 13 cases had liver metastasis, 11 had multiple organ metastasis, 8 had lymph node metastasis, 6 had peritoneal metastasis, 3 had local recurrence, 2 had pulmonary metastasis and 1 had distant muscle metastasis. All of these patients formed the early recurrence group. The observation period was from February 2009 to December 2016, with a median follow-up time of 15 months (range: 2–74 months).
G-CSF is not expressed in normal pancreatic tissue and increased in PDAC
To detect the G-CSF expression in pancreas, we examined the tissues of 100 patients with PDAC and 5 patients with normal pancreas resected. We examined G-CSF staining in normal pancreatic tissues and found that G-CSF expression was frequently absent in these tissues (Figure 1A). But G-CSF staining was observed in most PDAC tissues (90%) and stained predominantly in the cytoplasm of PDAC cells. The results showed that low G-CSF (0–1) expression and high G-CSF (2–3) expression were accounted for 41% (41) and 59% (59), respectively. Taken together, these findings demonstrated that G-CSF expression increased in PDAC tissues.
G-CSF expression was associated with clinicopathological variables and prognosis in PDAC
The relationships between G-CSF expression and clinicopathological variables are summarized in Table 2. The most obvious correlation was identified between G-CSF expression and early recurrence (P = 0.019). Similarly, neural invasion correlated with higher G-CSF expression (P = 0.042). However, G-CSF expression was not found to be related significantly with patients’ age (P = 0.285), sex (P = 0.414), location of the tumor (P = 0.493), T stage (P = 0.255), N stage (P = 0.685), AJCC stage (P = 0.317), G grade (P = 0.333), R status (P = 0.477) and vessel invasion (P = 0.484).
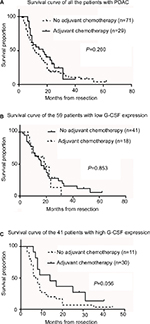
Further investigation was conducted to determine if G-CSF expression was correlated with patient survival. Patients with low or high G-CSF expression had median survival times of 18.0 and 8.0 months, respectively. Through Kaplan–Meier analysis, we found that the survival of patients with high G-CSF expressions was shorter than those with low G-CSF expressions (P = 0.006) (Table 3). In addition, lymph node metastasis (P = 0.001), AJCC stage (P = 0.013) and R status (P = 0.001) also predicted shorter overall survival for PDAC patients (Table 3). Patients with high G-CSF expression had 1.774 times elevated risk of death after adjusting for the remaining features in comparison with patients with low G-CSF expression by Cox regression model (Table 4). By contrast, no prognostic significance was found for overall survival with sex (P = 0.310), location of the tumor (P = 0.226), T stage (P = 0.076), vessel invasion (P = 0.262), neural invasion (P = 0.057), G grade (P = 0.173) and chemotherapy (P = 0.260) of the patients (Table 3 and Figure 2). Taken together, these results demonstrate that G-CSF is a factor of prognosis for PDAC patients.
High G-CSF expression was an indicator of adjuvant chemotherapy profit after radical resection in patients with PDAC
Among all the 100 patients, adjuvant chemotherapy was conducted in 41 patients, and there was no difference in survival time between patients who received adjuvant chemotherapy and those who did not (P = 0.260, Figure 3A). Listed separately, in the 59 patients with low G-CSF expression, there were also no differences in survival time between patients who received adjuvant chemotherapy and those who did not (P = 0.853, Figure 3B), while in the 41 patients with high G-CSF expression, 11 patients who received adjuvant chemotherapy demonstrated the tendency to have longer survival time (median survival time 14 months) than those who did not receive adjuvant chemotherapy (median survival time 7.5 months) (P = 0.056, Figure 3C).
The systemic level of G-CSF was no different between normal patients and pancreatic cancer patients
The systemic level of G-CSF in pancreatic cancer patients (104 cases) and control healthy individuals (10 cases) was 14.37 ± 13.06 pg/mL and 15.23 ± 10.75 pg/mL, respectively. There was no significant difference in systemic G-CSF levels between patients with PDAC and healthy individuals (P = 0.934).
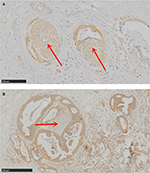
G-CSF expression cells demonstrate the association with neurogenesis in PDAC
Considering the correlation between G-CSF expression and neural invasion (P = 0.057) in our study, we further explored the relationship between G-CSF expression and neurogenesis. Interestingly, we observed that G-CSF expression cancer cells demonstrate the tendency to surround or invade into the nerve fibers with hypertrophy (Figure 2).
Discussion
The results of this study indicate clinical relevance of G-CSF expression in PDAC patients, not only from predicting of prognosis but also from a therapeutic point of view in clinical usage of chemotherapy, for routine usage of chemotherapy is controversial in patients with PDAC.
First, our results showed that G-CSF expression serves as a biomarker for the prognosis of PDAC. High G-CSF expression indicates an early recurrence and poor prognosis in PDAC patients compared with low expressed patients. In our cohort, early recurrence occurred in 75.6% (31/41) of patients with high G-CSF expression. These results are in line with previous reports,6,7 especially in accordance with studies from patients with PDAC.8–10 Unfortunately, the results from PDAC patients are case reports concentrating on patients with high systemic G-CSF levels. Plasma G-CSF detection fails to prove the correlation between patients with PDAC and healthy individuals in systemic G-CSF levels, confirms the preliminary report of Błogowski et al,13 who discover the same result of systemic G-CSF levels between PDAC patients and healthy individuals. This study is the first, to our knowledge, to specifically evaluate the association of G-CSF expression and early recurrence/prognosis and adjuvant regimens on outcomes for patients with PDAC. Our study first reported that high G-CSF expression is an indicator for early recurrence and poor prognosis in patients with PDAC.
Second, the simultaneous administration of G-CSF for the prevention of neutropenia in patients treated with chemotherapy should be done with caution. Many guidelines have recommended the use of prophylactic G-CSF for patients with an incidence of FN threshold of 20%; even simultaneous administration of G-CSF was adopted in some institution.4 A key point is whether G-CSF administration may bring protumor or prometastatic effects in patients. Indeed, G-CSF overexpression has been correlated with a poor prognosis in a variety of tumors,6,8–10,14,15 which is further confirmed in our study. These results emphasize the deleterious effects of G-CSF overexpression by tumors. Fortunately, the result of Kowanetz et al14 suggest that short-term administration of G-CSF, when given in conjunction with cytotoxic chemotherapy, does not increase the risk of metastasis.14 However, prolonged exposure to high levels of G-CSF, such as in tumors with constitutively G-CSF released, might result in enhanced metastasis.9,14
Third, chemotherapy is controversial in PDAC patients, especially in the first-line choice of regimen, including fluorouracil and gemcitabine. Among our patients, there was no difference in survival time between patients who received adjuvant chemotherapy and those who did not. But in the patients with high G-CSF expression, adjuvant chemotherapy results in longer survival time. This phenomenon raises the tantalizing possibility that G-CSF expression may be useful in selection of patients benefit for chemotherapy. Prognosis of G-CSF producing pancreatic cancers is extremely poor in patients who do not receive chemotherapy and receive FOLFIRINOX.9,15 Interestingly, Kataoka et al reported a case of patient with G-CSF-producing pancreatic cancer who responded to chemotherapy including the regimen of gemcitabine.10 The regimen of our center for adjuvant chemotherapy of pancreatic cancer is based on gemcitabine. These results indicate the privilege of gemcitabine in high G-CSF expression PDAC. Considering this is a retrospective study and limited number of the cohort, the results need to be further elucidated by in a cohort of more patients and prospective studies.
These results inferred us to consider that the potential mechanism of G-CSF on PDAC progression may not only depend on systemic effort but also involve local effort. Previous studies demonstrated several types of non-hematopoietic tumors secreting G-CSF that act on myeloid cells and tumor cells.16 The potential mechanism of G-CSF on tumor progression mainly depends on suppressing dendritic cell differentiation and activation and promoting increases in infiltration of neutrophil-like cells with high immunosuppressive activity.17–19 Intriguingly, the study of Dobrenis et al5 demonstrates G-CSF has an effect on parasympathetic nerve outgrowth and promotes prostate cancer development. Our previous study showed that parasympathetic neurogenesis is an independent adverse factor in PDAC prognosis.11 These results indicate that G-CSF in PDAC progression may also depend on parasympathetic neurogenesis. In our specimens, the sections with high G-CSF expression demonstrate concomitant nerve fibers with hypertrophy be around or infiltrated by cancer cells. These results show that G-CSF is important in tumor–nerve interactions and suggest that its impact on nerve fibers constitutes potential therapeutic targets in cancer progression.
Conclusion
In conclusion, G-CSF expressions were correlated with poor patient prognosis and indicate the treatment response to gemcitabine in adjuvant chemotherapy. High G-CSF expressions demonstrated the tendency to be around or infiltrated by nerve fibers with hypertrophy. Therefore, G-CSF expression is regarded as a novel predictor of prognosis and could be an effective target in the therapeutic strategy against PDAC.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81672862) and the Capital Characteristic Clinical Application Research and Achievement Promotion Project (grant no. Z171100001017121).
Disclosure
The authors report no conflicts of interest in this work.
References
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. | ||
Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140–2141. | ||
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. | ||
Terazawa T, Goto M, Miyamoto T, et al. Efficacy of prophylactic G-CSF in patients receiving folfirinox: a preliminary retrospective study. Intern Med. 2015;54(23):2969–2973. | ||
Dobrenis K, Gauthier LR, Barroca V, Magnon C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int J Cancer. 2015;136(4):982–988. | ||
Hollmén M, Karaman S, Schwager S, et al. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology. 2016;5(3):e1115177. eCollection 2016. | ||
Kumar J, Fraser FW, Riley C, Ahmed N, McCulloch DR, Ward AC. Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer. Br J Cancer. 2014;110(1):133–145. | ||
Kitade H, Yanagida H, Yamada M, et al. Granulocyte-colony stimulating factor producing anaplastic carcinoma of the pancreas treated by distal pancreatectomy and chemotherapy: report of a case. Surg Case Rep. 2015;1(1):46. | ||
Takami K, Miura K, Takeuchi H, et al. Granulocyte-colony stimulating factor-producing pancreatic cancer: report of a case. Surg Today. 2008;38(5):453–457. | ||
Kataoka K, Achiwa K, Minami Y, et al. A case of a patient with granulocyte-colony stimulating factor-producing pancreatic cancer who responded to nab-paclitaxel plus gemcitabine. Nihon Shokakibyo Gakkai Zasshi. 2017;114(7):1277–1284. Japanese [with English abstract]. | ||
Zhang L, Guo L, Tao M, Fu W, Xiu D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin J Cancer Res. 2016;28(2):180–186. | ||
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. | ||
Błogowski W, Deskur A, Budkowska M, et al. Selected cytokines in patients with pancreatic cancer: a preliminary report. PLoS One. 2014;9(5):e97613. | ||
Kowanetz M, Wu X, Lee J, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248–21255. | ||
Vinzens S, Zindel J, Zweifel M, Rau T, Gloor B, Wochner A. Granulocyte colony-stimulating factor producing anaplastic carcinoma of the pancreas: case report and review of the literature. Anticancer Res. 2017;37(1):223–228. | ||
Schweizerhof M, Stösser S, Kurejova M, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15(7):802–807. | ||
Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007;67(11):5479–5488. | ||
Pickup MW, Owens P, Gorska AE, et al. Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte colony stimulating factor secretion in carcinoma cells. Cancer Immunol Res. 2017;5(9):718–729. | ||
Phan VT, Wu X, Cheng JH, et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc Natl Acad Sci U S A. 2013;110(15):6079–6084. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.