Back to Journals » Drug Design, Development and Therapy » Volume 8
Fusion of cell-penetrating peptides to thermally responsive biopolymer improves tumor accumulation of p21 peptide in a mouse model of pancreatic cancer
Authors Walker L, Ryu JS, Perkins E, McNally L, Raucher D
Received 10 January 2014
Accepted for publication 30 May 2014
Published 7 October 2014 Volume 2014:8 Pages 1649—1658
DOI https://doi.org/10.2147/DDDT.S60451
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Leslie R Walker,1 Jung Su Ryu,1 Eddie Perkins,2 Lacey R McNally,3 Drazen Raucher1
1Department of Biochemistry, 2Department of Neurosurgery, University of Mississippi Medical Center, Jackson, MS, USA; 3Division of Hematology and Oncology, University of Louisville, Louisville, KY, USA
Abstract: Current therapies for the treatment of pancreatic cancer are limited. The limitations of this type of treatment are abundant. The majority of chemotherapeutic agents used in clinics are highly toxic to both tumor cells and normal tissues due to the lack of specificity. Resistance can develop due to overexposure of these agents. To address these issues, these agents must be made more exclusive toward the tumor site. We have developed a macromolecular carrier based on the sequence of the biopolymer elastin-like polypeptide (ELP) that is able to aggregate upon reaching the externally heated tumor environment. This carrier is specific to the tumor as it only aggregates at the heated tumor site. ELP is soluble below its transition temperature but will aggregate when the temperature is raised above its transition temperature. ELP was modified by p21, a cell cycle inhibitory peptide, and the addition of Bac, a cell-penetrating peptide with nuclear localization capabilities. In this study, p21-ELP-Bac and its control, ELP-p21, were used in cell proliferation studies using the pancreatic cancer cell lines Panc-1, MiaPaca-2, and S2013. ELP-p21 had little effect on proliferation, while the half maximal inhibitory concentration of p21-ELP-Bac was ~30 µM. As translocation across the plasma membrane is a limiting step for delivery of macromolecules, these polypeptides were utilized in a pancreatic xenograft model to study the plasma clearance, biodistribution, tumor accumulation, and tumor reduction capabilities of the polypeptide with and without a cell-penetrating peptide.
Keywords: elastin-like polypeptide, peptide, targeted drug delivery, macromolecule
Introduction
Pancreatic cancer is one of the most fatal cancers, with a five-year survival rate of only 6%.1 Studies aimed at improving treatment and thereby enhancing the quality of life for cancer patients are continuously underway. The timing and dosage for most existing therapies are hampered by narrow treatment windows, and individual variations in the growth and metastasis of primary tumors are considerable. The targeted delivery of drugs to solid tumors is a multipart problem because of the limitations caused by tumor heterogeneity and its blood vessel architecture. Macromolecules are gaining interest in drug delivery because they preferentially accumulate due to their ability to extravasate into the tumor much more readily compared to a normal cell well. Additionally, because of a tumor’s poor lymphatic drainage, the macromolecule will be retained longer than a smaller molecule.2 This phenomenon is known as the enhanced permeability and retention effect. Elastin-like polypeptide (ELP) is a macromolecular biopolymer that can be used as a drug delivery targeting system. ELP is made up of pentapeptide repeats in the motif Val-Pro-Gly-Xaa-Gly, where Xaa is a guest residue and can be any amino acid other than proline. In this study, the guest residue position consists of Val, Gly, and Ala in a 5:3:2 ratio, and the ELP molecule weighs ~60 kDa.3 At physiological temperature, ELP is soluble; but when the temperature is raised just a few degrees, ELP will aggregate. This causes ELP to not only accumulate in tumors due to the enhanced permeability and retention effect, but ELP also has the advantage of having an active targeting system through the use of hyperthermia.3–8 Therapeutic peptides target a process involved in the replication of cells in order to slow or stop the rapid proliferation of cells that leads to growing tumors and metastasis. The effectiveness of peptides in cancer therapy is limited by poor pharmacokinetics and their inability to accumulate in effective doses at the tumor site because of rapid degradation. Additionally, small molecules frequently accumulate in other organs, such as the liver and kidneys, and produce toxic metabolic products.9,10 This can be overcome by attaching the peptide to a macromolecular carrier, such as ELP. The p21 peptide, originally described in Mutoh et al, used here is derived from amino acids 139–164 of the C-terminus of the full length p21 protein and interferes with proliferating cell nuclear antigen (PCNA) function and inhibits cyclin-dependent kinase complex activity.11 This peptide was added to the N-terminus of ELP. The cell-penetrating peptide (CPP) Bac was added to the C-terminus of ELP to facilitate uptake into the cell. This CPP is important because, as p21 is a cell cycle inhibitory peptide, it must reach the nucleus in order to exert its inhibitory effect. When conjugated to ELP, Bac has been shown to localize to the nucleus in confocal microscopy experiments.12 As a control, a version without the CPP was also used. Previously, Bac-ELP-p21 was tested in vitro. These results showed that Bac was facilitating entry into the nucleus, as well as cytotoxic effects of Bac-ELP-p21 in combination with hyperthermia in several different cell lines.13
In this study, p21-ELP-Bac was shown to inhibit proliferation of three different pancreatic cancer cell lines. Additionally, the addition of the CPP enhances the uptake of ELP in the cell, as seen by fluorescence uptake experiments comparing p21-ELP-Bac and ELP-p21 in S2013 cells. This uptake was further increased in cells treated with hyperthermia. In animal studies, the polypeptide was shown to preferentially accumulate in the heated tumors and persisted in the heated tumors longer than in non-heated tumors.
Materials and methods
Synthesis and purification of ELP-based polypeptides
A pUC19 vector containing ELP was synthesized by recursive directional ligation as described previously in the sequence (VPGXG)n.14 To mediate intracellular uptake of ELP, the C-terminus was modified by the addition of the CPP Bac (RRIRPRPPRLPRPRPRPLPFPRP), and the therapeutic peptide p21 (GRKRRQTSMTDFYHSKRRLIFSKRKP) was added to the N-terminus. A control lacking the Bac CPP was also synthesized. Detailed descriptions of all peptides used in this study are included in Table 1.
pET25b+ expression vectors containing the desired constructs were transformed into Escherichia coli BLR(DE3) competent cells (EMD Millipore, Billerica, MA, USA) for hyperexpression of the protein.14 The expression strains were used to inoculate TB Dry plus ampicillin (100 μg/mL) and grown at 37°C, 220 rpm agitation for 18–24 hours. Cells were harvested by centrifugation (3,000 × g, 10 minutes), resuspended in phosphate-buffered solution (PBS), lysed by sonication (Fisher Scientific 550 Sonic Dismembrator; Thermo Fisher Scientific, Waltham, MA, USA), and centrifuged to remove cell debris (13,000× g, 45 minutes). Polyethyleneimine (0.5% w/v) was added to the lysate to precipitate nucleic acids, which were then removed by centrifugation at 10°C for 30 minutes. The phase transition of the polypeptide was induced by heating the lysate to 44°C and increasing NaCl concentration to 2 M. The polypeptide was collected by centrifugation (11,000× g, 10 minutes) and resuspended in PBS. Purification of ELP was achieved by inverse transition cycling which was repeated three to five times.15,16
Labeling with fluorescent probes
Quantitatively tracking fluorescence of a molecule can easily be done by various instruments. In the case of measuring uptake of ELP into the cell, a fluorescent probe was conjugated to ELP through a cysteine using a maleimide linker. In a typical fluorescent labeling procedure, protein was diluted to 100–200 μM in 50 mM Na2HPO4 buffer, pH 7.0, and incubated with ten-fold molar excess of tris(2-carboxyethyl)phosphine for 20 minutes at room temperature. Then, the fluorescent probe (tetramethylrhodamine-5-maleimide [Thermo Fisher Scientific], fluorescein-5-maleimide [Thermo Fisher Scientific], or AlexaFluor750 C5-maleimide [Thermo Fisher Scientific]) was dissolved in 10–20 μL dimethyl sulfoxide and added to the protein and incubated with continuous stirring overnight at 4°C. Free label was separated by inverse thermal cycling. The labeling efficiency was assessed by ultraviolet–visible spectrophotometry (UV-1600 Shimadzu; Shimadzu Corporation, Kyoto, Japan) at 540 nm for rhodamine, 768 nm for Alexa, 492 nm for fluorescein, and 280 nm for protein. The protein concentration was estimated by subtracting the percentage of absorbance by the dye. A typical molar ratio for label to protein was 0.15–0.30.
Characterization of the transition temperature
Both p21-ELP-Bac and ELP-p21 were heated at 1°C/minute at increasing concentrations in a multicell holder in an ultraviolet–visible spectrophotometer (Cary 100; Varian Instruments, Palo Alto, CA, USA). For comparison of the two constructs, the transition temperature (Tt) was determined at 20 μM in cell media containing 10% serum. Aggregation, induced by increasing temperature, was characterized by the turbidity measured at 350 nm.3 The Tt was defined as the temperature at which 50% of the solution was turbid.
Cell culture
Mia-Paca2 and Panc-1 (American Type Culture Collection [ATCC], Manassas, VA, USA) pancreatic carcinoma cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. Luciferase-transfected S2013 pancreatic cells were cultured in Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum and 100 units/mL penicillin, and 100 μg/mL streptomycin. Cultures were maintained at 37°C in a humidified atmosphere (5% CO2). For experiments, cells were removed from tissue culture flasks by treatment with 0.05% v/v trypsin-ethylenediaminetetraacetic-acid (Thermo Fisher Scientific) and counted using a Beckman Coulter counter Z series (Beckman Coulter, Inc., Brea, CA, USA).
For comparison of the potency of p21-ELP-Bac and ELP-p21, the three pancreatic cancer cell lines were plated in 96-well plates and allowed to grow for 24 hours. The cells were treated with varying concentrations of each polypeptide conjugate for 1 hour at 37°C or 42°C. After treatment, cells were washed and returned to the incubator with fresh media. 72 hours later, cell proliferation was measured using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) (Promega Corporation, Fitchburg, WI, USA).
Uptake analysis
For determining levels of p21-ELP-Bac and ELP-p21 taken up into the cell, S2013 cells were treated with 20 μM of each polypeptide for 1 hour at either 37°C or 42°C. Twenty-four hours after treatment, cells were washed with PBS, harvested with nonenzymatic cell dissociation buffer, centrifuged, and resuspended in 0.5 mL PBS. Intracellular uptake was measured by Beckman Coulter Gallios Flow Cytometer (Beckman Coulter, Inc.). Data were corrected for mean labeling efficiencies and analyzed by Kaluza Flow Cytometry Analysis Software (v 1.1) (Beckman Coulter, Inc.).
Tumor xenografts and measurements
Female athymic nu/nu mice (National Cancer Institute, Bethesda, MD, USA) with an average body weight of 25 g were housed in appropriate caging in a barrier room with a 12-hour light–dark rhythm under standard temperature and humidity conditions. Each mouse was implanted with 2×106 S2013 pancreatic carcinoma cells in the left flank and measured once palpable by caliper measurement using the formula: Volume = (Length * Width2)/2. Each group consisted of at least five mice. Mice were carefully monitored for weight, tumor size, and general welfare every day. During all treatments, animals were anesthetized using 1.5%–2.5% isoflurane. All animal experiments were performed in accordance with the University of Mississippi Medical Center’s Institutional Animal Care and Use Committee.
In order to actively target ELP to the tumor site, the tumor must be heated to ~41°C while the rest of the body stays at normal physiological temperature (37°C). To accomplish this, a Me 540L Laser Sys*Tim (Mettler Electronics Corp., Anaheim, CA, USA) was placed 2–3 mm above the skin over the tumor while the surrounding area was shielded. Heating was carried out in cycles of 20 minutes heating, 10 minutes cooling for a total of 2 hours, during which the animals were kept under isoflurane anesthetic.17
Pharmacokinetics
Mice bearing 250 mm3 S2013 subcutaneous tumors were anesthesized with isoflurane, and a cannula was placed in the femoral artery. Rhodamine-labeled p21-ELP-Bac or ELP-p21 was administered by intravenous injection in the femoral vein at a dose of 100 mg/kg. In the heated group, tumors were immediately heated by thermal cycling. At specific time points (5, 15, 30, 60, 90, 120, 150, 180, 210, 240 minutes), 30 μL of blood was collected via the arterial catheter in heparinized hematocrit tubes and centrifuged (13,000× g, 5 minutes) to separate cells and plasma. Five microliters of plasma was added to 95 μL of PBS in a black 96-well plate. Polypeptide fluorescence in the plasma was measured by fluorescence plate reader (BioTek FLx800; BioTek, Winooski, VT, USA). Rhodamine fluorescence was measured at 485 nm excitation and 590 nm emission. A standard curve was generated using known quantities of the labeled polypeptide. Fluorescence data were fit to the standard curve to calculate plasma levels in μg/mL at each time point. This data was then fit to a two-compartment pharmacokinetic model using Microcal Origin (OriginLab Corporation, Northampton, MA, USA). Plasma concentration with time were fit to the relationship described previously.5
Biodistribution and tumor accumulation
Levels of polypeptide fluorescence in the organs of the mice were quantified using the IVIS Spectrum (PerkinElmer Inc., Waltham, MA, USA). Once tumors reached 250 mm3, mice were administered 100 mg/kg of AlexaFluor750-labeled p21-ELP-Bac or ELP-p21 by intravenous injection in the femoral vein. Animals in the heated group began heating cycles immediately after injection. Four hours after injection, the mice were euthanized, and their organs (heart, lungs, liver, kidney, spleen) and tumors were harvested and placed in the IVIS for fluorescence measurement using the Living Image software (PerkinElmer Inc.). Fluorescence intensity for saline-treated animals was subtracted to correct for background fluorescence.
In order to determine the amount of polypeptide in the tumor over time, AlexaFluor750-labeled polypeptides were injected in the tail vein. Animals were imaged starting at 2 hours after injection and continued at 3, 4, 6, 24, and 48 hours after injection. Animals were placed under anesthesia during image capture then allowed to wake up until the next time point. Tumor fluorescence was measured using the Living Image software.
Results
Design and synthesis of p21 bound to ELP
The polypeptides used in this study were based upon ELP, a drug delivery vector comprised of the pentapeptide repeat VPGXG. Under the conditions listed previously, the temperature at which ELP changes from soluble to insoluble aggregates is ~40°C (Figure 1). During this change, ELP undergoes a structural rearrangement to expose the hydrophobic residues. This property was exploited to thermally target ELP carrying a therapeutic peptide to the tumor site. The thermally responsive p21 cell cycle inhibitory peptide was designed as described in Methods. The p21WAF1/CIP1-derived peptide that interferes with PCNA function and inhibits cyclin-dependent kinase complex activity was conjugated to Bac-ELP. This peptide is from the C-terminus of the full-length p21 protein and encompasses amino acids 139–164. Massodi et al originally designed the Bac-ELP-p21 polypeptide with the Bac CPP at the N-terminus and p21 at the C-terminus.13 However, this peptide was difficult to purify and provided low yields. By reversing the order, we increased the efficiency and yield in E. coli. The sequences of p21-ELP-Bac and ELP-p21 are shown in Table 1. The mechanism was confirmed by Mikecin et al.18 ELP-p21 without a CPP was designed to compare tumor uptake and biodistribution with and without a CPP.
Cytotoxic effects on pancreatic cancer cells by p21-ELP-Bac
Cytotoxicity in three pancreatic cell lines by Bac-ELP-p21 was determined by comparison of p21-ELP-Bac and ELP-p21. Additionally, the similar mechanism of Bac-ELP-p21 and p21-ELP-Bac was compared in S2013 cells. To evaluate these effects, S2013 cells were plated in 96-well plates. Cells were treated with 5–40 μM of Bac-ELP-p21, p21-ELP-Bac, ELP-p21, and p21-ELP for 1 hour at either 37°C or 42°C. After treatment, fresh media replaced polypeptides, and cells were incubated at 37°C for 72 hours. Cell viability was measured by MTT assay. The differences in cell survival between Bac-ELP-p21 and p21-ELP-Bac were minimal, with each half maximal inhibitory concentration being ~30 μM in cells treated in combination with hyperthermia (Table 2). ELP-p21 and p21-ELP had little effect on S2013 cells (Figure 2). Additionally, these effects were duplicated in both Panc-1 and Mia-Paca2 pancreatic cancer cells (Figure 3).
Uptake of p21-ELP-Bac and ELP-p21
S2013 cells were plated in six-well plates and treated with fluorescein-labeled p21-ELP-Bac or ELP-p21 at 37°C or 42°C for 1 hour. Immediately after treatment, cells were harvested using non-enzymatic cell dissociation buffer. The amount of fluorescence associated with the cell was almost triple for p21-ELP-Bac compared to ELP-p21, and hyperthermia further increased the amount of intracellular ELP. These results are shown in Figure 4.
Pharmacokinetics of ELP-p21 with and without CPP
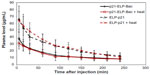
To determine the plasma kinetics of each polypeptide in this study, 2×106 S2013 cells were implanted in the flanks of mice. Catheters were placed in the femoral artery for drawing blood. ELP-p21 and p21-ELP-Bac (100 mg/kg) labeled with rhodamine were injected into the femoral vein. Next, 30 μL of blood was drawn at 5, 15, and 30 minutes, and continuing every 30 minutes up to 4 hours after injection. Plasma was separated from blood cells by centrifugation. Samples were read in a fluorescent plate reader (BioTek) and the numbers were fit to a standard curve generated from known quantities of peptide to calculate plasma levels (μg/mL) at each sampling point. As shown in Figure 5, the polypeptides displayed an initially rapid clearance from the blood, followed by a slower clearance. The construct without CPP had double the amount of peptide in the blood starting at 5 minutes and continuing for the first 2 hours. The terminal half-life for p21-ELP-Bac in combination with hyperthermia was ~195 minutes, which is considerably shorter than the half-life previously reported for unmodified ELP1,19 but this is sufficient time to apply hyperthermia to the tumor while the polypeptide is still in circulation. The pharmacokinetic parameters are shown in Table 3. The area under the curve was largest for p21-ELP-Bac-Rho plus heat (9,002.9±2,454.8 μg · min · mL−1) and smallest for ELP-p21 without heat (5,781.7±1,940.7 μg · min · mL−1). The terminal half-lives of each polypeptide were between 115.9 minutes and 194.8 minutes.
Tumor accumulation of p21 polypeptides
While the pharmacokinetic data were helpful in determining the amount of polypeptide in the blood and clearance rates, it was not useful in determining the amount of ELP in the tumor. Mice bearing 250 mm3 S2013 tumors in the flank were injected with AlexaFluor750-labeled p21-ELP-Bac and ELP-p21 at 100 mg/kg. Those in the heated groups began the heat cycling protocol immediately. Imaging in the IVIS began at 2 hours and continued at 3, 4, 6, 24, and 48 hours after injection. Mild hyperthermia triggers a phase transition in ELP to form aggregates which adhere to the tumor vasculature.20 Therefore, those mice with tumors that were heated by mild localized hyperthermia should have more ELP accumulation than those without heat. The IVIS allows for fluorescent monitoring over time in a live animal, making this system advantageous for these experiments. Quantification of levels of polypeptide in the tumors revealed a 1.6-fold increase in the level of p21-ELP-Bac in combination with hyperthermia compared to p21-ELP-Bac without heat and ELP-p21 with or without heat at 2 hours (Figure 6). This increase in fluorescent levels continued throughout the timecourse of the experiment. At 48 hours, there was still 1.3× more p21-ELP-Bac in the heated tumors than the non-heated tumors; this increased to 2.2× more when compared to ELP-p21 tumors with or without heat.
Ex vivo biodistribution
Finally, organs were examined by ex vivo fluorescence analysis. Mice bearing S2013 pancreatic cells were injected with AlexaFluor750-labeled p21-ELP-Bac and ELP-p21 via the femoral vein. Those in the heated groups immediately began the thermal cycling protocol. Four hours after injection, mice were euthanized, and tumors and major organs were harvested. These were placed in the IVIS to measure levels of fluorescence in the tumor, heart, kidney, lung, spleen, and liver. In the liver, kidney, spleen, heart, and lung, the levels of ELP were similar between both polypeptides, with or without heat (Figure 7A). Accumulation was highest in the kidney, which is likely due to the clearance of the polypeptide, which has been documented previously.4,5 Hyperthermia did not affect accumulation in the major organs. However, as shown in Figure 7B, fluorescence levels were highest in tumors treated with p21-ELP-Bac in combination with hyperthermia (1.7× more than p21-ELP-Bac, 1.3× more than ELP-p21 plus heat, and 1.7× more than ELP-p21).
Discussion
In this study, the thermal properties of ELP were exploited in order to enhance the abilities of the cell cycle inhibitory peptide p21. Rationally designed peptides that modulate aberrant protein function in cancer cells can make a significant contribution to the field of cancer therapy. As the knowledge of a protein’s structure, sequence, and interactions with other proteins is continually growing, a peptide library can be designed with exact specifications to either inhibit or stimulate a protein’s process in cancer cells. However, the use of peptides is riddled with difficulties. Accumulation of effective doses of peptides at a tumor site is limited by rapid degradation and poor tumor cell penetration by the peptides.21 Although there are ways to circumvent these problems, such as using non-natural amino acids, the use of macromolecules and CPPs alleviates issues both with stability and penetration and can be further developed into rational treatments for cancer.
Previous studies with the thermally responsive biopolymer ELP have shown that when conjugated to therapeutic peptides (TP), CPP-ELP-TP has potent activity against cancer cells. These studies included peptides that inhibited the Myc–Max interaction,22 a p21 mimetic inhibitory peptide,23 and a peptide that inhibits the interaction of Sm core proteins with SMN (survival of motor neuron) protein,24 and a proapoptotic peptide.25 Additionally, when hyperthermia was applied in these studies, the inhibitory effects were markedly enhanced. This study sought to extend the work of Massodi et al13 with p21 to a comparison of with and without a CPP as well as animal studies to examine the biodistribution and tumor accumulation capabilities. These results demonstrate that the p21-ELP-Bac and ELP-p21 proteins undergo a phase transition with the ideal targeting range of 39°C–42°C, which is the range required for an elevation in temperature but without the incidence of edema and necrosis in the healthy tissues surrounding the tumor site.19 Previous work with the Bac CPP illustrated that Bac crosses the cell membrane and is localized in the nucleus of murine monocytes.26 This localization is important because p21 exerts its cell cycle inhibitory effects in the nucleus. In conjugation with ELP, Bac has been used to inhibit tumor growth in both breast and glioma rodent models of cancer.4,5 When further modified by the addition of p21, Bac-ELP can inhibit proliferation of ovarian, breast, and pancreatic cancer cells in vitro. The delivery of Bac-ELP-p21 into the nucleus was seen under confocal microscopy, and it arrested cells in the S and G2/M phases.23
In this study, the configuration of the polypeptide was reversed, putting the p21 peptide on the N-terminus of ELP and Bac on the C-terminus. This allowed for better yields during protein purification. This reversal, however, did not affect the mechanism. In results by Mikecin et al,18 p21-ELP-Bac also inhibited the cell cycle at S phase, as well as increasing levels of caspases and binding with PCNA to promote apoptosis. Bac-ELP-p21 and p21-ELP-Bac had similar cytotoxic effects on the proliferation of S2013 pancreatic cells, while ELP-p21 showed modest toxicity to the cells. In an uptake experiment, Bac increased the uptake of fluorescein-labeled p21-ELP-Bac three-fold more than ELP-p21. Hyperthermia slightly increased this uptake.
Cell culture experiments showed that ELP-p21 had little cytotoxicity effect on Panc-1, Mia-Paca2, and S2013 pancreatic cancer cells. In contrast, the addition of a CPP allowed the peptide to be internalized and thereby reduced the proliferation of all three cell lines, especially in the presence of hyperthermia. To determine the plasma kinetics of each polypeptide in this study, S2013 cells were implanted in the flanks of mice. Plasma samples from time points starting with 5 minutes after injection and continuing until 4 hours after injection were taken and analyzed for fluorescence of the polypeptides labeled with rhodamine. From these studies, the plasma half-life of p21-ELP-Bac in the heated tumors was approximately 3.25 hours, which was vastly different than that of unmodified ELP (8.7 hours).27 This difference was most likely due to the addition of the positively-charged CPP as well as the addition of the p21 peptide. Of note, the difference in the level of fluorescence in the plasma differed significantly between p21-ELP-Bac and ELP-p21. The amount of circulating ELP labeled with rhodamine in the blood between 5 minutes and 180 minutes indicated that twice the amount of ELP-p21 was present in the plasma compared to p21-ELP-Bac. This is most likely due to the CPP’s ability to bind to the cell membrane and internalize the polypeptide and thereby remove it from circulation.
Because of the thermal properties of ELP, mild hyperthermia triggers ELP to undergo a phase transition to form aggregates which adhere to the tumor vasculature.20 Therefore, in a tumor with applied local hyperthermia, ELP will accumulate. Mice bearing subcutaneous pancreatic tumors were injected via tail vein with Alexa750-labeled p21-ELP-Bac or ELP-p21, and tumors were immediately heated using the thermal cycling protocol. Quantification of levels of polypeptide in the tumors revealed double the amount of p21-ELP-Bac when combined with hyperthermia than p21-ELP-Bac without hyperthermia and ELP-p21, with or without heat. This became a trend starting at 2 hours after injection and continuing until 48 hours after. Therefore, the CPP is most likely facilitating entry into the tumor cells and causing an increase in tumor uptake, and the application of hyperthermia induces protein aggregation, and this aggregation leads to increased tumor deposition. In the previous uptake experiment, hyperthermia accounted for only a modest increase in the amount of fluorescein-labeled polypeptide associated with the cell. However, the differences between an in vitro system and in vivo are vast. In cell culture, there is a limited amount of exposure to polypeptide as well as the homogeneity of the cells present. Therefore, results are expected to differ somewhat between the two models.
Finally, organs were examined by ex vivo fluorescence analysis. To determine biodistribution throughout the major organs, 4 hours after polypeptide injection, tumors and organs were removed and analyzed in IVIS. These results in tumors agreed with the previous tumor accumulation data versus time. Fluorescence levels were highest in tumors treated with p21-ELP-Bac and heat. In other organs, levels were similar between the two polypeptides. Polypeptide accumulation was highest in the kidney, which is likely due to clearance of the polypeptide. Hyperthermia did not affect accumulation in the other organs.
The work described here demonstrates the ongoing work for the use of ELP for thermally targeting both small-molecule drugs and therapeutic peptides. When aided by hyperthermia, ELP increases the therapeutic efficacy of conventional drugs, such as doxorubicin and methotrexate.7,8,28 Furthermore, the ability of a CPP to facilitate entry into the cell has been established in multiple studies in this work, such as the uptake by flow cytometry and tumor accumulation by IVIS. The results seen here, coupled with previous work,10,12,22,29 are promising in the development of the CPP-ELP moiety for producing more successful outcomes, leading to improved cancer therapy and reduced side effects. And, as is the goal of any cancer therapy, this technique will lead to a better quality of life for patients undergoing treatment.
Disclosure
The lead author and the corresponding author of this publication (Leslie R Walker and Drazen Raucher) have research support from Thermally Targeted Therapeutics, Inc., and the corresponding author Drazen Raucher also has equity ownership in Thermally Targeted Therapeutics, Inc., which is developing technology related to the research being reported. The authors report no other conflicts of interest in this work.
References
American Cancer Society. Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014. Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed January 6, 2014. | |
Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21(5):797–802. | |
Raucher D, Chilkoti A. Enhanced uptake of a thermally responsive polypeptide by tumor cells in response to its hyperthermia-mediated phase transition. Cancer Res. 2001;61(19):7163–7170. | |
Bidwell GL 3rd, Perkins E, Hughes J, Khan M, James JR, Raucher D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PLoS One. 2013;8(1):e55104. | |
Bidwell GL 3rd, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett. 2012;319(2):136–143. | |
Chilkoti A, Dreher MR, Meyer DE, Raucher D. Targeted drug delivery by thermally responsive polymers. Adv Drug Deliv Rev. 2002;54(5):613–630. | |
Moktan S, Perkins E, Kratz F, Raucher D. Thermal targeting of an acid-sensitive doxorubicin conjugate of elastin-like polypeptide enhances the therapeutic efficacy compared with the parent compound in vivo. Mol Cancer Ther. 2012;11(7):1547–1556. | |
Walker L, Perkins E, Kratz F, Raucher D. Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid-sensitive doxorubicin derivative. Int J Pharm. 2012;436(1–2):825–832. | |
Bidwell GL 3rd, Raucher D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv Drug Deliv Rev. 2010;62(15):1486–1496. | |
Massodi I, Bidwell GL 3rd, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J Control Release. 2005;108(2–3):396–408. | |
Mutoh M, Lung FD, Long YQ, Roller PP, Sikorski RS, O’Connor PM. A p21(Waf1/Cip1)carboxyl-terminal peptide exhibited cyclin-dependent kinase-inhibitory activity and cytotoxicity when introduced into human cells. Cancer Res. 1999;59(14):3480–3488. | |
Bidwell GL 3rd, Davis AN, Raucher D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135(1):2–10. | |
Massodi I, Moktan S, Rawat A, Bidwell GL 3rd, Raucher D. Inhibition of ovarian cancer cell proliferation by a cell cycle inhibitory peptide fused to a thermally responsive polypeptide carrier. Int J Cancer. 2010;126(2):533–544. | |
Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol. 1999;17(11):1112–1115. | |
McPherson DT, Xu J, Urry DW. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr Purif. 1996;7(1):51–57. | |
Daniell H, Guda C, McPherson DT, Zhang X, Xu J, Urry DW. Hyperexpression of a synthetic protein-based polymer gene. Methods Mol Biol. 1997;63:359–371. | |
Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67(9):4418–4424. | |
Mikecin AM, Walker LR, Kuna M, Raucher D. Thermally targeted p21 peptide enhances bortezomib cytotoxicity in androgen-independent prostate cancer cell lines. Anticancer Drugs. 2014;25(2):189–199. | |
Liu W, Dreher MR, Furgeson DY, et al. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J Control Release. 2006;116(2):170–178. | |
Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98(5):335–344. | |
Raucher D, Moktan S, Massodi I, Bidwell GL 3rd. Therapeutic peptides for cancer therapy. Part II – cell cycle inhibitory peptides and apoptosis-inducing peptides. Expert Opin Drug Deliv. 2009;6(10):1049–1064. | |
Bidwell GL 3rd, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4(7):1076–1085. | |
Massodi I, Bidwell GL 3rd, Davis A, et al. Inhibition of ovarian cancer cell metastasis by a fusion polypeptide Tat-ELP. Clin Exp Metastasis. 2009;26(3):251–260. | |
Bidwell GL 3rd, Whittom AA, Thomas E, Lyons D, Hebert MD, Raucher D. A thermally targeted peptide inhibitor of symmetrical dimethylation inhibits cancer-cell proliferation. Peptides. 2010;31(5):834–841. | |
Moktan S, Raucher D. Anticancer activity of proapoptotic peptides is highly improved by thermal targeting using elastin-like polypeptides. Int J Pept Res Ther. 2012;18(3):227–237. | |
Sadler K, Eom KD, Yang JL, Dimitrova Y, Tam JP. Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry. 2002;41(48):14150–14157. | |
Liu W, Dreher MR, Chow DC, Zalutsky MR, Chilkoti A. Tracking the in vivo fate of recombinant polypeptides by isotopic labeling. J Control Release. 2006;114(2):184–192. | |
Moktan S, Ryppa C, Kratz F, Raucher D. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Invest New Drugs. 2012;30(1):236–248. | |
Bidwell GL 3rd, Raucher D. Enhancing the antiproliferative effect of topoisomerase II inhibitors using a polypeptide inhibitor of c-Myc. Biochem Pharmacol. 2006;71(3):248–256. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.