Back to Journals » Clinical Ophthalmology » Volume 13
Functional outcome of repeat Descemet membrane endothelial keratoplasty (DMEK) for corneal decompensation following graft failure after primary DMEK
Authors Agha B, Shajari M, Slavik-Lencova A, Kohnen T , Schmack I
Received 29 October 2018
Accepted for publication 31 December 2018
Published 7 March 2019 Volume 2019:13 Pages 477—482
DOI https://doi.org/10.2147/OPTH.S192424
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Bishr Agha, Mehdi Shajari, Anna Slavik-Lencova, Thomas Kohnen, Ingo Schmack
Department of Ophthalmology, Goethe-University, Frankfurt am Main, Germany
Purpose: To evaluate if repeat Descemet membrane endothelial keratoplasty (DMEK) is appropriate to achieve functional improvements in patients with corneal decompensation from secondary graft failure after primary DMEK.
Methods: This is a retrospective monocentric cohort study including 13 eyes of 13 patients with repeat DMEK for corneal decompensation following primary DMEK. Eyes with primary DMEK only and comparable preoperative corrected distance visual acuity (CDVA) served as control. Main outcome parameter was CDVA. Secondary outcome measures were central corneal thickness (CCT), endothelial cell density, and rebubbling rate (RR).
Results: The average time interval (±SD) between primary and secondary DMEK was 12.5±6 months. Preoperative CDVA (logMAR) was 1.97±0.90 in the repeat DMEK group and 1.38±0.92 in the primary DMEK group. At 6 months, both groups showed significant improvement in visual acuity (repeat DMEK group, 0.49±0.35, P<0.01 and primary DMEK group, 0.40±0.36, P<0.01). CDVA did not differ significantly between both groups at all time points examined (1, 3, and 6 months postoperatively). Mean CCT values at 3 and 6 months postoperatively did not differ significantly between the two groups (P>0.05). The RR was
23% (n=3) in both groups.
Conclusion: Repeat DMEK is a useful therapeutic approach in the setting of corneal decompensation following primary DMEK. Functional results of repeat DMEK, visual acuity in particular, are comparable to patients with single DMEK only.
Keywords: DMEK, repeat DMEK, corneal edema, corneal transplantation
Introduction
Currently, Descemet membrane endothelial keratoplasty (DMEK) is considered to be the gold standard in the management of corneal endothelial disease such as Fuchs’ endothelial dystrophy (FED) and pseudophakic bullous keratopathy (BKP). It allows the selective replacement of the diseased corneal endothelium and adjacent Descemet membrane compared to alternative posterior lamellar techniques such as Descemet stripping (automated) endothelial keratoplasty (DS[A]EK).1,2 Main advantages are attributed to thinner corneal grafts without any remnants of corneal stroma, less structural alterations at the recipients corneal stroma interface, and reduced induction of higher-order aberrations.3–5 Previous work demonstrated that repeat DMEK is a valuable treatment option for patients with failed primary DMEK.6,7 Nevertheless, the knowledge about the functional outcome of repeat DMEK, especially in case of corneal decompensation, is limited. Data on this topic might have a high clinical impact since previous studies showed that visual acuity of patients with DMEK following corneal decompensation is reduced mainly due to ultrastructural changes in the corneal stroma.8 Comparable findings are already known from patients with secondary DMEK for poor visual function after DSEK.9 It is likely that similar structural changes might also occur in the setting of repeat DMEK for corneal decompensation following primary DMEK. Based on these observations, we addressed the question about the postoperative functional outcome of patients with repeat DMEK for corneal decompensation from secondary graft failure compared to primary DMEK.
Patients and methods
The study protocol was approved by the institutional review board of the Goethe-University and is in accordance with the tenets of the Declaration of Helsinki. The institutional review board waived the need for obtaining patient consent to review their medical records because patient data confidentiality was guaranteed throughout the study. Medical files of all eligible patients treated with repeat DMEK between April 26, 2016 and April 7, 2017 were reviewed.
Patients
The study cohort (repeat DMEK group) included 13 eyes of 13 patients (n=7 female, n=6 male, mean age: 73.2±7.6 years, range: 52–82 years) undergoing repeat DMEK surgery for corneal decompensation after primary DMEK. The control group (primary DMEK group) consisted of 13 eyes of 13 patients (n=7 female, n=6 male, mean age: 70.8±11.6 years, range: 51–88 years) with successful primary DMEK. The control group was chosen in regard to comparable preoperative corrected distance visual acuity (CDVA) and age. Additionally, care was taken not to exceed the number of patients with ocular comorbidities present in the repeat DMEK group. Besides a single patient in the repeat DMEK group, who underwent primary DMEK at a different institution, all DMEK surgeries were carried out at our department by two experienced surgeons (TK and IS). Mean preoperative CDVA (logMAR) was 1.97±0.90 (repeat DMEK group) and 1.38±0.92 (primary DMEK group).
Surgical technique
Failed DMEK grafts from the previous surgery were carefully mobilized and explanted via a 2.2 mm corneal incision. Peripheral remnants of the original recipient Descemet’s membrane were stained with trypan blue and subsequently removed with forceps within a diameter of 9.0 mm. Secondary DMEK grafts were prepared using a standardized technique and stained with trypan blue as previously described and transferred into a glass cartridge for further use.10 In all cases in situ, surgeon-prepared tissue was used. Diameter of the DMEK grafts was 7.75 mm or 8.0 mm depending on the white-to-white distance. Once all remnants of the recipient Descemet’s membrane were removed, the prepared lamellar grafts were injected into the anterior chamber, oriented (endothelium facing down), unfolded, and centered over the pupil. Finally, as in the primary DMEK procedures, 20% sulfur hexafluoride (SF6) gas was installed between the iris and DMEK graft until adherence of the DMEK graft to the recipient stroma was achieved. Anterior chamber gas fill ranged between 80% and 90%. Intraocular pressure control was performed two hours after surgery. Patients were asked to stay predominantly in a supine position for 2–3 days postoperatively.
Clinical evaluation
Main outcome parameter was CDVA. Secondary measurements included central corneal thickness (CCT) (Pentacam® AXL; Oculus GmbH, Wetzlar, Germany), endothelial cell density (ECD) (CEM-530; Oculus GmbH), and rebubbling rate (RR). Intergroup comparison of CDVA and CCT was performed preoperatively as well as at 1, 3, and 6 months postoperatively. ECD of the secondary DMEK graft was recorded prior to transplantation and 6 months postoperatively. The decision for additional intracameral gas injections (SF6 20%) (rebubbling) was based on clinical symptoms, functional outcome, and the presence of signs for graft detachment (1/3 of the lamella area).
Statistical analysis
Statistical analysis was performed using Excel for Mac (version 15.37; Microsoft, Inc., Redmond, WA, USA) and IBM SPSS software version 24 (SPSS, Armonk, NY, USA). Differences in parameter values within each group were assessed by Wilcoxon test, and differences between both groups were evaluated by Mann–Whitney U Test. A P-value of less than 0.05 was considered statistically significant.
Results
Clinical indications for DMEK surgery were almost similar between both groups (repeat vs primary DMEK group). In the majority of cases, DMEK was performed for corneal decompensation secondary to FED (n=11 vs n=10) and BKP (n=1 vs n=3). A single patient of the repeat DMEK group underwent DMEK for corneal decompensation of unknown reason (n=1). Both groups included the same number of eyes (n=3) with ocular comorbidities limiting the visual acuity (age-related macular degeneration and corneal subepithelial scarring) in addition to corneal decompensation. The average time interval between primary and repeat DMEK was 12.5±6 months (range: 3–25 months).
The average time interval from diagnosis of graft failure to repeat DMEK was 2.5±1.4 months (range: 1–6 months). No graft rejections were observed.
Corrected distance visual acuity (CDVA)
Preoperative mean CDVA was 1.97±0.90 in the repeat DMEK group and 1.38±0.92 in the primary DMEK group (P=0.161). At 6 months, visual acuity improved significantly compared to preoperative values in both groups to 0.49±0.35 in the repeat DMEK group (P<0.01) and 0.40±0.36 in the primary DMEK group (P<0.01). The results are summarized in Table 1 and illustrated in Figure 1. Constant improvement in visual acuity was present at all time points during follow-up in the repeat DMEK group. The primary DMEK group also showed continuous improvement in visual acuity apart from one insignificant increase from 0.38±0.35 to 0.40±0.36 at 3 and 6 months, respectively. There were no significant differences concerning CDVA between the repeat and primary DMEK group at all follow-up time points (intergroup comparison, P>0.05). When excluding the eyes with visual acuity limiting ocular comorbidities (n=3 in each group), preoperative mean CDVA was 1.96±0.99 in the repeat DMEK group and 1.20±0.78 in the primary DMEK group. At 6 months, there was an improvement to 0.33±0.20 and 0.29±0.25, respectively. Again, intergroup comparison showed no significant differences. Overall, there was a tendency toward better visual acuity data in the primary DMEK group.
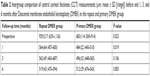
  | Table 1 Intergroup comparison of corrected distance visual acuity (CDVA) (mean ± SD [range], logMAR) before and 1, 3, and 6 months after (repeat) Descemet membrane endothelial keratoplasty (DMEK) |
Central corneal thickness (CCT)
One month after surgery, a significant decrease in CCT was present in both groups. Patients of the repeat DMEK group showed a significant reduction in CCT from 929±217 μm (preoperative) to 564±64 μm (1 month postoperatively) (P=0.01). In the primary DMEK group, CCT decreased from 683±114 μm (preoperative) to 484±22 μm (1 month postoperatively) (P=0.02). Over time (3 and 6 months postoperatively), reduction in CCT continued in the repeat DMEK group (Table 2, Figure 2).
  | Figure 2 Changes in central corneal thickness (CCT) after Descemet membrane endothelial keratoplasty (DMEK) in the repeat and primary DMEK group over time (1, 3, and 6 months). |
Regarding intergroup comparison, mean CCT was demonstrated to be significantly higher in the repeat DMEK group preoperatively as well as 1 month after secondary DMEK (P-values 0.023 and 0.019, respectively). However, mean CCT values at 3 and 6 months postoperatively did not differ significantly between the two groups (P>0.05).
Endothelial cell density
Six months after surgery, DMEK grafts showed a 30.1% (repeat DMEK group, 2,679±293 cells/mm² prior to transplantation and 1,866±331 cells/mm² 6 months after repeat DMEK) and a 30.7% (primary DMEK group, 2,612±268 cells/mm² prior to transplantation and 1,811±431 cells/mm² 6 months after DMEK) reduction in ECD, respectively. Differences between the two groups were statistically not significant (P=1.0, Table 3).
Rebubbling rate
Partial graft detachment requiring secondary intracameral gas injection was present in three patients (23%) of each group. Rebubbling was performed 8 and 9 days as well as 5 weeks after repeat DMEK. In the primary DMEK group, rebubbling was required at 2, 4, and 5 weeks after DMEK. In all patients, a single additional gas installation was sufficient. Nevertheless, despite complete graft adherence, a single patient of the primary DMEK group eventually required full-thickness keratoplasty for persistent graft dysfunction and progressive corneal scarring 7 months after DMEK. All other patients showed complete graft attachment during the entire postoperative follow-up period.
Discussion
Our study showed that repeat DMEK is a feasible and successful procedure in eyes with corneal decompensation following DMEK. These findings are in accordance to the common literature.6,7,11,12 However, analysis and emphasis of previous studies are different from our study proposal. Yoeruek and Bartz-Schmidt evaluated the clinical outcome of six patients with repeat DMEK for non-clearing corneal edema or graft detachment after primary DMEK. Based on their results, repeat DMEK is a feasible technique potentially resulting in full visual rehabilitation and clinical results comparable to patients with uneventful DMEK transplantation.11 In contrast to our study cohort, the average time interval between primary and repeat DMEK was markedly shorter (2.9 months vs 12.5 months). In addition, the overall CDVA (logMAR) prior to repeat DMEK surgery was superior compared to our patients (1.50±0.28 vs 1.97±0.90).11 Based on the assumption that there is a positive correlation between the degree of corneal decompensation and the amount of visual acuity, it can indirectly be concluded that corneal edema was more pronounced in our study. Overall, intraoperative handling of a DMEK graft is much more advanced in patients with marked corneal edema due to poor visualization and orientation of the corneal graft.13
Another study analyzing 17 eyes from a series of 550 consecutive DMEK surgeries undergoing repeat DMEK concluded that repeat DMEK represents an appropriate procedure for patients with complicated primary DMEK.12 Although the authors performed a comparison to an age-matched control group, a major difference between the aforementioned and our study is that repeat DMEK was mainly (n=14) performed for graft detachment after primary DMEK and not for corneal decompensation occurring with grafts completely attached like in our study. However, the results presented by Baydoun et al showed a lower visual acuity in eyes with repeat DMEK than in eyes with primary DMEK.12 These findings are in contrast to Yoeruek and Bartz-Schmidt, who have reported a similar clinical outcome between eyes after primary and repeat DMEK.11 Our study showed no statistically significant differences in the CDVA during the entire follow-up period of 6 months, although there was a tendency toward better CDVA in the primary DMEK group.
A study examining the ultrastructure of failed primary DMEK grafts in patients undergoing repeat DMEK suggests that there might be a pre-existing subclinical corneal endothelial dysfunction contributing to graft failure.7 Therefore, early repeat DMEK might be of advantage in primary graft failure, since the possibility to restore corneal transparency is rather low due to the presumed pre-existing endothelial dysfunction of the primary graft. Price et al emphasized the importance of prompt intervention by repeat DMEK surgery to minimize the duration of central corneal decompensation and development of anterior stromal scarring.6 In this study, it was reported that the visual outcomes with repeat DMEK matched the fellow-eye visual outcomes with primary DMEK when prompt regrafting was performed.6
A recent study suggests secondary ultrathin DSAEK (UT-DSAEK) as an alternative treatment option to repeat DMEK in eyes with primary graft failure after DMEK.14 However, the mean preoperative CDVA was 0.62 logMAR and was better than in our study population. In addition, the mean time interval between initial DMEK and UT-DSAEK was only 3 months. The preoperative situation therefore is very different, making transfer of these results to other patient groups difficult. Nevertheless, UT-DSAEK might be a reasonable option for surgeons not highly experienced with DMEK surgery.
Concerning rebubbling, the rate of 23%, all performed within 5 weeks after DMEK, was identical in both groups of our study. This indicates that the RR does not seem to be a limiting factor for the functional outcome of repeat DMEK for corneal decompensation. It must be taken into account that every rebubbling potentially leads to further endothelial cell loss and therefore should be indicated carefully. However, the time interval between DMEK and rebubbling seems to be of importance in the management of incomplete postoperative graft adherence. A series of 760 DMEK surgeries with 41 eyes requiring rebubbling (5.4%) investigating the clinical outcome after rebubbling for graft detachment after DMEK demonstrated that the visual outcomes may be similar to uncomplicated DMEK when rebubbling was performed within the first 6–8 weeks.15
The discrepancies in the postoperative outcome of patients with primary and repeat DMEK reported in previous reports clearly demonstrate that additional studies focusing on objective postoperative data after repeat DMEK are necessary. By focusing on repeat DMEK for corneal decompensation, we consider our case series a valuable contribution in this regard. Nevertheless, we are aware that the small sample size and heterogeneous preoperative visual acuity data represent important limiting factors. Larger studies are necessary to better judge if there are limitations regarding the suitability of visual restoration by repeat DMEK after failed primary DMEK, especially concerning the optimal timing for performing a secondary procedure.
In conclusion, the present study demonstrates that repeat DMEK is feasible and justified in eyes with corneal decompensation after failed DMEK. Primary and secondary outcome parameters, especially visual acuity, showed that the functional results of primary and repeat DMEK are comparable.
Disclosure
Mehdi Shajari is a consultant in Oculus, Oertli, Santen, Zeiss. Thomas Kohnen received research funding from Hoya, J&J Vision (Abbott), Novartis (Alcon), Oculentis, Oculus, Schwind, Zeiss; and he is a consultant or advisory board in Geuder, J&J Vision (Abbott), Novartis (Alcon), Oculus, Santen, Schwind, STAAR, TearLab, Thea Pharma, Thieme Compliance, Ziemer, Zeiss. The other authors report no conflicts of interest in this work.
References
Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25(8):886–889. | ||
Vedana G, Villarreal G, Jun AS. Fuchs endothelial corneal dystrophy: current perspectives. Clin Ophthalmol. 2016;10:321–330. | ||
Dapena I, Ham L, Melles GR. Endothelial keratoplasty: DSEK/DSAEK or DMEK – the thinner the better? Curr Opin Ophthalmol. 2009;20(4):299–307. | ||
Rudolph M, Laaser K, Bachmann BO, Cursiefen C, Epstein D, Kruse FE. Corneal higher-order aberrations after Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119(3):528–535. | ||
Suh LH, Dawson DG, Mutapcic L, et al. Histopathologic examination of failed grafts in Descemet’s stripping with automated endothelial keratoplasty. Ophthalmology. 2009;116(4):603–608. | ||
Price MO, Feng MT, Mckee Y, Price FW. Repeat Descemet membrane endothelial keratoplasty: secondary grafts with early intervention are comparable with fellow-eye primary grafts. Ophthalmology. 2015;122(8):1639–1644. | ||
Ćirković A, Schlötzer-Schrehardt U, Weller JM, Kruse FE, Tourtas T. Clinical and ultrastructural characteristics of graft failure in DMEK: 1-year results after repeat DMEK. Cornea. 2015;34(1):11–17. | ||
Turnbull AM, Tsatsos M, Hossain PN, Anderson DF. Determinants of visual quality after endothelial keratoplasty. Surv Ophthalmol. 2016;61(3):257–271. | ||
Ham L, Dapena I, van der Wees J, Melles GR. Secondary DMEK for poor visual outcome after DSEK: donor posterior stroma may limit visual acuity in endothelial keratoplasty. Cornea. 2010;29(11):1278–1283. | ||
Melles GR, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006;25(8):987–990. | ||
Yoeruek E, Bartz-Schmidt KU. Secondary Descemet membrane endothelial keratoplasty after failed primary Descemet membrane endothelial keratoplasty: clinical results. Cornea. 2013;32(11):1414–1417. | ||
Baydoun L, van Dijk K, Dapena I, et al. Repeat Descemet membrane endothelial keratoplasty after complicated primary Descemet membrane endothelial keratoplasty. Ophthalmology. 2015;122(1):8–16. | ||
Weller JM, Tourtas T, Kruse FE, Schlötzer-Schrehardt U, Fuchsluger T, Bachmann BO. Descemet membrane endothelial keratoplasty as treatment for graft failure after Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2015;159(6):1050–1057. | ||
Graffi S, Leon P, Nahum Y, et al. Outcomes of ultrathin Descemet stripping automated endothelial keratoplasty (UT-DSAEK) performed in eyes with failure of primary Descemet membrane endothelial keratoplasty (DMEK). Br J Ophthalmol. Epub 2018 May 29. | ||
Gerber-Hollbach N, Baydoun L, López EF, et al. Clinical outcome of rebubbling for graft detachment after Descemet membrane endothelial keratoplasty. Cornea. 2017;36(7):771–776. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.