Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Functional BDNF rs7124442 Variant Regulated by miR-922 is Associated with Better Short-Term Recovery of Ischemic Stroke
Received 31 July 2019
Accepted for publication 11 November 2019
Published 20 November 2019 Volume 2019:15 Pages 1369—1375
DOI https://doi.org/10.2147/TCRM.S225536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Binghui Liu,1,* Wei He,1,2,* Dinghua Liu2,3
1Department of Neurology, The First People’s Hospital of Huocheng, Yili, People’s Republic of China; 2Department of Neurology, The Affiliated Jiangyin People’s Hospital of Southeast University Medical College, Wuxi, People’s Republic of China; 3Department of Physical Medicine and Rehabilitation, The Affiliated Jiangyin People’s Hospital of Southeast University Medical College, Wuxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dinghua Liu
Department of Neurology, The Affiliated Jiangyin People’s Hospital of Southeast University Medical College Southeast University Medical College, Shoushan Road 163, Jiangyin, Wuxi 214400, People’s Republic of China
Tel +86-159 9535 2188
Email [email protected]
Background: Brain-derived neurotrophic factor (BDNF) is the most abundant neurotrophin, which contributes to the neuronal survival and synaptic plasticity. This study investigated the associations of BDNF polymorphisms at the 3ʹ-untranslated region with risk and outcome of ischemic stroke in a Chinese Han population.
Methods: 500 patients and 520 controls were enrolled for BDNF rs7124442 genotyping. The binding of miR-922 to BDNF rs7124442 was examined by luciferase assay; BDNF expression was assessed using qRT-PCR.
Results: Alcohol consumption, cigarette smoking, diabetes, hypertension (all P < 0.001) and higher serum triglycerides concentration (P = 0.009) were associated with an increased risk of developing ischemic stroke. After adjusted for age and sex, logistic regression analysis showed that IS patients harbored with rs7124442 TC genotype had a milder initial stroke (Dominant model: OR = 0.45, 95% CI = 0.25–0.81, P = 0.015), and also showed a better short-term recovery (Dominant model: OR = 0.39, 95% CI =0.24–0.68, P = 0.003). Furthermore, we found that co-transfection of hsa-miR-922 mimics with BDNF 3ʹ-UTR containing the mutated allele C changed luciferase activity when compared to co-transfection with BDNF 3ʹ-UTR containing the wild-type allele. Besides, patients carrying BDNF rs712444 TC or CC genotype had an increased level of BDNF compared with patients with the TT genotype.
Conclusion: Our study demonstrates that the SNP rs7124442 in BDNF 3ʹ-UTR, through affecting the regulatory role of miR-922 in BDNF expression, might act as a protective factor for the outcome of patients with ischemic stroke.
Keywords: ischemic stroke, polymorphism, brain-derived neurotrophic factor, BDNF, miR-922, outcome
Introduction
Stroke is a major reason for death and disability of people in the world.1 Ischemic stroke (IS), which is usually caused by blockage of blood vessels, accounts for about 80% of all strokes. Previous data demonstrated that 6.9 million patients had ischemic stroke in 2013.2 In China, the estimated mortality rate caused by stroke is 157 per 100,000 every year,3 and the stroke burden continues to increase. Traditional risk factors of stroke include diabetes mellitus, hypertension, high blood cholesterol, etc., suggesting the important role of genetic factors in stroke.4
Brain-derived neurotrophic factor (BDNF) is the most abundant neurotrophin, which contributes to the neuronal survival, synaptic plasticity, angiogenesis and outgrowth of peripheral and central neurons.5,6 In experimental model studies of stroke, both intravenous and intraventricular BDNF injections could reduce infarct size and showed neuroprotective effects.7 In addition, evidence showed that BDNF is associated with some neuropsychiatric disorders.8 The most well-studied BDNF single nucleotide polymorphism (SNP) rs6265 has been reported to be related to brain morphology changes and cortical plasticity.9 In the context of stroke, Qin et al6 and Vliet et al10 reported that BDNF rs6265 polymorphism was associated with motor recovery of stroke patients. Thus, this study mainly focused on the contributions of BDNF genetic variants to ischemic stroke.
Micro RNAs (miRNA) can degrade mRNA by binding to its 3ʹ-untranslated region (3ʹ-UTR), which is one of the post-transcriptional gene regulation mechanisms.11 Previous studies have reported that SNPs in 3ʹ-UTR of mRNA were associated with the occurrence of different diseases.12,13 In this study, we investigated the unreported SNPs in the 3ʹ-UTR of BDNF. With the bioinformatics software (http://www.bioguo.org/miRNASNP/), we predicted all candidate SNPs that may regulate BDNF expression. Polymorphism rs7124442 was identified as the only potential SNP via regulating the binding of miR-922 and its relationship with ischemic stroke was further investigated.
Materials and Methods
Study Subjects
In this study, we included 500 ischemic stroke patients from Jiangyin People’s Hospital (Jiangyin, China) between June 2010 and August 2016. 520 age- and gender-matched healthy controls were also recruited. Ischemic stroke was diagnosed based on the World Health Organization criteria.14 A questionnaire was filled by all patients and controls. It contains demographical information, medical history such as diabetes mellitus and hypertension, and other vascular risk factors. The definition of vascular risk factors has been detailed described in a previous study.16 This study was conducted in accordance with the Declaration of Helsinki. An informed consent was signed by all participants and the Ethics Committee of Jiangyin People’s Hospital approved this study (Project identification code: 2016–011).
Stroke Severity and Outcome Measures
At the time of presentation and 3 months post-stroke, the National Institutes of Health Stroke Scale (NIHSS) was scored to measure stroke severity and functional changes of patients. The NIHSS score was skewed and not normally distributed, and thus it was dichotomized for logistic regression analysis. The mild and severe IS cutoff score was set at 6, which is consistent with previous studies.15,16 The score change from presentation to discharge (ΔNIHSS) was used to measure the recovery of ischemic stroke. It was also skewed and dichotomized for logistic regression analysis. Its cutoff score was set at 0 and <0 means clinical improvement.
DNA Extraction and Genotyping
Total DNA of patients and controls was extracted on the basis of a standard protocol.17 The TaqMan allelic discrimination assay was used to genotype the SNP (Applied Biosystems, San Diego, CA, USA). The PCR conditions were 50°C for 2 mins, 95°C for 10 mins followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. The allelic discrimination mode of the SDS 2.3 software was used to calculate the results.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
The BDNF mRNA levels were determined by qRT-PCR. Briefly, a Trizol reagent was used to isolate total RNA from 293T cells (Invitrogen, Carlsbad, CA, USA). After that, a TaqMan Reverse Transcription Kit was used to reverse transcribe the RNA into cDNA (ABI, CA, USA). With Realmaster Mix (SYBR Green I) (TAKARA, Dalian, China), the qPCR amplifications were then performed under the following condition: 95°C for 10 mins followed by 40 cycles of 95°C for 30 s, 55°C for 40 s and 72°C for 30 s, and finally 4°C for 30 mins.
Prediction of miRNAs Binding to the SNP
The potential miRNAs that could be affected by SNPs in the 3ʹ-UTR of BDNF were predicted by a bioinformatics software (http://bioinfo.life.hust.edu.cn/miRNASNP2/index.php). In brief, the target sites of SNPs in 3ʹ-UTR were predicted using two different methods. Four kinds of results are categorized as SNP targetscan site (ST), SNP miranda site (SM), wild targetscan site (WT), wild miranda site (WM). If a target shows both in ST and SM, but not in WT or WM, we called the gene gained the target site. On the other side, the gene lost the target site.
Construction of Plasmids and 3ʹ-UTR Reporter Assay
The 3ʹ-UTR fragments containing the C or T allele were amplified using PCR and the results were verified by DNA sequencing. Then, we cloned the PCR productions into the pGL3-promoterless luciferase-based plasmid (Promega, CA, USA) at the cloning site between BamHI and XhoI. For reporter assay, Lipofectamine 2000 (Invitrogen Corp, CA, USA) was used to transfect cells with 100 ng of pGL3- BDNF wild, pGL3- BDNF mutant and miR-922 mimics, respectively. Renilla luciferase vector pRL-SV40 (5 ng) was co-transfected as a control for transfection efficiency.
ELISA of Serum BDNF Levels
ELISA kit (Green Stone, Bern, Switzerland) was used to measure BDNF levels according to the manufacturer’s instructions. Concentrations of serum BDNF were calculated by mean absorbance of each sample at 460 nm.
Statistical Analysis
Demographic data were compared using two-sample t-tests and chi-square (χ2) test. Univariate and multivariate logistic regression analysis were used to analyze the relationships between different genotypes and IS risk, severity and outcome. Luciferase activities and serum BDNF levels between different genotypes were compared using independent-sample t-test. All statistical analyses were performed using Stata/SE (V.12.0 for Windows; StataCorp LP, College Station, TX, USA) and two-tailed P < 0.05 was considered statistically significant.
Results
Demographic Characteristics of Patients and Control Subjects
Table 1 shows the demographic characteristics of IS patients and controls. Significantly higher proportion of drinking (P < 0.001), smoking (P < 0.001), suffering from diabetes (P = 0.003) and hypertension (P < 0.001) were observed in patient group compared with control group. As expected, these observations confirmed that drinking, smoking, hypertension and diabetes were risk factors for IS. As compared with control group, a significant higher serum triglycerides concentration was also observed in the patient group (P = 0.009).
 |
Table 1 Demographic Characteristics of IS Patients and Controls |
Identification of BDNF SNPs in 3ʹ-UTR
To identify candidate BDNF SNPs in 3ʹ-UTR, NCBI single nucleotide polymorphism database (https://www.ncbi.nlm.nih.gov/snp) was searched. The following searching parameters were used: Organism (Homo sapiens); Function Class (3ʹ-UTR); Global MAF (0.05–0.4); Validation Status (by-1000 Genomes). Finally, three BDNF SNPs were identified (Table 2).
 |
Table 2 SNPs Located in the BDNF Gene 3ʹ-UTR |
Associations of BDNF rs7124442 with IS Risk, Severity, Outcome
We then assessed genotype frequencies of these three BDNF SNPs in 500 ischemic stroke patients and 520 healthy controls and further investigated their associations with IS severity and outcome in patient group. There were no significant differences observed in the genotype distributions of the three SNPs between patients and controls before and after adjusting the risk factors including age, sex, smoking, drinking, diabetes, hypertension, total cholesterol, triglycerides. Moreover, when the rs7124442 was further analyzed under dominant and additive models, still no significant difference was found between patients and controls (Table 3). This suggested that BDNF rs11030099, rs7124442 and rs11030100 were not risk factors for ischemic stroke. However, when we next investigated the associations of the three SNPs with IS severity and outcome, there was a significant difference of rs7124442 distribution observed in mild and severe patient groups. After adjusted for age and sex, logistic regression analysis showed that patients harbored with rs7124442 TC genotype had a milder initial stroke (Dominant model: OR = 0.45, 95% CI = 0.25–0.81, P = 0.015) (Table 4), and also showed a better short-term recovery (Dominant model: OR = 0.39, 95% CI =0.24–0.68, P = 0.003) (Table 5).
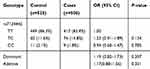 |
Table 3 Genotype Frequencies of the BDNF rs7124442 Polymorphism Among Patient Group and Control Group |
 |
Table 4 Association of BDNF rs7124442 Polymorphism with IS Severity |
 |
Table 5 Association of BDNF rs7124442 Polymorphism with IS Short-Term Recovery |
miR-922 Binding Altered by rs7124442
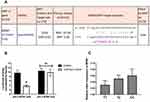
We next performed a bioinformatics analysis to determine whether this BDNF rs7124442 SNP could have miRNA binding sites. The results showed that rs7124442 polymorphism may potentially alter miR-922 binding to BDNF mRNA (Figure 1A). Then, a dual luciferase assay was conducted to confirm this potential regulation. The results showed that co-transfection of hsa-miR-922 mimics with BDNF 3ʹ-UTR containing the mutated allele C changed luciferase activity when compared to co-transfection with BDNF 3ʹ-UTR containing the wild-type allele (Figure 1B). BDNF mRNA levels in IS patients were then examined. The results showed that patients carrying BDNF rs712444 TC or CC genotype had an increased level of BDNF mRNA when compared to patients carrying TT genotype (Figure 1C). Next, we tested the patients’ serum BDNF concentrations. Mean BDNF serum concentrations of 21.53 ± 6.05 ng/mL in the rs7124442 TT group and 25.06 ± 3.27 ng/mL in the rs7124442 TC/CC group were measured. Student’s t-test revealed significantly higher BDNF protein concentrations in the TC/CC group compared to TT group (t = 5.165, df = 498, P < 0.001). Considering the BDNF rs7124442 polymorphism was associated with IS severity and outcome; we then analyzed the serum BDNF concentrations in patients with different stroke severity and outcome. Mean BDNF serum concentrations of 27.11 ± 7.25 ng/mL in mild stroke group and 20.32 ± 4.67 ng/mL in severe stroke group were measured. Student’s t-test revealed significantly higher BDNF protein concentrations in mild stroke group compared to severe stroke group (t = 10.91, df = 498, P < 0.001). In addition, mean BDNF serum concentrations of 26.78 ± 6.19 ng/mL in IS improvement group and 19.37 ± 5.81 ng/mL in IS deterioration group were measured. Student’s t-test revealed significantly higher BDNF protein concentrations in IS improvement group compared to IS deterioration group (t = 13.68, df = 498, P < 0.001). These results showed some consistency with previous rs7124442 association analysis.
Discussion
In this study, the relationships between BDNF rs7124442 polymorphism and IS risk and outcome were investigated in a Chinese Han population. We observed that drinking, smoking, hypertension and diabetes were all associated with an increased IS risk. We further found that BDNF rs7124442 C-carriers had a milder initial stroke and a better short-term recovery. In addition, our data were first to show that BDNF rs7124442 polymorphism could alter the binding of miR-922 to positively regulate BDNF expression, thus providing a post-transcriptional mechanism for gene regulation.
Previously, clinical studies have demonstrated that low circulating concentrations of BDNF are associated with poor long-term functional outcome,18 as well as short-term functional outcome and mortality of ischemic stroke.19 Genetic studies have revealed that BDNF Val66Met (rs6265), the most well-studied BDNF polymorphism so far, is associated with several neurological disorders. The BDNF rs6265 polymorphism involves a G to A substitution at position 196 (G196A), which results in the conversion of valine to methionine in coding area.6 Evidence suggests rs6265 is associated with cortical and hippocampal plasticity, as well as brain morphology changes.9,20–22 Clinical studies have also shown that this polymorphism is associated with motor recovery in stroke patients.6,10 In addition, the less studied rs7124442 polymorphism is reported to affect acute mortality in patients with traumatic brain injury.23
Mounting evidence suggests that SNPs localize in 3ʹ-UTRs may affect the binding of miRNAs to their target genes, causing post-translational expression changes of target mRNAs and alteration of susceptibility to disease.13 For example, previous studies showed that rs4143815 and rs4819388 SNPs in the 3ʹ-UTR of B7-H1 and B7-H2 genes, respectively, contribute to development of gastric cancer.24–26 Our study firstly demonstrates the interactions between miR-922 and SNP in BDNF 3ʹ-UTR.
There are some limitations needing to be considered. Our study only studied the short-term outcome of patients, and the contributions of the SNPs to the long-term recovery and functional outcomes need to be further studied. Our results should therefore be confirmed in independent samples or samples from another ethnic population. Moreover, our findings also need to be further verified in future studies with larger sample sizes.
Conclusion
In summary, our current study showed that the genetic variant of BDNF rs7124442 was associated with milder initial stroke severity, and also with a better short-term recovery after ischemic stroke. Furthermore, miR-922 could regulate SNP rs7124442, resulting in increased BDNF expression in C-allele mutants. Thus, BDNF rs7124442 might act as a protective factor for the outcome of patients with ischemic stroke.
Acknowledgment
This work was supported by the Natural Science Foundation Program of WuXi Health and Family Planning Commission (NO. Q201616).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann Trop Med Parasitol. 2006;100(5–6):481–499. doi:10.1179/136485906X97417
2. Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4
3. Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381(9882):1987–2015.
4. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–1623. doi:10.1016/S0140-6736(08)60694-7
5. Kermani P, Rafii D, Jin DK, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115(3):653–663. doi:10.1172/JCI200522655
6. Qin L, Jing D, Parauda S, et al. An adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic stroke. J Neurosci. 2014;34(7):2493–2502. doi:10.1523/JNEUROSCI.4140-13.2014
7. Schabitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000;31(9):2212–2217. doi:10.1161/01.STR.31.9.2212
8. Caputo V, Sinibaldi L, Fiorentino A, et al. Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allele-specific binding. PLoS One. 2011;6(12):e28656. doi:10.1371/journal.pone.0028656
9. McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cerebral Cortex. 2010;20(5):1254–1262. doi:10.1093/cercor/bhp189
10. van der Vliet R, Ribbers GM, Vandermeeren Y, Frens MA, Selles RW. BDNF Val66Met but not transcranial direct current stimulation affects motor learning after stroke. Brain Stimul. 2017;10(5):882–892. doi:10.1016/j.brs.2017.07.004
11. Plasterk RH. Micro RNAs in animal development. Cell. 2006;124(5):877–881. doi:10.1016/j.cell.2006.02.030
12. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
13. Wang Y, Zhou L, Chen J, et al. Association of the 3ʹUTR FOXO3a polymorphism rs4946936 with an increased risk of childhood acute lymphoblastic leukemia in a Chinese population. Cell Physiol Biochem. 2014;34(2):325–332. doi:10.1159/000363002
14. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol. 1988;41(2):105–114. doi:10.1016/0895-4356(88)90084-4
15. Zhao J, Wu H, Zheng L, Weng Y, Mo Y. Brain-derived neurotrophic factor G196A polymorphism predicts 90-day outcome of ischemic stroke in Chinese: a novel finding. Brain Res. 2013;1537:312–318. doi:10.1016/j.brainres.2013.08.061
16. Stanne TM, Tjarnlund-Wolf A, Olsson S, Jood K, Blomstrand C, Jern C. Genetic variation at the BDNF locus: evidence for association with long-term outcome after ischemic stroke. PLoS One. 2014;9(12):e114156. doi:10.1371/journal.pone.0114156
17. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi:10.1093/nar/16.3.1215
18. Stanne TM, Aberg ND, Nilsson S, et al. Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke. 2016;47(7):1943–1945. doi:10.1161/STROKEAHA.115.012383
19. Wang J, Gao L, Yang YL, et al. Low serum levels of brain-derived neurotrophic factor were associated with poor short-term functional outcome and mortality in acute ischemic stroke. Mol Neurobiol. 2017;54(9):7335–7342. doi:10.1007/s12035-016-0236-1
20. Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi:10.1016/S0092-8674(03)00035-7
21. Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–737. doi:10.1038/nn1699
22. Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24(45):10099–10102. doi:10.1523/JNEUROSCI.2680-04.2004
23. Failla MD, Conley YP, Wagner AK. Brain-Derived Neurotrophic Factor (BDNF) in traumatic brain injury-related mortality: interrelationships between genetics and acute systemic and central nervous system BDNF profiles. Neurorehabil Neural Repair. 2016;30(1):83–93. doi:10.1177/1545968315586465
24. Wu D, Wang F, Dai WQ, et al. The miR-146a rs2910164 G > C polymorphism and susceptibility to digestive cancer in Chinese. Asian Pac J Cancer Prev. 2013;14(1):399–403. doi:10.7314/APJCP.2013.14.1.399
25. Wang W, Li F, Mao Y, et al. A miR-570 binding site polymorphism in the B7-H1 gene is associated with the risk of gastric adenocarcinoma. Hum Genet. 2013;132(6):641–648. doi:10.1007/s00439-013-1275-6
26. Yang P, Tang R, Zhu J, et al. A functional variant at miR-24 binding site in B7-H2 alters susceptibility to gastric cancer in a Chinese Han population. Mol Immunol. 2013;56(1–2):98–103. doi:10.1016/j.molimm.2013.04.010
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.