Back to Journals » Journal of Pain Research » Volume 14
Full-Endoscopic Lumbar Decompression versus Open Decompression and Fusion Surgery for the Lumbar Spinal Stenosis: A 3-Year Follow-Up Study
Authors Song Q, Zhu B, Zhao W, Liang C, Hai B , Liu X
Received 5 March 2021
Accepted for publication 3 May 2021
Published 20 May 2021 Volume 2021:14 Pages 1331—1338
DOI https://doi.org/10.2147/JPR.S309693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Qingpeng Song,1,* Bin Zhu,2,* Wenkui Zhao,3,* Chen Liang,3 Bao Hai,1 Xiaoguang Liu1
1Department of Orthopaedics, Peking University Third Hospital, Beijing, People’s Republic of China; 2Department of Orthopaedics, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Pain Medicine Center, Peking University Third Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoguang Liu
Department of Orthopedics, Peking University Third Hospital, Beijing, People’s Republic of China
Email [email protected]
Purpose: Compare the efficacy of full-endoscopic lumbar decompression surgery (FELDS) and open decompression and fusion surgery (ODFS) for lumbar spinal stenosis (LSS).
Patients and Methods: A retrospective analysis of 358 LSS patients treated by FELDS (“FELD” group) or ODFS (“open” group) was undertaken. There were 177 patients in the FELDS group with a mean age of 65.47± 9.26 years and 181 patients in the open group with a mean age of 64.18± 10.24 years. Duration of follow-up was 38.63± 11.88 months in the FELDS group and 38.56± 12.29 months in the open group. Visual analog scale (VAS) score, Oswestry Disability Index (ODI), and Modified MacNab criteria were used to access clinical outcomes. Surgical outcomes (duration of surgical procedure, blood loss, complications, duration of postoperative hospital stay (DOPHS), prevalence of revision procedures) were evaluated. Magnetic resonance imaging was used to evaluate the change in the Pfirrmann grade at adjacent segments.
Results: VAS score (leg and back) and ODI improved significantly in both groups (P< 0.001). Success rate reached 86.55% and 90.60% in the FELDS group and open group (P> 0.05), respectively. Procedure duration (84.12 vs 112.08 min), blood loss (7.97 vs 279.67 mL), and DOPHS (2.68 vs 4.78 days) of the FELDS group were significantly better than those of the open group (P< 0.05). Total prevalence of complications and procedure revisions was 14.69% and 10.73% in the FELD group, respectively, but did not show a significant difference with that in the open group (12.15% and 9.39%, respectively). The Pfirrmann grade increased in 13.04% of adjacent segments in the FELDS group, significantly better than that in the open group (32.67%) (P< 0.05).
Conclusion: FELDS had the same efficacy as ODFS for LSS treatment. FELDS had the advantages of minimal invasiveness, less surgical trauma, rapid recovery, and lower risk of degeneration of adjacent segments compared with that of ODFS.
Keywords: lumbar spinal stenosis, full-endoscopic lumbar decompression, fusion, clinical outcome, adjacent segments degeneration
Introduction
Lumbar spinal stenosis (LSS) is caused by gradual degenerative changes of the lumbar spine. These include hypertrophy of facet joints and ligamentum flavum, disk herniation, collapse of the intervertebral space, and osteophyte formation.1–3 LSS can result in pain in the lower back, leg, as well as intermittent claudication that can seriously affect the daily life of patients.1,3,4 Studies have shown that surgical treatment is better than conservative treatment if patients are selected carefully.5,6 With the prolonged life expectancy and increased number of older people in developed countries, LSS has become the most common indication for spinal surgery among older people.3,7
Open decompression and fusion surgery (ODFS) is considered the “gold standard” treatment for LSS.8,9 However, it carries some disadvantages: surgical trauma, complications related to screws and cages, and degeneration of adjacent spinal segments.3,10 More minimally invasive surgical treatments are required for LSS.
Full-endoscopic lumbar-decompression surgery (FELDS) has several advantages: minimally invasive, less surgical trauma, can be undertaken under local anesthesia, rapid postoperative recovery and, theoretically, non-degeneration of adjacent spinal segments.11–14 In recent years, with the development of surgical methods and instruments, FELDS has been used for LSS treatment.12,14 However, there have been three main concerns about FELDS application for LSS: (i) whether the extent of decompression is sufficient to achieve satisfactory clinical outcomes; (ii) decompression without fusion might cause instability of the lumbar spine and influence clinical efficacy; (iii) whether degeneration of adjacent spinal segments is prevented.
Few studies have compared FELDS and ODFS for LSS treatment. We aimed to evaluate and compare the safety and efficacy of FELDS and ODFS for LSS treatment. The 3-year follow-up outcomes of FELDS and ODFS for LSS were compared. Furthermore, changes in degeneration of adjacent spinal segments in the two groups were compared.
Patients and Methods
Ethical Approval of the Study
This study was conducted according to the Declaration of Helsinki and approved by the Peking University Third Hospital Medical Science Research Ethics Committee (approval number: IRB00006761-M2020022). The written informed consent was waived due to the retrospective nature of the review, and the data was anonymized.
Inclusion Criteria
The inclusion criteria were: (i) age >18 years; (ii) diagnosis of LSS (including stenosis of the central canal, lateral recess, or foramen); (iii) single level was affected; (iv) symptoms with no relief from conservative treatment for ≥12 weeks.
Exclusion Criteria
The exclusion criteria were: (i) symptoms caused only by herniation of lumbar disks; (ii) instability at responsibility level; (iii) grade-D stenosis of a lumbar central canal;15 (iv) isthmic lumbar spondylolisthesis or degree of degenerative lumbar spondylolisthesis >1; (v) concomitant conditions affecting the lumbar spine (fracture, infection, tumor, or neurological disease).
Study Design
This was a retrospective propensity score-matched study comparing the efficacy of LSS treatment using FELDS (“FELDS group”) and OFDS (“open group”). Patients who met the inclusion and exclusion criteria were enrolled in the study between 1 January 2015 and 31 December 2018.
Data Collection
Preoperative clinical-evaluation data and radiological-evaluation data were collected during hospitalization. Postoperative clinical-evaluation data and radiological-evaluation data were collected through telephone questionaries and in the outpatient setting during follow-up.
Outcome Measurement
Clinical Evaluation
A visual analog scale (VAS),16 Oswestry Disability Index (ODI),17 and Modified MacNab criteria were used to access clinical outcomes. The VAS score and ODI were evaluated at baseline, as well as at 3, 6, 12, 24, and 36 months (final follow-up). The modified MacNab criteria were evaluated at the final follow-up. Procedure-related measures (procedure duration, blood loss, complications, 1-day postoperative VAS score, duration of postoperative hospital stay (DOPHS), and prevalence of revision procedures) were evaluated.
Radiological Evaluation
The grade of degeneration of the adjacent level was evaluated by magnetic resonance imaging (MRI) preoperatively and at the final follow-up according to the Pfirrmann grade.18
Surgical Procedure
FELDS Group
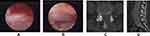
Surgery was undertaken under local anesthesia. Patients were placed in a lateral position for the transforaminal approach, or placed prone for the interlaminar approach (Figure 1). The operating level and entry point were guided by fluoroscopy. An 18-G spinal needle was inserted to the superior articular process or posterior to the interlaminar ligamentum flavum. A guidewire, a series of obturators, working cannula, and the endoscope system were inserted in sequence. Decompression of the foramen, lateral recesses, and central canal were carried out according to the stenosis (Figure 2).
Open Group
Surgery was undertaken under general anesthesia. Patients were placed prone. After exposure to the lamina, a pedicle screw was inserted. Decompression (laminectomy, resection of lateral recesses, and foraminotomy) was done according to the stenosis. Interbody fusion and posterolateral fusion were undertaken.
Matching of Propensity Scores
Matching of propensity scores was carried out to minimize a selection bias between the FELDS group and open group at baseline. The factors of propensity-score matching were age, sex, LSS duration, and the affected level. According to a suggestion in a previous study, the caliper width was set to 0.2.19 Cases in the FELDS group were matched in a 1:1 ratio to those in the open group based on the propensity score.
Statistical Analyses
For variables measured at baseline and follow-up (VAS score and ODI), the paired Student’s t-test was used for variables with a normal distribution. Non-parametric tests were used for variables with a non-normal distribution.
For comparison between the two groups, the Student’s t-test was employed for variables with a normal distribution, and the Mann–Whitney U-test was employed for variables with a non-normal distribution. The chi-square test or Fisher’s exact test were used for categorical variables.
Statistical analyses were conducted using SPSS 22.0 (IBM, Armonk, NY, USA). P < 0.05 was considered significant.
Results
Baseline Data
A total of 207 patients in the FELDS group and 694 patients in the open group met the inclusion and exclusion criteria. All 207 patients in the FELDS group could be matched (1:1) to patients in the open group. A total of 177 patients (85.51%) in the FELDS group and 181 patients (87.44%) in the open group who completed final follow-up were enrolled in our study.
In the FELDS group, the mean age was 65.47±9.26 years, the mean duration of LSS was 52.63±61.07 months, and the mean duration of follow-up was 38.63±11.88 months. In the open group, the mean age was 64.18±10.24 years, the mean duration of LSS was 53.78±67.26 months, and the mean duration of follow-up was 38.56±12.29 months. The difference in data at baseline (age, sex, LSS duration, follow-up period, affected level, and percentage of cases followed up) in the two groups was not significant (Table 1).
 |
Table 1 Baseline Data in 2 Groups |
Surgical Results
Compared with the open group, the FELDS group had a significantly reduced duration of the surgical procedure (84.12 vs 112.08 min), blood loss (7.97 vs 279.67 mL), and DOPHS (2.68 vs 4.78 days). The overall prevalence of complications in the FELDS group was 14.69% (26 cases): new neurological deficit (seven), postoperative dysesthesia (15), wound infection (one), and dura tear (three). The overall prevalence of complications in the open group was 12.15% (22 cases): new neurological deficit (four), postoperative dysesthesia (11), wound infection (four), postoperative hematoma (one), implant-related (one), and dura tear (one). The prevalence of a revision procedure in the FELDS group was 10.73% (19 cases): insufficient decompression (three), new neurological symptoms caused by the adjacent level (seven), and new neurological symptoms caused by the index level (nine). The prevalence of a revision procedure in the open group was 9.39% (17 cases): postoperative hematoma (one), insufficient decompression (one), wound infection (two), and new neurological symptoms caused by the adjacent level (13). The difference in the total prevalence of complications (14.69% vs 12.15%) and revision of the procedure (10.73% vs 9.39%) in the two groups was not significant (Table 2).
 |
Table 2 Surgical Outcomes in 2 Groups |
Clinical Outcomes
The preoperative VAS score for leg pain in the FELDS group (6.46±0.94) and open group (6.72±0.92) improved significantly at 1 day (2.98±0.72 and 2.94±0.75), 3 months (2.71±0.90 and 2.62±0.93), 6 months (2.38±0.90 and 2.17±1.05), 12 months (2.16±0.94 and 2.11±1.04), and 24 months (2.16±0.92 and 2.11±1.03) after the procedure, and at the final follow-up (2.15±0.92 and 2.11±1.03) (P < 0.001 for all) (Figure 3A). The preoperative VAS score for back pain decreased significantly from 3.73±1.10 to 1.97±0.80, 2.37±0.72, 2.15±0.70, 2.11±0.70, 2.11±0.71, and 2.12±0.70 in the FELDS group, and decreased from 3.85±1.09 to 2.57±0.74, 2.45±0.68, 2.18±0.76, 2.14±0.75, 2.13±0.74, and 2.12±0.72 in the open group, at 1 day as well as 3, 6, 12, and 24 months after the procedure, and at the final follow-up (P < 0.001 for all) (Figure 3B). The preoperative ODI in the FELDS group (51.60%±6.49%) and open group (51.92%±6.77%) improved significantly at 3 months (28.18%±4.53% and 30.08%±4.63%), 6 months (26.06%±4.48% and 26.82%±4.22%), 12 months (25.62%±4.43% and 25.82%±4.39%), and 24 months (25.52%±4.39% and 25.82%±4.43%) after the procedure, and at the final follow-up (25.52%±4.39% and 25.82%±4.43%) (P < 0.001 for all) (Figure 3C). The difference in the VAS score (back and leg) and ODI in the two groups was below the minimum clinically important difference.20
The difference in the preoperative VAS score (leg pain) (t = 2.590, P = 0.010), VAS score (back pain) 1 day after the procedure (t = 7.458, P < 0.001), VAS score (leg pain) 6 months after the procedure (t = 2.002, P = 0.046), and ODI 3 months after the procedure (t = 3.939, P < 0.001) between the two groups was significant.
At the final follow-up, according to the modified MacNab criteria, the success rate (“excellent” and “good” outcomes) reached 86.55% (148 cases) in the FELDS group, and 90.60% (164 cases) in the open group (Figure 3D). The difference in the score according to the modified MacNab criteria in the two groups was not significant.
Radiological Outcomes
Forty-eight patients (92 adjacent spinal segments) in the FELDS group and 53 patients in the open group (101 adjacent segments) completed MRI examination. At the final follow-up, the Pfirrmann grade increased in 12 segments (13.04%) in the FELDS group, and 33 segments (32.67%) in the open group. The difference between the two groups was significant (χ2 = 10.376, P = 0.01).
Discussion
FELDS for LSS
Application of FELDS used to be limited for herniation of soft disks. In recent years, with the development of surgical skills and instruments, FELDS has been used to treat LSS.11,21 Lateral or posterolateral approaches, including transforaminal and extraforaminal full-endoscopic lumbar decompression, were employed mainly for lateral-recess stenoses and foraminal stenoses. The posterior approach, including unilateral laminotomy bilateral decompression and interlaminar full-endoscopic lumbar decompression, was suitable for stenoses of the central canal and lateral recess.
McGrath et al22 reported the 1-year follow-up outcomes of 45 patients who underwent minimally invasive laminectomy and 50 patients who underwent FELDS for LSS. They discovered that FELDS had the same efficacy as that of minimally invasive laminectomy. Calvin et al12 compared the long-term efficacy of unilateral laminotomy for bilateral decompression and fusion surgery for LSS and degenerative spondylolisthesis. They showed that the two methods had similar efficacy, and that the prevalence of reoperation was lower in the group that underwent unilateral laminotomy for bilateral decompression.
We undertook a large sample, propensity score-matched cohort study comparing the long-term efficacy of FELDS and conventional ODFS for LSS. The 3-year follow-up outcomes of 177 patients who underwent FELDS and 181 patients who underwent ODFS showed that FELDS had the same efficacy as that of ODFS, and could decrease the development of degeneration of adjacent segments.
Clinical and Radiological Outcomes
In our study, early, mid-term, and long-term postoperative VAS scores (back and leg) and ODI showed considerable improvement in both groups. According to the modified MacNab criteria, the success rate (excellent and good outcomes) was >85% in both groups. The VAS scores (back and leg) and ODI 1 year after surgery showed no significant difference between the two groups. These clinical outcomes demonstrated that the endoscopic decompression surgery used for LSS had satisfactory mid-term and long-term efficacy.
Although the mid- and long-term clinical outcomes were similar in the two groups, the immediate and early postoperative outcomes were different. Compared with ODFS, FELDS reduced back pain immediately and significantly, and improved the quality of life at the early stage after the procedure. This might have resulted from the reduced surgical trauma and rapid recovery after FELDS. These clinical outcomes were similar to those reported previously that compared endoscopic decompression surgery and other types of surgical procedures for LSS.23,24 We also found that recovery from leg pain was quicker at the early stage after the procedure in the open group. This might have been caused by the shorter time of waistline protection and rapid return to normal activities of cases in the FELDS group. However, the results at ≥1 year showed the relief from leg pain in the two groups to be similar.
The results for back pain also demonstrated that FELDS did not lead to symptomatic spinal instability.25,26 The radiological outcomes also showed that, compared with ODFS, FELDS could prevent degeneration of adjacent segments. The mean duration of the procedure, DOPHS, and blood loss in the FELDS group were better than those in the open group.
Complications and Influencing Factors
There are several concerns about the potential complications of FELDS for LSS. Some surgeons are concerned that the limited visualization of the dura and nerve roots as well as the relatively narrow operative space might lead to dura tears, iatrogenic neural injuries, and insufficient decompression.27,28 In our study, the total prevalence of complications in the FELDS group (14.69%) was similar to that in the open group (12.15%). The prevalence of revision procedures was 10.73% in the FELDS group, which was not significantly different to that in the open group.
The total prevalence of complications and revision procedures was similar to that reported previously.12,24,29,30 The success rate of FELDS reached 86.55%, which was similar to that for ODFS. Furthermore, we undertook FELDS to treat LSS at a relatively earlier phase; a lack of experience and appropriate instruments could lead to a prolonged procedure, neural injury, and insufficient decompression. Moreover, as pointed out previously, FELDS has a steep learning curve (especially in the early stage of learning).31 In our study, most complications and revisions occurred in the early stage. However, with the accumulation of experience and the development of instruments (including visualized burrs and trephines32,33), efficient and adequate decompression could be achieved. The success rate of FELDS for LSS after the early stage of learning could be >86.55%. The relatively narrow surgical field of FELDS increased the difficulty of the procedure. Besides, bony decompression under the endoscope angle can hamper the manipulation of endoscopic instruments, which could result in neurovascular injury and insufficient decompression, especially at the early stage of learning.
Limitations
Our study had three main limitations. First, the prevalence of radiological follow-up was relatively low. Due to the pandemic of coronavirus disease-19, many patients were unwilling to return to hospital to complete the radiological evaluation. Second, the different endoscopic approaches (transforaminal and interlaminar) and kinds of stenosis (foraminal, lateral recess and central) were included in the study, which might have influenced the final results. However, compared with ODFS, the overall efficacy of FELDS was satisfactory. Third, this was a retrospective study and not randomized. Although we matched propensity scores to balance the baseline, there was some selection bias between the two groups at baseline. Large-sample randomized controlled trials will be needed to evaluate the efficacy of FELDS for LSS.
Conclusions
FELDS is a safe and efficacious treatment for LSS. Our 3-year follow-up results showed that FELDS had the same efficacy as ODFS for LSS treatment. Compared with ODFS, FELDS was minimally invasive, led to rapid recovery, caused less surgical trauma, had a shorter duration, and could prevent degeneration of adjacent segments.
Declaration
This study was conducted according to the Declaration of Helsinki and approved by the Peking University Third Hospital Medical Science Research Ethics Committee (approval number: IRB00006761-M2020022). The written informed consent was waived due to the retrospective nature of the review, and the data was anonymized.
Funding
This study was funded by Peking University Third Hospital Key Clinical Program (Code: BYSYZD2019001) and Beijing Municipal Science and Technology Commission (Code: 2020-2-4091).
Disclosure
Qingpeng Song, Bin Zhu, and Wenkui Zhao are co-first authors for this study. The authors have no conflicts of interest.
References
1. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–1265. doi:10.1001/jama.2010.338
2. Du Bois M, Szpalski M, Donceel P. A decade’s experience in lumbar spine surgery in Belgium: sickness fund beneficiaries, 2000–2009. Eur Spine J. 2012;21(12):2693–2703. doi:10.1007/s00586-012-2381-1
3. Försth P, Ólafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374(15):1413–1423. doi:10.1056/NEJMoa1513721
4. Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi:10.1136/bmj.h6234
5. Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;2016(1):Cd010264.
6. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794–810. doi:10.1056/NEJMoa0707136
7. Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine. 2013;38(11):916–926. doi:10.1097/BRS.0b013e3182833e7c
8. Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurgery. 2017;80(5):701–715. doi:10.1093/neuros/nyw162
9. Resnick DK, Watters WC
10. Peul WC, Moojen WA. Fusion for lumbar spinal stenosis–safeguard or superfluous surgical implant? N Engl J Med. 2016;374(15):1478–1479. doi:10.1056/NEJMe1600955
11. Lee CH, Choi M, Ryu DS, et al. Efficacy and safety of full-endoscopic decompression via interlaminar approach for central or lateral recess spinal stenosis of the lumbar spine: a meta-analysis. Spine. 2018;43(24):1756–1764. doi:10.1097/BRS.0000000000002708
12. Calvin KC, Merchant M, Kardile MP, Yacob A, Majid K, Bains RS. In degenerative spondylolisthesis, unilateral laminotomy for bilateral decompression leads to less reoperations at 5 years when compared to posterior decompression with instrumented fusion: a propensity-matched retrospective analysis. Spine. 2019;44(21):1530–1537. doi:10.1097/BRS.0000000000003121
13. Liu X, Yuan S, Tian Y, et al. Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: minimum 2-year follow-up results. J Neurosurg Spine. 2018;28(3):317–325. doi:10.3171/2017.6.SPINE172
14. Sun F, Liang Q, Yan M, et al. Unilateral laminectomy by endoscopy in central lumbar canal spinal stenosis: technical note and early outcomes. Spine. 2020;45(14):E871–e877. doi:10.1097/BRS.0000000000003478
15. Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine. 2010;35(21):1919–1924. doi:10.1097/BRS.0b013e3181d359bd
16. Rodriguez CS. Pain measurement in the elderly: a review. Pain Manag. 2001;2(2):38–46. doi:10.1053/jpmn.2001.23746
17. Liu H, Tao H, Luo Z. Validation of the simplified Chinese version of the Oswestry disability index. Spine. 2009;34(11):1211–1216; discussion 1217. doi:10.1097/BRS.0b013e31819e2b34
18. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi:10.1097/00007632-200109010-00011
19. Lonjon G, Porcher R, Ergina P, Fouet M, Boutron I. Potential pitfalls of reporting and bias in observational studies with propensity score analysis assessing a surgical procedure: a methodological systematic review. Ann Surg. 2017;265(5):901–909. doi:10.1097/SLA.0000000000001797
20. Parker SL, Mendenhall SK, Shau DN, et al. Minimum clinically important difference in pain, disability, and quality of life after neural decompression and fusion for same-level recurrent lumbar stenosis: understanding clinical versus statistical significance. J Neurosurg Spine. 2012;16(5):471–478. doi:10.3171/2012.1.SPINE11842
21. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices. 2014;11(6):605–616. doi:10.1586/17434440.2014.940314
22. McGrath LB, White-Dzuro GA, Hofstetter CP. Comparison of clinical outcomes following minimally invasive or lumbar endoscopic unilateral laminotomy for bilateral decompression. J Neurosurg Spine. 2019;1–9. doi:10.3171/2018.9.SPINE18689
23. Lee CW, Yoon KJ, Jun JH. Percutaneous endoscopic laminotomy with flavectomy by uniportal, unilateral approach for the lumbar canal or lateral recess stenosis. World Neurosurg. 2018;113:e129–e137. doi:10.1016/j.wneu.2018.01.195
24. Lee CW, Yoon KJ, Ha SS. Comparative analysis between three different lumbar decompression techniques (microscopic, tubular, and endoscopic) in lumbar canal and lateral recess stenosis: preliminary report. Biomed Res Int. 2019;2019:6078469. doi:10.1155/2019/6078469
25. Leone A, Guglielmi G, Cassar-Pullicino VN, Bonomo L. Lumbar intervertebral instability: a review. Radiology. 2007;245(1):62–77. doi:10.1148/radiol.2451051359
26. Khalsa SS, Kim HS, Singh R, Kashlan ON. Radiographic outcomes of endoscopic decompression for lumbar spinal stenosis. Neurosurg Focus. 2019;46(5):E10. doi:10.3171/2019.2.FOCUS18617
27. Kim JE, Choi DJ, Park EJ. Evaluation of postoperative spinal epidural hematoma after biportal endoscopic spine surgery for single-level lumbar spinal stenosis: Clinical and Magnetic Resonance Imaging Study. World Neurosurg. 2019;126:e786–e792. doi:10.1016/j.wneu.2019.02.150
28. Kamson S, Trescot AM, Sampson PD, Zhang Y. Full-endoscopic assisted lumbar decompressive surgery performed in an outpatient, ambulatory facility: report of 5 years of complications and risk factors. Pain Physician. 2017;20(2):E221–e231. doi:10.36076/ppj.2017.E231
29. Hu D, Fei J, Chen G, Yu Y, Lai Z. Treatment for lumbar spinal stenosis in elderly patients using percutaneous endoscopic lumbar discectomy combined with postoperative three-dimensional traction. Expert Rev Med Devices. 2019;16(4):317–323. doi:10.1080/17434440.2019.1599282
30. Yang F, Chen R, Gu D, et al. Clinical comparison of full-endoscopic and microscopic unilateral laminotomy for bilateral decompression in the treatment of elderly lumbar spinal stenosis: a retrospective study with 12-month follow-up. J Pain Res. 2020;13:1377–1384. doi:10.2147/JPR.S254275
31. Park SM, Kim HJ, Kim GU, et al. Learning curve for lumbar decompressive laminectomy in biportal endoscopic spinal surgery using the cumulative summation test for learning curve. World Neurosurg. 2019;122:e1007–e1013. doi:10.1016/j.wneu.2018.10.197
32. Song QP, Hai B, Zhao WK, et al. Full-endoscopic foraminotomy with a novel large endoscopic trephine for severe degenerative lumbar foraminal stenosis at L5S1 level: an advanced surgical technique. Orthop Surg. 2021;13(2):659–668. doi:10.1111/os.12924
33. Tang S, Jin S, Liao X, Huang K, Luo J, Zhu T. Transforaminal percutaneous endoscopic lumbar decompression by using rigid bendable burr for lumbar lateral recess stenosis: technique and clinical outcome. Biomed Res Int. 2018;2018:2601232. doi:10.1155/2018/2601232
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.