Back to Journals » Drug Design, Development and Therapy » Volume 15
FTY720 Inhibits the Development of Collagen-Induced Arthritis in Mice by Suppressing the Recruitment of CD4+ T Lymphocytes
Authors Zhu C, Wen S , Li J, Meng H, Zhang J, Zhao K, Wang L, Zhang Y
Received 25 November 2020
Accepted for publication 6 April 2021
Published 11 May 2021 Volume 2021:15 Pages 1981—1992
DOI https://doi.org/10.2147/DDDT.S293876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Manfred Ogris
Supplementary video 1 of "FTY720 inhibits the development of collagen-induced arthritis" [ID 293876].
Views: 230
Chao Zhu,1– 5 Shuang Wen,6 Junyong Li,1– 3,5 Hongyu Meng,1– 3,5 Junzhe Zhang,1– 3,5 Kuo Zhao,1– 3,5 Ling Wang,1– 3,5 Yingze Zhang1– 3,5,7
1Department of Orthopaedic Surgery, Third Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Key Laboratory of Biomechanics of Hebei Province, Shijiazhuang, People’s Republic of China; 3NHC Key Laboratory of Intelligent Orthopaedic Equipment, Third Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 4Department of Orthopedics, The Affiliated Jiangning Hospital with Nanjing Medical University, Nanjing, People’s Republic of China; 5Orthopaedic Research Institution of Hebei Province, Shijiazhuang, People’s Republic of China; 6Department of Immunology, Nanjing Medical University, Nanjing, People’s Republic of China; 7Chinese Academy of Engineering, Beijing, People’s Republic of China
Correspondence: Yingze Zhang Email [email protected]
Background: Fingolimod (FTY720), a novel immunomodulator, was found to suppress the severity of collagen-induced arthritis (CIA) in mice. However, the potential molecular mechanisms are still unknown, and the effect of FTY720 on the recruitment of immune cells in the affected joints in the CIA model is not clear.
Materials and Methods: Following the oral administration of FTY720 (2 mg/kg) was treated into CIA mice per day for 35 days, intravital microscopy and immunofluorescence assays were performed to examine immune cell recruitment in the affected joints. Human MH7A synoviocytes were stimulated with tumour necrosis factor (TNF)-α and incubated with FTY720. Interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-8 (IL-8) mRNA and protein expression were evaluated using RT-PCR and enzyme-linked immunosorbent assay, respectively. Signal transduction pathway protein expression was measured by Western blotting. Nuclear translocation of nuclear factor (NF)-κB was also analyzed by fluorescence microscopy.
Results: In vivo experiments showed that FTY720 inhibited the recruitment of CD4+ lymphocytes in the affected joints of CIA mice. FTY720 reduced the secretion of IL-1β, IL-6, and IL-8 from TNF-α-stimulated MH7A cells in a dose-dependent manner. FTY720 also inhibited TNF-α-induced phosphorylation of NF-κBp65 and IκBα, as well as NF-κBp65 nuclear translocation, in a dose- and time-dependent manner. Interestingly, FTY720 blocked PI3K/Akt, the upstream targets of the NF-κB pathway.
Conclusion: Our findings demonstrated that oral administration of FTY720 exerted beneficial effects in CIA mice by inhibiting CD4+ T lymphocyte recruitment to the affected joints. Our data also indicated that FTY720 inhibited TNF-α-induced inflammation by suppressing the AKT/PI3K/NF-κB pathway in MH7A cells.
Keywords: FTY720, rheumatoid arthritis, NF-κB, MH7A, CD4+ T lymphocytes
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease of unknown aetiology that is characterized by inflammation of the synovial tissues leading to destruction of periarticular bone and cartilage, which can lead to irreparable joint destruction if not properly treated.1 At present, the main drugs for RA are disease-modifying antirheumatic drugs (DMARDs) and biologics.2 However, approximately one-third of RA patients still have disease that is not adequately controlled. Therefore, new antirheumatic drugs with high efficacy are needed to meet RA therapy requirements.3
Sphingosine-1-phosphate (S1P) is a metabolite of membrane sphingolipids, which can be phosphorylated by sphingosine kinase 1 (Sphk1) and Sphk2,4 is a metabolite of membrane sphingolipids. The transport and migration of various types of immune cells can be controlled by the S1P/S1P receptor axis, such as lymphocytes, neutrophils and macrophages. FTY720 is developed from the natural product ISP-1 (myriocin), the extract of Cordyceps sinensis. FTY720-P, the phosphorylated form of FTY720, is a structural analogue of S1P and exerts its effects by binding with the S1P receptor in vivo.5
The effects of FTY720 which is positive have been measured in a murine rheumatoid arthritis model. Researchers found that FTY720 could inhibit arthritis by sequestering CD4+ T lymphocytes in the thymus and inhibiting PGE2 production in synovial cells.6 However, the effect of FTY720 on the recruitment of immune cells in the affected joint was not well understood, and the detailed molecular mechanisms of the therapeutic effects of FTY720 in RA have also not been fully elucidated.
Fibroblast-like synoviocytes (FLSs) play an important role in the development of RA, and the abnormal activation of FLSs eventually leads to synovial inflammation and joint damage.7,8 We performed intravital microscopy to evaluate the role of FTY720 in immune cell recruitment in the affected joints of CIA mice in this study. In addition, we examined the anti-inflammatory effect and mechanism of FTY720 in human MH7A synoviocytes as well.
Our results suggested that FTY720 inhibited the recruitment of CD4+ T lymphocytes in the affected joints of CIA mice. Our results also showed that the production of inflammatory cytokines was inhibited by FTY720 in human MH7A synoviocytes, which may be related to the inhibition of the PI3K/Akt/NF-κB pathway.
Materials and Methods
Mice
Male DBA/1J mice were purchased from Shanghai Slac Laboratory Animal (Shanghai, China) at 6–8 weeks of age and maintained under specific pathogen-free conditions at Nanjing Medical University Animal Facility, China. Experimental protocols were approved by the Animal Research Ethics Committee of Nanjing Medical University. The guidelines followed for the welfare of the laboratory animals were the UK Animals Act, 1986.
Induction of Collagen-Induced Arthritis
The induction of collagen-induced arthritis (CIA) was showed by Brand et al9 which was described previously. First, collagen was dissolved in 0.1 M acetic acid (2 mg/mL) by gently stirring overnight at 4 °C and then emulsified with an equal volume of CFA containing mycobacteria to produce the inducing agent. On the day 0, 0.1mL of emulsion was injected subcutaneously 1–1.5 cm from the root of tail, and on day 21, near the initial injection site, a booster injection was executed.
Grouping of Animals
The animals were randomly divided into three groups (6 animals each) as follows: Group A: Control; Group B: Collagen-induced arthritis (CIA); and Group C: CIA + FTY720. The mice in group C received 2 mg/kg FTY720 each day by oral from the day of immunization until the mice were sacrificed.10,11 In the model group of CIA model and the control group, mice accepted saline instead.
Clinical Assessment of Arthritis
Beginning on day 25, arthritis severity was assessed every two or three days by two specially trained observers in a blinded manner using a 16-point scoring method as follows: no joint redness and swelling, 0 points; joint redness and mild swelling limited to the tarsal or ankle joint, 1 point; joint redness and swelling, mild swelling, swelling from the tarsal bone to ankle joint, 2 points; joint redness and swelling, moderate swelling extending from the tarsal bone to ankle joint, 3 points; and joint redness and swelling, severe swelling extending from toe to ankle joint, joint ankylosis and dyskinesia 4 points.12 In case of obstruction, 4 points were scored. The total arthritis score was the sum of the limb joint score.
Intravital Confocal Microscopy
The surgical preparations for joint capillary imaging were based on previously described methods.13 By using a cocktail of ketamine (50 mg/kg), xylazine (10 mg/kg), and acepromazine (1.7 mg/kg) which were injected intraperitoneally, the mice were anaesthetized. Beginning 45 min later, anaesthesia was maintained by half-dose boosts administered subcutaneously every 30 min. The animals were intravenously (i.v.) administered PE/Dazzle™ 594 anti-mouse CD4 (Biolegend, San Diego, California, USA) (0.5 mg/kg, body weight) via the tail vein to label CD4+ lymphocytes, and FITC anti-mouse CD8 (Biolegend) (0.5 mg/kg, body weight) was used to label CD8+ lymphocytes. After anaesthetizing the mice, depilatory cream was used for hair removal and skin preparation, and the mice were sterilized with 75% alcohol on absorbent cotton for 5 seconds. Sterile ophthalmic surgical instruments were used to open the mouse ankle joint tissue and expose the joint capillaries under a stereo dissection microscope. Images were acquired using an inverted epifluorescence Zeiss LSM 880 confocal laser system equipped with a 10×/0.3 Plan Neofluar objective or a Fluar 40×/1.3 objective. The microscope objective was thermostatically controlled to maintain a temperature of 37 °C. Videos were obtained by consecutive frames using appropriate combinations of 488-nm, 561-nm, and 633-nm laser lines. Cells that remained stationary for at least 30 seconds were considered to be adhered to the inner wall of the vessel.
Immunofluorescence Assay
Fresh samples of lower extremity joint synovium of the mice were embedded with OCT, and then frozen sections were prepared. After the frozen sections were naturally dried for 30 min, they were fixed with 4% paraformaldehyde prepared in 0.1 mol/L phosphate buffer (PBS, pH 7.2 ~ 7.4) for 30 min, washed with PBS 3 times, and permeabilized with 1% Triton X-100 for 20 min. The frozen sections were blocked with 3% bovine serum albumin (BSA) and 10% bovine serum albumin (BSA). PBS containing normal goat serum (250 μL) was added and incubated at 4 °C for 1 h in a wet box; the incubation solution was gently removed, and then the primary antibody against CD4+ T lymphocytes (1:150) (Abcam, Cambridge, MA, USA) was added. PBS was utilized as a negative control instead of primary antibody and was incubated overnight in a 4 °C wet box. The sections were washed three times with PBS, and FITC-conjugated secondary antibody (1:200) (Alexa Fluor® 594, Abcam) and DAPI were added to the wet box and incubated at 4 °C for 1 h. the sections were washed three times with PBS and sealed with an anti-fluorescence quencher. A fluorescence microscope (Nikon Eclipse TE2000-S, Nikon, Tokyo, Japan) was used to observe and photograph the samples in the darkroom. In each slide, three visual fields at ×200 magnification were selected, and the positively stained spots were counted by two pathologists in a blinded manner.
Cell Culture
MH7A cells were provided by Professor David Yu at the University of California, Los Angeles. This was approved by the institutional ethics committee of the Third Hospital of Hebei Medical University. Cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM, GE Healthcare HyClone, Logan, UT, USA) containing 10% FBS (Gibco, Gaithersburg, MD, USA) and 1% penicillin/streptomycin in a 37 °C incubator with 5% CO2.
Cell Viability Assay
CellTiter-Blue® Cell Viability Assay (Promega Corporation, Madison, WI, USA) was applied to assess the cell viability. The 96-well culture plates at 3000–5000 cells/well received MH7A cells for 24 h and then treated with or without the indicated concentrations of FTY720 (Cayman, Ann Arbor, MI, USA) for 24 h. Then, CellTiter 96® AQueous One Solution Cell Proliferation Assay reagent (20 µL/well) was added and incubated at 37 °C for 1–2 h. The absorbance was measured at 490 nm by a microplate reader.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
MH7A cells were pretreated without or with various concentrations of FTY720 for 1 h and then incubated for 8 h in the use of 10 ng/mL TNF-α (PeproTech Rocky Hill, NJ, USA) or not. Total RNA was extracted from the cells in the use of TRIzol reagent (Invitrogen Life Technologies, Gaithersburg, MD, USA), and reverse transcription was applied in order to obtain single-stranded cDNA. In a 20-µL volume system which contained 4 µL of cDNA, 2 µL of each primer (forward and reverse), 10 µL of mix and 2 µL of ddH2O, real-time PCR was performed in the use of the SYBR green PCR reagent (Takara). The primer sets which were used for amplification were as follows:
β-ACTIN: 5′-TGA CGT GGA CAT CCG CAA AG-3′ (forward)
5′-CTG GAA GGT GGA CAG CGA GG-3′ (reverse)
IL-1β: 5′- CCT GTC CTG CGT GTT GAA AGA −3′ (forward)
5′- GGG AACTGG GCA GAC TCA AA −3′ (reverse)
IL-6: 5′- CCT GAC CCA ACC ACA AAT GC −3′ (forward)
5′- ATC TGA GGT GCC CAT GCT AC −3′ (reverse)
IL-8: 5′-GGT GCA GTT TTG CCA AGG AG −3′ (forward)
5′- TTC CTT GGG GTC CAGACA GA −3′ (reverse).
Cytokine Quantification by ELISA
In the 6-well plates at 0.5×106 cells/well, MH7A cells were seeded for 24 h. The cells were pretreated with various concentrations of FTY720 for 1 h and then the cells were incubated with or without 10 ng/mL TNF-α for another 24 h. And then the culture medium was gathered, and the concentrations of IL-1β, IL-6 and IL-8 were determined by ELISA using a commercial kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instructions.
Immunofluorescence Staining
After pretreatment with or without FTY720 (10 nM) for 1 h, MH7A cells were incubated for 30 min when treated with TNF-α (10 ng/mL). The cells were incubated with anti-NF-κB-p65 antibody (Cell Signaling Technology, Beverly, MA, USA) overnight and incubated with FITC-conjugated secondary antibody for 1 h at room temperature. After being washed in PBS, the cells were stained with 0.1 mg/mL DAPI. The stained cells were visualized using fluorescence microscope (Nikon Eclipse TE2000-S, Nikon, Tokyo, Japan).
Western Blotting
In order to extract proteins, the cells were lysed in 1 mL of RIPA buffer supplemented with 1% PMSF and 1% phosphatase inhibitor, and the protein concentration was tested by using BCA kit (Beyotime Institute of Biotechnology, Shanghai, China). Then, in the use of 10% SDS-PAGE, 20 µg of the protein was separated and transferred to a PVDF membrane. Then, at room temperature, the membrane was blocked with 5% skim milk for 2 h and incubated at 4 °C overnight using the following primary antibodies: anti-PI3Kp85 (Cell Signaling Technology), anti-p-PI3K p85 (Cell Signaling Technology), anti-Akt (Cell Signaling Technology), anti-p-Akt (Cell Signaling Technology), anti-NF-κBp65 (Cell Signaling Technology), anti-p-NF-ΚBp65 (Cell Signaling Technology), anti-P-IκBα (Abcam, MA, USA), and anti-GAPDH (Cell Signaling Technology). The membrane was incubated with HRP-labelled secondary antibodies at room temperature for 2 h, after three times washed with PBST. The membranes were washed again (three times) in use of PBST and visualized utilizing enhanced chemiluminescence.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). A one-way analysis of variance (ANOVA) was used for the statistical analysis among different groups. Each value is presented as the mean ± standard deviation (SD). The results with P < 0.05 were considered statistically significant.
Results
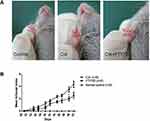
FTY720 Suppressed the Development of CIA
The FTY720 group were immunized with CII/CFA and received oral FTY720 (2 mg/kg/d), in order to observe the inhibitory effect of FTY720 on CIA, in DBA/1 mice. In the CIA group, signs of arthritis came into develop 4–6 days after the booster immunization. FTY720 treatment reduced the mean articular scores. On days 40 and 42 after the first immunization, there were significant differences in the mean articular scores between the groups (Figure 1).
T Lymphocytes into the Affected Joints of CIA Mice but Did Not Affect the Recruitment of CD8 T Lymphocytes
By using intravital microscopy, we found that CD4+ T lymphocyte adhesion in the peripheral vessels of the lower extremity joint was obviously increased in the CIA group compared with the normal control group. Notably, after FTY720 treatment, CD4+ T lymphocyte recruitment in the peripheral vessels of the lower extremity joint was significantly reduced (Supplementary Video S1, Figure 2A). There were no significant differences in the recruitment of CD8+ T lymphocytes in the peripheral blood vessels of the lower extremity joint in any of the groups (Supplementary Video S2, Figure 2B).
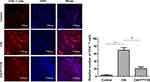
The Influence of FTY720 Treatment on the Number of CD4+ T Lymphocytes in the Synovial Tissue of CIA Mice
The immunofluorescence results showed that the number of CD4+ T lymphocytes in the CIA model group (69.7±6.9) was higher than that in the normal control group. Compared with that of the CIA group, the number of CD4+ T lymphocytes in the FTY720 group was significantly decreased (Figure 3).
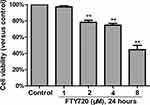
Cytotoxicity of FTY720 on MH7A Cells (IC50)
We investigated the cytotoxicity of FTY720 in MH7A cells by using the MTS assay (Promega, Madison, WI, USA). The cytotoxicity was not observed until the cells were exposed to up to 2 μM FTY720 for 24 hours (Figure 4). Treatment with increasing concentrations of FTY720 for 24 or 48 h resulted in a dose- and time-dependent reduction in MH7A cell viability (Table 1).
 |
Table 1 IC50 and IC80 of FTY720 for MH7A Cells |
Effects of FTY720 on IL-1β, IL-6, IL-8 mRNA and Protein Expression in MH7A Cells Induced by TNF-α
As shown in Figure 5A–C), TNF-α (10 ng/mL) significantly increased the mRNA expression of IL-1β, IL-6, and IL-8 in MH7A cells compared with that of the control group, which could be attenuated by FTY720 pretreatment in a dose-dependent manner. Similarly, the protein expression of IL-1β, IL-6 and IL-8 in the supernatant of the control group was low but increased significantly with TNF-α stimulation (10 ng/mL), and FTY720 decreased the TNF-α-induced increases in these factors in a dose-dependent manner (Figure 5D–F).
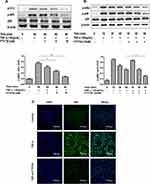
FTY720 Attenuated the TNF-α-Induced Activation of NF-κB in MH7A Cells
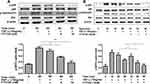
NF‐κB pathway plays a key role in inflammatory responses and NF‐κB is activated by phosphorylated IκB‐α in pervious studies. Therefore, the expression levels of p65 and p‐p65 in whole cell extracts and phosphorylated IκB‐α in the cytosolic fraction were detected by Western blotting. As shown in Figure 6A and B, FTY720 treatment inhibited the TNF-α-induced expression of p‐p65 and p‐IκBα in MH7A cells compared with that in control cells. We also found that TNF- α-induced NF-κB p65 nuclear translocation was significantly inhibited by treatment with FTY720 (Figure 6C)
FTY720 Inhibited TNF-α-Induced PI3K/Akt Activation in MH7A Cells
We further examined the effect of FTY720 on the PI3K/Akt pathway by measuring the expression levels of Akt, p‐Akt, p85, and p‐p85 using Western blotting. As shown in Figure 7, FTY720 treatment decreased the expression levels of p‐Akt and p‐p85 compared with those of the control. These results indicated that FTY720 treatment inhibited the PI3K/Akt pathway in MH7A cells.
Discussion
Sphingosine is one of the components of the cell membrane. Sphingosine is phosphorylated to form sphingosine-1-phosphate (S1P), which is catalysed by sphingosine kinase 1 (SphK1) and sphingosine kinase 1 (SphK2) in vivo.4 S1P is synthesized in the cell and transported to the extracellular environment or cell surface by transporters.14 S1P can bind to sphingosine-1-phosphate receptors (S1P1-S1P5) on the cell membrane surface by autocrine or paracrine signalling and mediate various biological effects, such as cell activation, proliferation, differentiation, migration and angiogenesis.15,16
There was a high level of S1P receptor in the peripheral blood of rheumatoid arthritis patients.17 Lai et al found that the expression of S1P in the synovial fluid of RA patients was 5 times higher than that of the synovial fluid of normal patients.18 Kitano found that S1P could independently enhance TNF-α- and IL-1β-induced production of COX-2 and PGE2 in MH7A cells;19 Zhao found that S1P could induce rheumatoid synovial cells to secrete inflammatory cytokines (IL-6 and IL8), and S1P could promote the proliferation of rheumatoid synovial cells through anti-apoptotic effects.20 Takeshita found that S1P/S1P1 signalling plays a role in the pathogenesis of RA by regulating the expression of RANKL. S1P can independently enhance the expression of RANKL in MH7A cells and has a synergistic effect with TNF-α.21 Because of the role of S1P/S1P1 signalling in the occurrence and development of RA, S1P/S1P1 is a new target in terms of the treatment of rheumatoid arthritis.22 The phosphorylated form of FTY720 is a structural analogue of S1P, which can be recognized by the S1P receptor at very low concentrations (10−9 M) and can suppress the downstream signal of S1P1.23 In this study, FTY720 was shown to inhibit CIA development through both in vitro and in vivo experiments.
In the process of inflammation, circulating immune cells roll, adhere, crawl and cross the vascular wall on the surface of the vascular endothelium and finally migrate to the tissue parenchyma.24 This process widely exists in the migration of immune cells, tumour cells and other cells. We can directly observe and analyze the behaviours of immunocytes labelled by fluorescence through visualization by exposing the blood vessels of the affected joints of anaesthetized animals to intravital microscopy.
The mechanism by which FTY720 is believed to play a strong immunosuppressive role is through the reversible and dose-dependent reduction in the number of peripheral blood lymphocytes, especially CD4+ T lymphocytes.6 FTY720 has immunomodulatory effects on dendritic cells (DCs), innate lymphoid cells (ILCs) and has important consequences for cytokines that have major impact on RA pathogenesis.25,26 Several studies have presented that FTY720 can decrease inflammation in a murine rheumatoid arthritis model. Tsunemi et al found that FTY720 could inhibit arthritis in an SKG mouse RA model via the sequestration of autoimmune CD4+ T lymphocytes in the thymus.6 Han et al demonstrated that FTY720 inhibited arthritis in CIA mice by hindering DC migration to local lymph nodes.12 However, the recruitment of immune cells in the affected joint after FTY720 treatment in CIA mice was not well understood. Our results suggested that FTY720 inhibited the recruitment of CD4+ T lymphocytes in the lower extremity joint in CIA mice. Increasing evidence shows that the occurrence and development of RA is the result of many abnormal lymphocyte effects, among which the imbalance in CD4+ T lymphocyte subsets is an important factor leading to the occurrence and development of RA.27,28 We also confirmed that the infiltration of CD4+ T lymphocytes in the synovium of CIA mice was significantly higher than that of normal subjects. The mechanism of action of FTY720 is believed to be mainly through the reversible and dose-dependent reduction in the number of peripheral blood lymphocytes, especially CD4+ T lymphocytes, downregulating the ratio of CD4+/CD8+ lymphocytes. The S1P/S1P receptor axis has been found to control the transport and migration of various types of immune cells, such as lymphocytes, neutrophils and macrophages.29,30 Eken et al found that when S1P receptor 1 is knocked out other disease models such as EAE does not develop and the Th17 cells are unable to home to tissues.25
In our study, by using intravital microscopy, we observed a significant reduction in the number of infiltrating CD4+ lymphocytes in the peripheral vessels of the lower extremity joint in CIA mice after FTY720 treatment. We also found that after FTY720 treatment, the number of CD8+ lymphocytes adhering to the vessels around the joints in CIA mice decreased, but the difference was not statistically significant.
FLSs play a key role in the development of RA.31,32 Tsunemi et al found that FTY720 inhibited PGE2 production by MH7A cells and cytokine expression in synovial tissues of a mouse RA model.6 The results of our study showed that in MH7A cells, FTY720 inhibited TNF-α-induced production of inflammatory cytokines. Furthermore, our results also demonstrated that the anti-inflammatory properties of FTY720 were mediated by the inhibition of the Akt/PI3K/NF-κB signalling pathway.
The significance of RA treatment is to control inflammation and pain.33 The pathogenesis of rheumatoid synovitis is closely related to the release of cytokines such as TNF - α, IL-1 β, IL-6 and IL-8. The higher the concentration of cytokines, the more serious the clinical symptoms and the more active the RA. The levels of TNF-α, IL-1β, IL-6 and IL-8 were significantly reduced after DMARD treatment. Our results showed that FTY720 significantly inhibited the production of TNF-α-activated inflammatory cytokines, such as IL-1β, IL-6 and IL-8, at the mRNA and protein levels in MH7A cells. In addition, we found that the effective concentration of FTY720 on MH7A cells was 0.001 µM to 0.01 µM, far lower than the IC50 value of FTY720 against the proliferation of MH7A.
The transcription factor NF-κB plays an important role in inflammation.34 Several studies have shown that NF-κB is stimulated in the development of RA.35,36 It is well established that NF-κB is activated via the canonical pathway in RA pathogenesis. In the resting stage, the NF-κB protein dimer (p65/p50) is complexed with the inhibitory subunit IκBα and is sequestered in the cytoplasm. After stimulation with TNF-α, IκBα is rapidly phosphorylated by IKKα/β at Ser32 and Ser36 residues and subsequently ubiquitinated and degraded by the proteasome. Once released from IκB, free NF-κB translocates to the nucleus and initiates the transcription of numerous inflammatory gene products. In this study, we found that FTY720 remarkablely restrained IκBα and NF-κBp65 phosphorylation induced by TNF-α and abated nuclear NF-κBp65 levels in MH7A cells.
The PI3K/Akt pathway is a major regulator of inflammation in inflammatory diseases.37 Moreover, PI3k/Akt axis has also been shown in the context of Treg cells.38
Several studies have shown that the PI3K/Akt signalling pathway probable the principal upstream pathway of the NF-κB signalling cascade.37,39 Previous studies have shown that TNF-α could activate the PI3K/Akt pathway in synovial cells.40 Interestingly, our results showed that FTY720 could inhibit the PI3K/Akt pathway, which was accompanied by the suppression of IκBα and NF-κB phosphorylation. Other studies have shown that the phosphorylation of NF-κBp65 was also hindered by the PI3K inhibitor LY294002 in TNF-α-activated MH7A cells. Based on these results, we hypothesised that FTY720 suppressed NF-κB activity through the PI3K/Akt signalling pathway in MH7A cells.
In an inflammatory environment, endothelial cells express high levels of adhesion molecules. P65 and Akt are key signalling molecules that mediate endothelial cell activation. Zhao et al found that in an LPS-induced central nervous system (CNS) inflammation model, the phosphorylation of FTY720 could inhibit the phosphorylation of Akt and NF-κB in vivo and in vitro by binding to S1P1 on the surface of endothelial cells, thus significantly inhibiting the activation of mouse cerebral vascular endothelial cells.41 Kitano et al also found that S1P1 was upregulated in vascular endothelial cells in RA.19 Based on these conclusions and our experimental results, we speculated that FTY720 could inhibit the recruitment of CD4+ lymphocytes by inhibiting S1P/S1P1 signalling and restraining the activation of vascular endothelial cells in CIA mice, but further research is needed to confirm this hypothesis.
In conclusion, our study showed that FTY720 reduced CD4+ lymphocyte adhesion in peripheral vessels of lower extremity joints in CIA mice, and in vitro experiments showed that FTY720 reduced the production of inflammatory cytokines by inhibiting the PI3K/Akt/NF-κB signalling pathways in MH7A cells. We speculated that the inhibitory effect of FTY720 on CD4+ lymphocyte recruitment in RA was related to the inhibition of p65 and Akt phosphorylation; however, further experiments are needed to clarify the mechanism.
Acknowledgments
The study was supported by the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2019PT320001) and the Major Research Plan of National Natural Science Foundation of China (91949203).
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Li J, Gang D, Yu X, et al. Genistein: the potential for efficacy in rheumatoid arthritis. Clin Rheumatol. 2013;32(5):535–540. doi:10.1007/s10067-012-2148-4
2. Tatangelo M, Tomlinson G, Paterson JM, et al. Association of patient, prescriber, and region with the initiation of first prescription of biologic disease-modifying antirheumatic drug among older patients with rheumatoid arthritis and identical health insurance coverage. JAMA Netw Open. 2019;2(12):e1917053. doi:10.1001/jamanetworkopen.2019.17053
3. Li J, Li J, Yue Y, et al. Genistein suppresses tumor necrosis factor alpha-induced inflammation via modulating reactive oxygen species/Akt/nuclear factor kappaB and adenosine monophosphate-activated protein kinase signal pathways in human synoviocyte MH7A cells. Drug Des Devel Ther. 2014;8:315–323. doi:10.2147/DDDT.S52354
4. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4(5):397–407. doi:10.1038/nrm1103
5. Adachi K, Chiba K. FTY720 story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspect Medicin Chem. 2007;1:11–23. doi:10.1177/1177391X0700100002
6. Tsunemi S, Iwasaki T, Kitano S, Imado T, Miyazawa K, Sano H. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin immunol. 2010;136(2):197–204. doi:10.1016/j.clim.2010.03.428
7. Liu YR, Yang L, Xu QQ, et al. Long noncoding RNA MEG3 regulates rheumatoid arthritis by targeting NLRC5. J Cell Physiol. 2019;234(8):14270–14284. doi:10.1002/jcp.28126
8. Mosquera N, Rodriguez-Trillo A, Blanco FJ, Mera-Varela A, Gonzalez A, Conde C. All-trans retinoic acid inhibits migration and invasiveness of rheumatoid fibroblast-like synoviocytes. J Pharmacol Exp Ther. 2020;372(2):185–192. doi:10.1124/jpet.119.261370
9. Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2(5):1269–1275. doi:10.1038/nprot.2007.173
10. Shi D, Tian T, Yao S, et al. FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain Behav Immun. 2018;70:293–304. doi:10.1016/j.bbi.2018.03.009
11. Yao S, Li L, Sun X, et al. FTY720 inhibits mpp+-induced microglial activation by affecting NLRP3 inflammasome activation. J Neuroimmune Pharmacol. 2019;14(3):478–492. doi:10.1007/s11481-019-09843-4
12. Han Y, Li X, Zhou Q, et al. FTY720 abrogates collagen-induced arthritis by hindering dendritic cell migration to local lymph nodes. J Immunol. 2015;195(9):4126–4135. doi:10.4049/jimmunol.1401842
13. Lee WY, Sanz MJ, Wong CH, et al. Invariant natural killer T cells act as an extravascular cytotoxic barrier for joint-invading Lyme Borrelia. Proc Natl Acad Sci U S A. 2014;111(38):13936–13941. doi:10.1073/pnas.1404769111
14. Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2011;36(2):97–107. doi:10.1016/j.tibs.2010.08.001
15. Salas A, Ponnusamy S, Senkal CE, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117(22):5941–5952. doi:10.1182/blood-2010-08-300772
16. Anelli V, Gault CR, Snider AJ, Obeid LM. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J. 2010;24(8):2727–2738. doi:10.1096/fj.09-150540
17. Pi X, Tan SY, Hayes M, et al. Sphingosine kinase 1-mediated inhibition of Fas death signaling in rheumatoid arthritis B lymphoblastoid cells. Arthritis Rheum. 2006;54(3):754–764. doi:10.1002/art.21635
18. Lai WQ, Irwan AW, Goh HH, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181(11):8010–8017. doi:10.4049/jimmunol.181.11.8010
19. Kitano M, Hla T, Sekiguchi M, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54(3):742–753. doi:10.1002/art.21668
20. Zhao C, Fernandes MJ, Turgeon M, et al. Specific and overlapping sphingosine-1-phosphate receptor functions in human synoviocytes: impact of TNF-alpha. J Lipid Res. 2008;49(11):2323–2337. doi:10.1194/jlr.M800143-JLR200
21. Takeshita H, Kitano M, Iwasaki T, et al. Sphingosine 1-phosphate (S1P)/S1P receptor 1 signaling regulates receptor activator of NF-kappaB ligand (RANKL) expression in rheumatoid arthritis. Biochem Biophys Res Commun. 2012;419(2):154–159. doi:10.1016/j.bbrc.2012.01.103
22. Dinges J, Harris CM, Wallace GA, et al. Hit-to-lead evaluation of a novel class of sphingosine 1-phosphate lyase inhibitors. Bioorg Med Chem Lett. 2016;26(9):2297–2302. doi:10.1016/j.bmcl.2016.03.043
23. Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi:10.1126/science.1070238
24. Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24(6):327–334. doi:10.1016/S1471-4906(03)00117-0
25. Eken A, Duhen R, Singh AK, et al. S1P1 deletion differentially affects TH17 and Regulatory T cells. Sci Rep. 2017;7(1):12905. doi:10.1038/s41598-017-13376-2
26. Thomas K, Sehr T, Proschmann U, et al. Fingolimod additionally acts as immunomodulator focused on the innate immune system beyond its prominent effects on lymphocyte recirculation. J Neuroinflammation. 2017;14(1):41. doi:10.1186/s12974-017-0817-6
27. Sumitomo S, Nagafuchi Y, Tsuchida Y, et al. Transcriptome analysis of peripheral blood from patients with rheumatoid arthritis: a systematic review. Inflamm Regen. 2018;38:21. doi:10.1186/s41232-018-0078-5
28. Fonseka CY, Rao DA, Teslovich NC, et al. Mixed-effects association of single cells identifies an expanded effector CD4 + T cell subset in rheumatoid arthritis. Sci Transl Med. 2018;10(463):eaaq0305. doi:10.1126/scitranslmed.aaq0305
29. Weigert A, Olesch C, Brune B. Sphingosine-1-phosphate and macrophage biology-how the sphinx tames the big eater. Front Immunol. 2019;10:1706. doi:10.3389/fimmu.2019.01706
30. Burg N, Swendeman S, Worgall S, Hla T, Salmon JE. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol. 2018;70(11):1879–1889. doi:10.1002/art.40558
31. Su Z, Sun H, Ao M, Zhao C. Atomic force microscopy study of the anti-inflammatory effects of triptolide on rheumatoid arthritis fibroblast-like synoviocytes. Microsc Microanal. 2017;23(5):1002–1012. doi:10.1017/S1431927617012399
32. Lee CJ, Moon SJ, Jeong JH, et al. Kaempferol targeting on the fibroblast growth factor receptor 3-ribosomal S6 kinase 2 signaling axis prevents the development of rheumatoid arthritis. Cell Death Dis. 2018;9(3):401. doi:10.1038/s41419-018-0433-0
33. McWilliams DF, Ferguson E, Young A, Kiely PD, Walsh DA. Discordant inflammation and pain in early and established rheumatoid arthritis: Latent Class Analysis of Early Rheumatoid Arthritis Network and British Society for Rheumatology Biologics Register data. Arthritis Res Ther. 2016;18(1):295.
34. Christian F, Smith EL, Carmody RJ. The regulation of NF-kappaB subunits by phosphorylation. Cells. 2016;5(1). doi:10.3390/cells5010012
35. Bogunia-Kubik K, Wysoczanska B, Piatek D, Iwaszko M, Ciechomska M, Swierkot J. Significance of polymorphism and expression of miR-146a and NFkB1 genetic variants in patients with rheumatoid arthritis. Arch Immunol Ther Exp (Warsz). 2016;64(Suppl 1):131–136. doi:10.1007/s00005-016-0443-5
36. Wang L, Song G, Zheng Y, et al. Expression of Semaphorin 4A and its potential role in rheumatoid arthritis. Arthritis Res Ther. 2015;17:227. doi:10.1186/s13075-015-0734-y
37. Wang L, Ma H, Xue Y, Shi H, Ma T, Cui X. Berberine inhibits the ischemia-reperfusion injury induced inflammatory response and apoptosis of myocardial cells through the phosphoinositide 3-kinase/RAC-alpha serine/threonine-protein kinase and nuclear factor-kappaB signaling pathways. Exp Ther Med. 2018;15(2):1225–1232. doi:10.3892/etm.2017.5575
38. Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11(11):1047–1056. doi:10.1038/ni.1939
39. Venkatesan B, Valente AJ, Prabhu SD, Shanmugam P, Delafontaine P, Chandrasekar B. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 2010;49(4):655–663. doi:10.1016/j.yjmcc.2010.05.007
40. Morel J, Audo R, Hahne M, Combe B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen-activated protein kinases and phosphatidylinositol 3-kinase/Akt. J Biol Chem. 2005;280(16):15709–15718. doi:10.1074/jbc.M414469200
41. Zhao Y, Shi D, Cao K, et al. Fingolimod targets cerebral endothelial activation to block leukocyte recruitment in the central nervous system. J Leukoc Biol. 2018;103(1):107–118. doi:10.1002/JLB.3A0717-287R
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.