Back to Journals » Drug Design, Development and Therapy » Volume 9
Urinary kallidinogenase for the treatment of cerebral arterial stenosis
Authors Zhao L, Zhao Y, Wan Q, Zhang H
Received 26 July 2015
Accepted for publication 21 September 2015
Published 13 October 2015 Volume 2015:9 Pages 5595—5600
DOI https://doi.org/10.2147/DDDT.S93150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Liandong Zhao,1,2 Ying Zhao,2 Qi Wan,1 Haijun Zhang3
1Department of Neurology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 2Department of Neurology, The Second People’s Hospital of Huai’an and The Affiliated Huai’an Hospital of Xuzhou Medical College, Huai’an, Jiangsu, 3Department of Oncology, Zhongda Hospital, Medical School, Southeast University, Nanjing, People’s Republic of China
Aim: Urinary kallidinogenase (UK) has shown promise in improving cerebral perfusion. This study aimed to examine how UK affects cognitive status and serum levels of amyloid betas (Aβs) 1-40 and 1-42 in patients with cerebral arterial stenosis.
Methods: Ninety patients with cerebral arterial stenosis were enrolled, of whom 45 patients received UK + conventional treatment (UK group), and 45 patients received conventional treatment alone as control group. Cognitive status and Aβ1-40 and Aβ1-42 serum levels were determined before treatment and at 4 weeks and 8 weeks after treatment.
Results: At 4 weeks after treatment, cognitive status in patients treated with UK clearly improved accompanied by Aβ1-40 serum levels decreasing while there was no change of Aβ1-42. Cognitive status in patients receiving UK continued to improve, Aβ1-40 serum levels declined further as well as Aβ1-42 serum levels began to decrease dramatically at 8 weeks after treatment.
Conclusion: UK could improve cognitive status and decrease both Aβ1-40 and Aβ1-42 serum levels to prevent ischemic cerebral injury, which represents a good option for patients with cerebral arterial stenosis.
Keywords: urinary kallidinogenase, arterial stenosis, Alzheimer’s disease, Aβ1-40, Aβ1-42
Introduction
Urinary kallikrein, a glycoprotein extracted from urine, can activate kallikrein–kinin system (KKS) and thus transfer kininogen hydrolysis into kallidin and kinin.1 Kinin in turn could activate B1 and B2 receptors induced by ischemic brain tissue and trigger a series of biological effects, releasing nitric oxide and relaxing vascular smooth muscle.1 Thus, it can expand blood vessels in the ischemic area, improve cerebral blood supply of penumbra, and restore the neurological deficit.1 Urinary kallidinogenase (UK) for injection (Kailikang®; Techpool Bio-Pharma Co, Ltd, Guangdong, People’s Republic of China) has been approved by Chinese State Food and Drug Administration and used clinically in People’s Republic of China to treat patients with stroke2 (molecular formula and structure are shown in Figure 1). In addition, studies on hind limb ischemia, cardiac infarction, and renal ischemia confirmed that kallikrein attempts to maintain an adequate tissue perfusion.3 However, whether the commercially available KKS-regulating medicine, UK for injection (Kailikang®), is capable to improve the outcome of the cerebral arterial stenosis has not been reported yet.
  | Figure 1 Molecular formula (A) and structure (B) of urinary kallidinogenase. |
Cerebral arterial stenosis, including intracranial and/or extracranial artery stenosis, is characterized by narrowed arteries that supply blood to the brain, resulting in cerebral hypoperfusion.4 Patients with severe cerebral artery stenosis run a higher risk of distal embolization, hemodynamic changes, and sudden vascular occlusion.5–7 Alzheimer’s disease (AD) is associated with decreased cerebral blood flow. It was found that AD cases displayed a degree of cerebral artery occlusion and that there was a positive correlation between the degree of arterial stenosis and neurofibrillary tangle (NFT) score.8 Chronic brain hypoperfusion leading to cerebral hypoxia represents an important aspect of neurodegenerative processes in AD.9 The current hypothesis of the pathogenesis of familial AD results mainly from aberrant cleavage of amyloid beta (Aβ) precursor protein, which produces cytotoxic Aβ fragments, causes progressive degeneration and dysfunction of neurons, and ultimately leads to the development of AD.4 The contribution of Aβ deposition in the pathogenesis of AD has been well established, and Aβ appears to have a role in ischemic cerebral damage.10
For patients with cerebral arterial stenosis, improving the cerebrovascular perfusion remains a good option to rescue injured tissue and alleviate neurological deficits. In this study, we evaluated the cognitive status and the serum levels of Aβ1-40 and Aβ1-42 in patients with cerebral arterial stenosis treated with or without UK before treatment and at 4 weeks and 8 weeks after treatment. Our intention was to provide additional evidence on the efficacy of UK in the application of patients with cerebral arterial stenosis.
Materials and methods
Patients
Ninety patients were recruited from the Second People’s Hospital of Huai’an from June 1, 2013, to May 31, 2015. The study was approved by the Ethics Committee of the Second People’s Hospital of Huai’an, and informed consents were obtained from all the participants. Patients with dizziness, transient ischemic attack, and 70% or more internal carotid artery stenosis on one or both sides as determined by neuroimaging examination such as digital subtraction angiography and magnetic resonance imaging were included in the study.4 Patients were excluded if they had a history of major medical illness, such as heart failure, pulmonary insufficiency, renal insufficiency, hepatic dysfunction, or blood diseases unfit for antiplatelet therapy.4 Participants were randomly assigned (1:1) to receive either a UK treatment (UK group) or a conventional treatment (control group) using a computer-generated random sequence. The UK group consisted of 45 patients (24 men and 21 women) aged from 42 years to 84 years, including 21 cases of unilateral internal carotid artery stenosis, twelve cases of bilateral internal carotid artery stenosis, eight cases of unilateral internal carotid artery stenosis associated with vertebral artery stenosis, and four cases of unilateral internal carotid artery stenosis associated with middle cerebral artery stenosis. The control group also consisted of 45 patients (26 men and 19 women) aged from 40 years to 79 years, including 19 cases of unilateral internal carotid artery stenosis, eleven cases of bilateral internal carotid artery stenosis, ten cases of unilateral internal carotid artery stenosis associated with vertebral artery stenosis, and five cases of unilateral internal carotid artery stenosis associated with middle cerebral artery stenosis. All patients had experienced at least 1 month of nervous-system dysfunctions. No severe cerebrovascular problems such as stroke occurred during the study period. Clinical symptoms of cerebral ischemia, such as paralysis of limbs and/or disturbance of consciousness, were observed in most of the cases.
Procedures
The study procedure is shown in Figure 2. During the study period, all patients were given 75 mg of clopidogrel and 100 mg of enteric-coated aspirin orally once a day in addition to the use of salvia, puerarin (Conba Phamaceuticals, Zhejiang, People’s Republic of China), and an anti-free-radical agent, edaravone (Lijun Phamaceuticals, Xian, People’s Republic of China), as conventional treatment in the control group. In addition, UK was injected into patients in the UK group at a dose of 0.15 paranitroanilinum (PNA)/d every day for 21 days. There was no daily and total effect of a daily injection of UK on blood pressure (BP), pulse rate, and heart rate. Mini-mental state examination (MMSE) scale was performed to evaluate the efficacy of UK on the cognitive status before treatment and at 4 weeks and 8 weeks after treatment in all patients. MMSE scale was scored by experienced neurologists, who had over 10 years of experience in professional neuropsychological scale assessment and were blinded to the clinical findings. Moreover, 5 mL blood samples were collected via cubital vein from patients before treatment and at 4 weeks and 8 weeks after treatment. The blood samples were centrifuged at 1,000 rpm for 10 minutes, and sera were immediately collected and stored at -70°C. Then, serum concentrations of Aβ were determined using solid-phase sandwich enzyme-linked immunosorbent assay kit as per the manufacturer’s instructions (Adlitteram Diagnostic Laboratories, San Diego, CA, USA). Furthermore, the evaluator of Aβ was blind to the results of grouping.
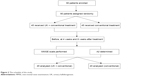  | Figure 2 The schedule of the study. |
Statistical analysis
Statistical analyses were performed using SPSS software (Version 10.0; IBM Corporation, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were expressed as percentage. The F-test was used for significance testing of continuous variables, and the χ2 statistic was used for testing of categorical variables. P<0.05 was considered to be statistically significant.
Results
Characteristics of enrolled patients
As shown in Table 1, 90 patients were enrolled, of whom 45 patients received conventional treatment + UK (UK group) and another 45 patients only received conventional treatment (control group), and the two groups were similar in basic characteristics for analysis.
  | Table 1 Characteristics of enrolled patients |
Comparison of MMSE before and after treatment
To investigate the effect of UK on the cognitive status in patients with cerebral arterial stenosis, we first evaluated the cognitive status with MMSE scale before any treatment. As shown in Figure 3, there was no obvious difference of the score of MMSE between the UK group and the control group before any treatment. The score of MMSE obviously improved at 4 weeks and 8 weeks after patients treated with UK. However, during the study, the MMSE score of patients with conventional treatment still remained similar to that of patients before treatment.
  | Figure 3 MMSE scores before and after treatment. |
Effect of UK on serum levels of Aβ
As shown in Table 2, no significant difference was found in the serum levels of Aβ1-40 and Aβ1-42 between the UK group and the control group before treatment. There were no significant changes of both Aβ1-40 and Aβ1-42 in the control group throughout the study. However, the serum levels of Aβ1-40 in the UK group decreased significantly at 4 weeks after treatment and further 8 weeks after treatment. The serum levels of Aβ1-42 in the UK group display different manners than that of Aβ1-40 that began to decrease significantly at 8 weeks after treatment.
Discussion
Perfusion and hypoxia due to cerebral arterial stenosis may promote β-site cleavage of Aβ precursor protein and cleavage activity of γ-secretase, resulting in increased levels of various Aβ fragments such as Aβ1-40 and Aβ1-42 in the brain.4 An imbalance between production and clearance of Aβ fragments may cause abnormal accumulation of these peptides inside the brain and the subsequent development of AD.11–13 Various mechanisms are involved in the detrimental effects of Aβ. For example, Aβ could activate astrocytes and microglias and cause inflammatory reactions via stimulating expression and release of tumor necrosis factor-alpha, interleukin-1, interleukin-6, and NO.14,15 Aβ might also induce nerve-cell apoptosis and disrupt the automatic regulation of vasomotion, collectively aggravating ischemic cerebral damage.4 Meanwhile, vascular risk factors can predict the clinical development of cognitive dysfunction. Cerebral hypoperfusion can lead to major reductions in suboptimal delivery of high-energy nutrients to the brain, with lethal consequences to brain cells that participate in cognitive function.16–20 Thus, expanding the blood vessels, improving the cerebral blood supply, and restoring the neurological deficit are urgent for the patients with cerebral arterial stenosis.
UK is a positive regulatory substance in KKS consisting of kinins, kallikreins, and kininogens by producing kallidin and kinin, and has been identified to protect against ischemic brain injury through multiple signaling pathways, including anti-inflammation and antiapoptosis, and to promote angiogenesis and neurogenesis.21,22 UK could supply kallikrein to patients’ salvages brain tissue by expanding blood vessels, recanalizing, and improving perfusion. The study on the efficacy of UK in stroke demonstrated that patients could benefit from it with 87% efficacy rate.2 In this study, we studied 45 patients with cerebral arterial stenosis that received UK could improve the cognitive status at 4 weeks after treatment than conventional treatment. Meanwhile, improved cognitive status in patients accompanied by Aβ1-40 decrease at 4 weeks after treatment and by Aβ1-42 decrease at 8 weeks after treatment, possibly through decreased production or increased clearance of Aβ fragments in cerebral tissues due to improved cerebral blood flow by UK. The findings in this study are consistent with most of the previous studies about UK for ischemic cerebral injury such as stroke.2 Furthermore, in this study, there is no side effect of hypotension, the most common adverse event of UK, on the patients using the new treatment method, which provides the evidence on the safety of UK in the application of patients with cerebral arterial stenosis.
Taken together, this study at first confirmed that UK could improve cognitive status and decrease Aβs to prevent the development and progression of AD and ischemic cerebral injury, which could represent an approach for patients with cerebral arterial stenosis. Due to the limitation of the short follow-up period, further investigation is needed in future.
Conclusion
In conclusion, UK successfully improved cognitive status in patients with cerebral arterial stenosis and decreased both Aβ1-40 and Aβ1-42 serum levels. Therapeutic strategy of the supplement of kallikreins to increase cerebral blood flow may prevent the development and progression of AD and ischemic cerebral injury.
Acknowledgment
We thank Xinyong Zhang, Zengjun Wang, Jingming Lu, and Guang Yang for assistance with patient recruitment.
Disclosure
The authors report no conflicts of interest in this work.
References
Wang YX, Chen Y, Zhang CH, et al. Study on the effect of urinary kallidinogenase after thrombolytic treatment for acute cerebral infarction. Eur Rev Med Pharmacol Sci. 2015;19(6):1009–1012. | ||
Zhang C, Tao W, Liu M, Wang D. Efficacy and safety of human urinary kallidinogenase injection for acute ischemic stroke: a systematic review. J Evid Based Med. 2012;5(1):31–39. | ||
Porcu P, Emanueli C, Kapatsoris M, Chao J, Chao L, Madeddu P. Reversal of angiogenic growth factor upregulation by revascularization of lower limb ischemia. Circulation. 2002;105(1):67–72. | ||
Zhao L, Zhao Y, Zhang H. Effect of stent-assisted angioplasty on cognitive status and serum levels of amyloid beta in patients with intracranial and/or extracranial artery stenosis. Neuropsychiatr Dis Treat. 2015;11:471–475. | ||
Wang PQ, Wang AP, Cao ZH, Wang P, Wang S, Zhang GB. The study of the distribution character of cerebral arterial stenosis in patients with ischemic cerebrovascular disease by means of 64 slices CT. Eur Rev Med Pharmacol Sci. 2015;19(12):2287–2292. | ||
Chen CC, Chung CY, Lee TH, Chang WH, Tang SF, Pei YC. Increased risk of posterior circulation infarcts among ischemic stroke patients with cervical spondylosis. Neuropsychiatr Dis Treat. 2015;11:273–278. | ||
Kolukisa M, Ozdemir Gültekin T, Eryigit Baran G, et al. One-year follow-up in patients with brainstem infarction due to large-artery atherothrombosis. Neuropsychiatr Dis Treat. 2015;11:379–384. | ||
Roher AE, Esh C, Kokjohn TA, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055–2062. | ||
Heinzel S, Liepelt-Scarfone I, Roeben B, et al. A neurodegenerative vascular burden index and the impact on cognition. Front Aging Neurosci. 2014;6:161–169. | ||
Zhang X, Li L, Zhang X, et al. Prenatal hypoxia may aggravate the cognitive impairment and Alzheimer’s disease neuropathology in APPSwe/PS1A246E transgenic mice. Neurobiol Aging. 2013;34(3):663–678. | ||
Al-Qazzaz NK, Ali SH, Ahmad SA, Islam S, Mohamad K. Cognitive impairment and memory dysfunction after a stroke diagnosis: a post-stroke memory assessment. Neuropsychiatr Dis Treat. 2014;10: 1677–1691. | ||
Ghezzi L, Scarpini E, Galimberti D. Disease-modifying drugs in Alzheimer’s disease. Drug Des Devel Ther. 2013;7:1471–1478. | ||
Zhang LF, Zhou ZW, Wang ZH, et al. Coffee and caffeine potentiate the antiamyloidogenic activity of melatonin via inhibition of Aβ oligomerization and modulation of the Tau-mediated pathway in N2a/APP cells. Drug Des Devel Ther. 2014;9:241–272. | ||
Zhang F, Jiang L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr Dis Treat. 2015;11:243–256. | ||
Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des Devel Ther. 2014;8:2221–2239. | ||
de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer’s disease. J Alzheimers Dis. 2012;32:553–567. | ||
Silvestrini M, Viticchi G, Falsetti L, et al. The role of carotid atherosclerosis in Alzheimer’s disease progression. J Alzheimers Dis. 2011;25:719–726. | ||
Silvestrini M, Viticchi G, Altamura C, Luzzi S, Balucani C, Vernieri F. Cerebrovascular assessment for the risk prediction of Alzheimer’s disease. J Alzheimers Dis. 2012;32:689–698. | ||
Balestrini S, Perozzi C, Altamura C, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology. 2013;80:2145–2150. | ||
Gupta A, Iadecola C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci. 2015;7:115. | ||
Chen ZB, Huang DQ, Niu FN, Zhang X, Li EG, Xu Y. Human urinary kallidinogenase suppresses cerebral inflammation in experimental stroke and downregulates nuclear factor-kappaB. J Cereb Blood Flow Metab. 2010;30:1356–1365. | ||
Albert-Weiβenberger C, Sirén AL, Kleinschnitz C. Ischemic stroke and traumatic brain injury: the role of the kallikrein-kinin system. Prog Neurobiol. 2013;10(1–102):65–82. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

