Back to Journals » Drug Design, Development and Therapy » Volume 13
Ubenimex induces apoptotic and autophagic cell death in rat GH3 and MMQ cells through the ROS/ERK pathway
Authors Wang Y, Pang B, Zhang R, Fu Y, Pang Q
Received 5 June 2019
Accepted for publication 20 August 2019
Published 12 September 2019 Volume 2019:13 Pages 3217—3228
DOI https://doi.org/10.2147/DDDT.S218371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Yanjun Wang,1,* Bo Pang,2,* Rui Zhang,1 Yibing Fu,3 Qi Pang1
1Department of Neurosurgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan 250021, Shandong, People’s Republic of China; 2Department of Neurosurgery, Shandong University, Jinan 250021, People’s Republic of China; 3Department of Obstetrics and Gynecology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan 250021, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Pang
Department of Neurosurgery, Shandong Provincial Hospital Affiliated to Shandong University, 324 Jingwu Road, Jinan 250021, Shandong Province, People’s Republic of China
Tel +86 136 1531 6688
Email [email protected]
Yibing Fu
Department of Obstetrics and Gynecology, Shandong Provincial Hospital Affiliated to Shandong University, 324 Jingwu Road, Jinan 250021, Shandong Province, People’s Republic of China
Tel +86 151 6888 9696
Email [email protected]
Purpose: Ubenimex, an aminopeptidase N (APN) inhibitor, is widely known for its use as an adjunct therapy for cancer therapy. However, in recent studies, it has also conferred antitumour effects in many cancers, but its anticancer mechanism is largely unknown. This study aims to investigate the specific anticancer activities and mechanisms of ubenimex in GH3 and MMQ cells.
Materials and methods: In this study, we investigated the anticancer effects of ubenimex in GH3 and MMQ cells. Cell viability and cell death were assessed by the Cell Counting Kit-8 kit (CCK-8) and a LIVE/DEAD cell imaging kit. Apoptosis and intracellular reactive oxygen species (ROS) generation were assessed by flow cytometry and fluorescence microscopy. Autophagosome formation was detected by transmission electron microscopy, and autophagic flux was measured with mRFP-GFP-LC3 adenoviral transfection. The protein expression level was detected by Western blotting.
Results: The results revealed that treatment with ubenimex induced apoptotic and autophagic cell death in GH3 and MMQ cells, which resulted in decreased viability, an increased proportion of apoptotic cells, and autophagosome formation. Further experiments showed that ubenimex induced ROS generation and activated the ROS/ERK pathway. The ROS scavenger NAC could attenuate ubenimex-induced apoptosis and autophagy.
Conclusion: Our studies revealed that ubenimex exerted anticancer effects by inducing apoptotic and autophagic cell death in GH3 and MMQ cells, rendering it a possible effective adjunctive therapy for pituitary treatment.
Keywords: pituitary, ubenimex, autophagy, apoptosis, ROS
Introduction
Pituitary adenoma is one of the most prevalent intracranial tumours, and prolactinomas account for approximately 40% of them.1 Currently, radical resection, radiotherapy and dopamine agonist (DA) medication are the common treatment strategies.2–4 Bromocriptine (BRC) and cabergoline (CAB) are the first-line DA treatment for prolactinomas, which can selectively activate the D2R short isoform, inhibit prolactin gene transcription and effectively restore gonadal function and reduce prolactin (PRL) hypersecretion and tumour size.5–7 However, the tumour can recur after drug discontinuation.8 Moreover, the drugs have side effects; they can increase the risk of cardiac valve regulation, induce retroperitoneal and pulmonary fibrosis9 and reduce the likelihood of complete adenoma resection due to preoperative DA therapy-induced fibrosis.10 Therefore, the development of adjuvant therapy for pituitary adenoma is urgently needed.
Ubenimex, an adjunct therapy medicine for many cancers, has shown anticancer effects by enhancing the function of immunocompetent cells.11,12 Aminopeptidase N (APN), a potential target of ubenimex, participates in various cellular processes in different cancers, including cell cycle control, cell motility, cell differentiation, cellular attachment and angiogenesis.13 It has been reported that ubenimex exerts antineoplastic effects through different mechanisms. However, the efficacy of ubenimex in pituitary adenomas have not been reported to date.
Apoptotic and autophagic cell death are the two common mechanisms by which chemotherapeutic drugs induce cytotoxicity. Apoptosis is recognized by cell shrinkage, cell membrane blebbing, nuclear and DNA fragmentation, chromatin condensation, and formation of apoptotic bodies.14 However, none of these aforementioned characteristics are associated with autophagic cell death, which is accomplished by autophagic double-layered membranes in the cytoplasm.15 In many cases, autophagy serves as a cytoprotective mechanism,16,17 but it can also lead to cell death under specific circumstances.18 Although these two forms of cell death are different, there are no clear boundaries between them, and they determine cell fate in a coordinated manner.
Reactive oxygen species (ROS) play an important role in the occurrence and development of tumours. Previous studies have shown that cancer cells contain higher ROS levels and more unregulated antioxidant activities than normal cells.19,20 Due to these traits, cancer cells create excessive oxidative stress. ROS are important signalling molecules that can mediate apoptosis, autophagy, and activation of cell signalling kinases.21 Many therapeutic medicines have been indicated to be effective in the treatment of human cancers through ROS-related signalling pathways. However, the effects of ubenimex-induced ROS damage and the regulation of related signalling pathways in pituitary adenoma cells remain unknown.
In the present study, we aimed to determine the anticancer activities and the potential mechanisms of ubenimex in two different rat pituitary adenoma cell lines. This study may provide a potentially novel drug treatment for pituitary adenomas.
Materials and methods
Chemicals and reagents
Ubenimex were provided by Shenzhen Main Luck Pharmaceuticals, Inc. (Shenzhen, China). LIVE/DEADTM Cell Imaging Kit and Total Reactive Oxygen Species (ROS) Assay Kit were purchased from Thermo Fisher Scientific (USA). Cell Counting Kit-8 was purchased from Dojindo (Japan). Annexin V-FITC/PI kit was purchased from BD Biosciences (USA). NAC, 3-MA and Z-VAD-FMK were purchased from Selleck (USA). Primary antibodies: caspase-3 (1:500; catalog # ab13847), parp1 (1:2000; catalog #ab32138), cleaved parp1 (1:2000; catalog #ab32064) and β-actin (1:5000; catalog # ab8226) were purchased from Abcam (UK). ERK (1:1000; catalog # 4695T) and p-ERK (1:1000; catalog # 4370T) were purchased from Cell Signaling Technology (USA). Caspase-9 (1:1000; catalog # 10380-1-AP) was purchased from Proteintech (USA).
Cell lines and cell culture
The rat pituitary adenoma cell lines GH3 and MMQ were obtained from the Chinese Academy of Sciences Cell Bank. These cells were cultured in Dulbecco’s modified Eagle’s medium/F12K culture medium supplemented with 15% horse serum, 2.5% foetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin and cultured at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air.
Cell viability measurement
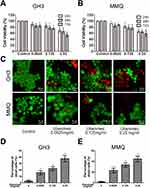
Cell viability was measured by CCK-8 assays. Cells in the exponential growth phase were harvested and seeded into 96-well plates at a density of 20,000 cells per well. The cells were treated with different concentrations of ubenimex for another 24 hrs, 48 hrs and 72 hrs, and the complete medium was used as a control. Then, 10 µL of CCK-8 solution was added into each well. Two hours later, the absorbance was determined by a microplate reader (EL340; Bio-Tek Instruments, MA, USA) at 450 nm.
Cell live/dead assay
GH3 and MMQ cells were seeded into pretreated 24-well plates with poly-L-lysine and administered different concentrations of ubenimex for 48 hrs; then, the cells were treated with a LIVE/DEADTM Cell imaging kit according to the instructions. After another 15 mins of culture in the dark, the cells were observed using fluorescence microscopy (Olympus) and measured at 488 nm excitation and 515 nm emission for live cells (green), 570 nm excitation and 602 nm emission for dead cells (red).
Annexin v-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
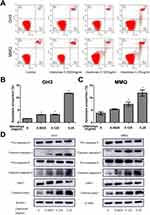
An annexin V-FITC/PI kit was used to determine the effect of ubenimex on apoptosis. Briefly, the cells were treated with different concentrations of ubenimex for 48 hrs and then collected. The cells were resuspended in 500 µL binding buffer and incubated with 5 µL annexin V-FITC and 5 µL PI for 15 mins in the dark. The results were analysed using a FACS Calibur or an EPICS XL flow cytometer (BD Biosciences).
Autophagic flux measurement
GH3 and MMQ cells were cultured in poly-L-lysine-treated 96-well plates for 24 hrs and then transfected through mRFP-GFP-LC3 adenoviral vectors (purchased from Hanbio Biotechnology Co., Ltd). Then, the cells were treated with different doses of ubenimex for 48 h. Next, the cells were observed under a fluorescence microscope (Nikon Inc., Japan) and measured at 488 nm excitation and 515 nm emission for green puncta, 570 nm excitation and 602 nm emission for red puncta. The experiment was performed in triplicate.
Intracellular ROS generation measurement
GH3 and MMQ cells were cultured in a six-well plate and incubated with 2ʹ, 7ʹ-dichlorofluorescein-diacetate (DCFH-DA) for 1 hr. Following the treatment, the cells were washed with serum-free medium three times. Next, the cells were treated for different time intervals and at various concentrations of ubenimex. The level of ROS was analysed using a flow cytometer (BD Biosciences) and imaged using fluorescence microscopy (Olympus) and detected at 495 nm excitation and 520 nm emission.
Transmission electron microscopy
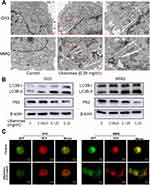
The GH3 and MMQ cells were treated with ubenimex (0.25 mg/mL) for 48 hrs. After incubation, the cells were fixed with 2.5% glutaraldehyde for 1.5 hrs and post-fixed with 1% osmium tetroxide for 1.5 hrs. Next, the cells were washed and stained in 3% aqueous uranyl acetate for 1 hr. Subsequently, the cells were dehydrated with increasing ethanol and acetone and then embedded and sectioned. Finally, the sections were double-stained with uranium acetate and 0.3% lead citrate and examined using a JEOL 1200EX electron microscope (Japan).
Western blot analysis
Cells were incubated with different treatments before lysis. Then, the cells were washed with cold PBS and lysed in cold RIPA lysis buffer containing 1% phosphatase inhibitor and 1% protease inhibitor for 30 mins. The protein samples were centrifuged at 12,000 rpm for another 30 mins at 4 °C. The supernatants were collected for continued analysis. The Bradford protein method and a bicinchoninic acid (BCA) protein assay kit were used for the measurement of protein concentration. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were incubated with different primary antibodies overnight at 4 °C, followed by horseradish peroxidase (HRP)‑conjugated secondary antibodies for 1 hr at room temperature. Immunoblots were visualized using enhanced chemiluminescence (LAS-4000).
Statistical analysis
The experiments were performed independently at least three times. The data were statistically analysed using ANOVA, followed by Tukey’s test for multiple comparisons. The analysis was performed using Prism Graph Pad v4.0 (Graph Pad Software Inc., San Diego, CA, USA). P-values of less than 0.05 were considered statistically significant.
Results
Ubenimex inhibits the viability of pituitary adenoma cells and induces cell death
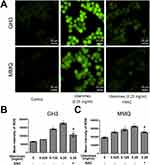
To analyse and test the cytotoxicity induced by ubenimex, CCK-8 assays were performed in GH3 and MMQ cell lines. As shown in Figure 1A and B, the viability of the GH3 and MMQ cells was reduced in a dose-dependent manner after treatment with ubenimex for 24, 48 and 72 hrs. Therefore, we concluded that ubenimex could inhibit cell viability in both the GH3 and MMQ cells. Next, we detected the ratios between live and dead cells using a LIVE/DEAD cell imaging kit. The results showed that the density of dead cells (red) was significantly increased compared with that of control cells (Figure 1C–E).
Ubenimex induces apoptotic effects in GH3 and MMQ cells
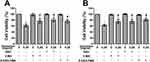
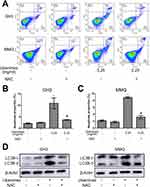
To quantify the level of apoptosis, the cells were subjected to annexin V-FITC/PI double staining. The results showed that the proportion of early and late apoptotic cells increased with increasing concentrations of ubenimex (Figure 2A–C). We further detected the expression of the main apoptotic proteins, namely, caspase-3, caspase-9 and parp1, by Western blot analysis. As shown in Figure 2D, the levels of the cleaved forms of caspase-3, caspase-9 and parp1 were significantly increased in the ubenimex-treated GH3 and MMQ cells, which further proved that ubenimex induced apoptotic effects. To further elucidate the ubenimex-induced apoptosis, we investigated the effect of Z-VAD-FMK, a pan-caspase inhibitor, using CCK-8 assay. As shown in Figure 4A and B, pre-treatment with Z-VAD-FMK attenuated the ubenimex-induced cell viability decrease. Taken together, these results illustrate that ubenimex-induced cell death in GH3 and MMQ cells is apoptosis-dependent.
Ubenimex induces autophagic cell death in GH3 and MMQ cells
Previous studies have demonstrated that ubenimex can induce not only apoptosis but also autophagic cell death in malignant melanoma and bladder cancer cells.22,23 In the present study, we directly detected autophagosome formation by transmission electron microscopy. As shown in Figure 3A, we found that the formation of double-membrane autophagosomes was increased in the ubenimex-treated cells compared to that observed in the untreated cells. During the process of autophagy, microtubule-associated protein light chain 3-I (LC3B-I) is converted to lipidated LC3B-II, which is the classic hallmark of autophagy.24 We next investigated the expression level of LC3B-II by Western blotting after gradually increasing the ubenimex treatment concentration. The results showed that LC3B-II levels in the ubenimex-treated GH3 and MMQ cells were increased compared with the level in the control cells (Figure 3B). Next, we used mRFP-GFP-LC3 adenovirus transfection to determine whether the accumulation of LC3B-II resulted from autophagosome formation or the inhibition of autophagosome-lysosome fusion. As shown in Figure 3C, in the ubenimex-treated GH3 and MMQ cells, the number of autophagosomes-lysosomes (red point in merged images) was significantly increased compared to that observed in the control cells. This suggested that the increased expression of LC3B-II was due to the considerable autophagosome formation rather than the inhibition of lysosomal degradation. The same result was found by decreasing the expression of P62 after ubenimex treatment. In addition, combined treatment with ubenimex and 3-methyladenine (3-MA) significantly increased the cell viability compared to that found with ubenimex treatment alone, according to results from the CCK-8 assay (Figure 4A and B). All the results described above indicated that ubenimex could induce autophagy-related cell death in GH3 and MMQ cells.
Ubenimex induces ROS generation and activates the ROS/ERK pathway
The death-inducing capacity of many anticancer drugs is related to the generation of reactive oxygen species (ROS). Surplus ROS can induce oxidative stress damage to cells, and intracellular ROS levels that exceed the maximum threshold cause cell death.25 In this study, we determined the level of ROS in ubenimex-treated GH3 and MMQ cells with DCFH-DA probes using flow cytometric analysis and images from fluorescence microscopy. The results showed that ubenimex enhanced ROS generation in a dose-dependent manner. In addition, pretreatment with the ROS inhibitor N-acetyl-L-cysteine (NAC) significantly attenuated the increase in ROS generation in GH3 and MMQ cells (Figure 5A–C).
Accumulating evidence suggests that ROS could affect factors in the MAPK pathway, including P38, N-terminal kinase (JNK) and ERK.26 We detected the expression level of p-ERK by Western blotting. After treatment with ubenimex (0.25 mg/mL) for different time intervals (0, 3 h, 6 h, 12 h, 24 h, and 48 h), the level of p-ERK was found to significantly increase after the 12 hr treatment (Figure 6A). To further detect the relationship between ROS and the ERK pathway, the GH3 and MMQ cells were pretreated with NAC for 2 hrs and then exposed to ubenimex. The results showed that NAC eliminated the ubenimex-mediated increase in ERK phosphorylation (Figure 6B).
Ubenimex induces apoptosis and autophagic activity via ROS generation
To investigate whether ROS generation is involved in ubenimex-induced cell death, we pretreated the GH3 and MMQ cells with NAC (5 mM) for 2 hrs before ubenimex exposure. The results showed that pretreatment with NAC increased the viability of the GH3 and MMQ cells compared with treatment with ubenimex alone, as determined by the CCK-8 assay (Figure 4A, B). The apoptotic effects were detected by flow cytometry, and the results showed that pretreatment with NAC could alleviate ubenimex-induced apoptosis in the GH3 and MMQ cells (Figure 7A–C). The results from the Western blot analysis indicated that pretreatment with NAC decreased the expression levels of LC3B-II in the GH3 and MMQ cells (Figure 7D). In summary, these results revealed that ROS generation induced by ubenimex mediated apoptosis and autophagic activity in GH3 and MMQ cells.
Discussion
Previous reports have found that ubenimex shows cytotoxicity and suppresses cell proliferation in leukaemia cell lines,27 glioma,28 malignant melanoma and bladder cancer.22,23 In addition, ubenimex can also synergistically enhance the efficacy of anticancer drugs in many human tumour-derived cell lines, such as those from hepatocellular carcinoma and osteosarcoma.29–31 And many clinical studies were performed to investigate the therapeutic efficacy, survival benefit in acute myeloid leukemia (AML) and lymphomas32 and many solid cancers, for instance, in carcinoma of the lung,33 gastric,34 bladder35 and cervical.36 However, the effect of ubenimex on pituitary adenoma is unknown. In our present study, we exposed GH3 and MMQ cells to different doses of ubenimex and then studied the effects on cell proliferation, apoptosis, and autophagy and deduced the possible involved pathway. The results clearly indicated that ubenimex could decrease cell viability, induce apoptotic and autophagic cell death and activate the ERK pathway. All of these effects were due to ROS generation.
Apoptosis, which is the most important cause of cell death in all clinically relevant cell lines, plays an important role in the mechanism of anticancer drugs. Five main signalling pathways have been identified, and the intrinsic pathway (referenced as the type II or mitochondrial pathway) is of vital importance.37 Our results from the annexin V-FITC/PI staining showed that the apoptotic population of cells increased with increasing concentration; the results from the Western blot analysis demonstrated that cleaved caspase-3, cleaved caspase-9 and cleaved paep1 were increased in this context.
In addition to apoptosis, autophagy, which is also called type II programmed cell death, is also due to mitochondria dysfunction.38 Autophagy is a highly conserved catabolic process that packages and traffics damaged organelles and unnecessary proteins to lysosomes for degradation, which helps to maintain cellular homeostasis.39 Currently, the role of autophagy in regulating or ameliorating cytotoxic effects through pro-death or pro-survival pathways is debated. This controversy inspired us to investigate whether autophagy is involved in the ubenimex-induced cell death of GH3 and MMQ cells. Autophagy is often accompanied by the formation of autophagic vesicles and the conversion of LC3B-I to LC3B-II. In our present study, all the phenomena described above were clearly shown in ubenimex-treated GH3 and MMQ cells by TEM and Western blotting. Moreover, autophagic flux is of vital importance in the successful degradation of autophagic substrates via fusion of autophagosomes with lysosomes, where they undergo complete degradation. SQSTM/P62, which is essential for cargo recognition and for specific degradation at the later stages of autophagy, is the hallmark of autophagic flux. Our present data showed that P62 was downregulated in a dose-dependent manner after GH3 and MMQ cells were exposed to ubenimex. The autophagic flux was further confirmed by mRFP-GFP-LC3 adenovirus transfection. To illustrate the pro-death effect, 3-MA, which can interfere with the PI3KC3 signalling pathway during the formation of autophagosomes, was used. The results showed that pretreatment with 3-MA partially alleviated GH3 and MMQ cell viability compared with treatment with ubenimex alone. Consequently, we concluded that ubenimex indeed played a key role in inducing both apoptotic and autophagic cell death in the GH3 and MMQ cells.
Previous studies have suggested that ROS represent important signalling molecules that play a critical role in the cytotoxicity induced by anticancer drugs. ROS accumulation causes mitochondrial dysfunction, which in turn induces apoptotic and autophagic cell death. It has been demonstrated that apoptosis and autophagy, the two main cellular death processes, are related to ROS generation.40,41 Ubenimex has been proven to increase intracellular ROS levels by inhibiting APN/CD13 in hepatocellular carcinoma cells.31,42 However, the role of ROS in ubenimex-induced cell death in GH3 and MMQ cells remains unknown. Consistent with previous studies, our results demonstrated that ubenimex treatment could upregulate ROS generation in GH3 and MMQ cells. To further test whether ROS upregulation was related to the autophagy and apoptosis induced by ubenimex treatment, NAC, an ROS scavenger, was used before ubenimex treatment. The results showed that NAC pretreatment could indeed alleviate ubenimex-mediated autophagy and cell death, indicating that ROS generation plays a key role in ubenimex-induced autophagy and apoptosis.
In general, MAPKs, including ERK1/2, P38 and JNK, have pleiotropic effects that help determine cell fate. Different portions of the MAPK pathway are diversely regulated in different cells by ubenimex. Liang29 demonstrated that ubenimex could decrease migration and invasion in human osteosarcoma cells by decreasing the phosphorylation of ERK1/2. However, in another study, ubenimex showed its anti-leukaemic effect by inhibiting the phosphorylation of P38.43 In addition, ubenimex induced apoptosis and autophagic cell death, which was accompanied by increased phosphorylation of JNK.23 In our present study, we found that, as time passed, ERK1/2 was increasingly phosphorylated after ubenimex treatment. To determine whether ROS were upstream of mediated ERK1/2 phosphorylation, we pretreated the GH3 and MMQ cells with NAC for 2 hrs. The results indicated that the p-ERK1/2 levels were decreased compared to those in cells that were treated with ubenimex alone. Therefore, we concluded that the ROS/ERK1/2 pathway played a role in ubenimex-mediated cell inhibition in the GH3 and MMQ cells. However, the detailed mechanism should be further investigated.
Conclusion
Our findings demonstrate that ubenimex can inhibit cell proliferation and induce apoptosis and autophagy in GH3 and MMQ cells, and these effects might be associated with the activation of the ROS/ERK1/2 pathway. These novel findings provide a new perspective for the possible use of ubenimex in pituitary adenoma chemotherapeutic interventions.
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant No. 81771270) and Shandong Provincial Natural Science Foundation (Grant No. ZR2015HM015).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu ZB, Yu CJ, Su ZP, Zhuge QC, Wu JS, Zheng WM. Bromocriptine treatment of invasive giant prolactinomas involving the cavernous sinus: results of a long-term follow up. J Neurosurg. 2006;104(1):54–61. doi:10.3171/jns.2006.104.1.54
2. Ciccarelli A, Daly AF, Beckers A. The epidemiology of prolactinomas. Pituitary. 2005;8(1):3–6. doi:10.1007/s11102-005-5079-0
3. Colao A, Savastano S. Medical treatment of prolactinomas. Nat Rev Endocrinol. 2011;7(5):267–278. doi:10.1038/nrendo.2011.37
4. Buchfelder M. Management of aggressive pituitary adenomas: current treatment strategies. Pituitary. 2009;12(3):256–260. doi:10.1007/s11102-008-0153-z
5. Molitch ME. Medical management of prolactin-secreting pituitary adenomas. Pituitary. 2002;5(2):55–65.
6. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi:10.1124/pr.110.002642
7. Li Q, Su Z, Liu J, et al. Dopamine receptor D2S gene transfer improves the sensitivity of GH3 rat pituitary adenoma cells to bromocriptine. Mol Cell Endocrinol. 2014;382(1):377–384. doi:10.1016/j.mce.2013.10.021
8. Wang X, Du Q, Mao Z, et al. Combined treatment with artesunate and bromocriptine has synergistic anticancer effects in pituitary adenoma cell lines. Oncotarget. 2017;8(28):45874–45887.
9. Serratrice J, Disdier P, Habib G, Viallet F, Weiller PJ. Fibrotic valvular heart disease subsequent to bromocriptine treatment. Cardiol Rev. 2002;10(6):334–336. doi:10.1097/01.CRD.0000031463.83977.15
10. Tamasauskas A, Sinkunas K, Bunevicius A, Radziunas A, Skiriute D, Deltuva VP. Transsphenoidal surgery for microprolactinomas in women: results and prognosis. Acta Neurochir (Wien). 2012;154(10):1889–1893. doi:10.1007/s00701-012-1450-x
11. Liu S, Wang X, Lu J, et al. Ubenimex enhances the radiosensitivity of renal cell carcinoma cells by inducing autophagic cell death. Oncol Lett. 2016;12(5):3403–3410. doi:10.3892/ol.2016.5036
12. Wickstrom M, Larsson R, Nygren P, Gullbo J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011;102(3):501–508. doi:10.1111/j.1349-7006.2010.01826.x
13. Liu S, Xie F, Wang H, et al. Ubenimex inhibits cell proliferation, migration and invasion in renal cell carcinoma: the effect is autophagy-associated. Oncol Rep. 2015;33(3):1372–1380. doi:10.3892/or.2014.3693
14. Burgess DJ. Apoptosis: refined and lethal. Nat Rev Cancer. 2013;13(2):79. doi:10.1038/nrc3462
15. Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–1010. doi:10.1038/nrm2529
16. Kinarivala N, Patel R, Boustany RM, Al-Ahmad A, Trippier PC. Discovery of Aromatic Carbamates that Confer Neuroprotective Activity by Enhancing Autophagy and Inducing the Anti-Apoptotic Protein B-Cell Lymphoma 2 (Bcl-2). J Med Chem. 2017;60(23):9739–9756. doi:10.1021/acs.jmedchem.7b01199
17. Makoukji J, Saadeh F, Mansour KA, et al. Flupirtine derivatives as potential treatment for the neuronal ceroid lipofuscinoses. Ann Clin Transl Neurol. 2018;5(9):1089–1103. doi:10.1002/acn3.625
18. Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1(2):66–74. doi:10.4161/auto.1.2.1738
19. Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–252. doi:10.1016/j.ccr.2006.08.009
20. Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10(3):175–176. doi:10.1016/j.ccr.2006.08.015
21. Zou J, Zhang Y, Sun J, et al. Deoxyelephantopin Induces Reactive Oxygen Species-Mediated Apoptosis and Autophagy in Human Osteosarcoma Cells. Cell Physiol Biochem. 2017;42(5):1812–1821. doi:10.1159/000479537
22. Wang X, Liu Y, Liu W, et al. Ubenimex, an APN inhibitor, could serve as an antitumor drug in RT112 and 5637 cells by operating in an Aktassociated manner. Mol Med Rep. 2018;17(3):4531–4539. doi:10.3892/mmr.2018.8402
23. Wang X, Liu Y, Wu R, et al. Role of ubenimex as an anticancer drug and its synergistic effect with Akt inhibitor in human A375 and A2058 cells. Onco Targets Ther. 2018;11:943–953. doi:10.2147/OTT.S157480
24. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi:10.1016/j.cell.2010.01.028
25. Ogasawara N, Matsushima M, Kawamura N, et al. Modulation of immunological activity on macrophages induced by diazinon. Toxicology. 2017;379:22–30. doi:10.1016/j.tox.2017.01.014
26. Hung CM, Liu LC, Ho CT, Lin YC, Way TD. Pterostilbene Enhances TRAIL-Induced Apoptosis through the Induction of Death Receptors and Downregulation of Cell Survival Proteins in TRAIL-Resistance Triple Negative Breast Cancer Cells. J Agric Food Chem. 2017;65(51):11179–11191. doi:10.1021/acs.jafc.7b02358
27. Sekine K, Fujii H, Abe F. Induction of apoptosis by bestatin (ubenimex) in human leukemic cell lines. Leukemia. 1999;13(5):729–734.
28. Han L, Zhang Y, Liu S, et al. Autophagy flux inhibition, G2/M cell cycle arrest and apoptosis induction by ubenimex in glioma cell lines. Oncotarget. 2017;8(64):107730–107743. doi:10.18632/oncotarget.22594
29. Liang W, Gao B, Xu G, Weng D, Xie M, Qian Y. Possible contribution of aminopeptidase N (APN/CD13) to migration and invasion of human osteosarcoma cell lines. Int J Oncol. 2014;45(6):2475–2485. doi:10.3892/ijo.2014.2664
30. Li J, Wang X, Hou J, Huang Y, Zhang Y, Xu W. Enhanced anticancer activity of 5-FU in combination with Bestatin: evidence in human tumor-derived cell lines and an H22 tumor-bearing mouse. Drug Discov Ther. 2015;9(1):45–52. doi:10.5582/ddt.2015.01006
31. Yamashita M, Wada H, Eguchi H, et al. A CD13 inhibitor, ubenimex, synergistically enhances the effects of anticancer drugs in hepatocellular carcinoma. Int J Oncol. 2016;49(1):89–98. doi:10.3892/ijo.2016.3496
32. Bauvois B, Dauzonne D. Aminopeptidase-N/CD13 (EC 3. 4.11.2)inhibitors: chemistry, biological evaluations, and therapeutic prospects. Med Res Rev. 2006;26(1):88–130. doi:10.1002/med.20044
33. Ichinose Y, Genka K, Koike T, et al. Randomized double-blind placebo-controlled trial of bestatin in patients with resected stage I squamous-cell lung carcinoma. J Natl Cancer Inst. 2003;95(8):605–610. doi:10.1093/jnci/95.8.605
34. Niimoto M, Hattori T. Prospective randomized controlled study on bestatin in resectable gastric cancer. Biomed Pharmacother. 1991;45(2–3):121–124.
35. Uchibayashi T, Kunimi K, Yamamoto H, Koshida K. Adjuvant therapy with 5-fluoro-1-(2-tetrahydrofuryl)-2,4 (1H,3H)-pyrimidinedione (UFT) and Bestatin in patients with transitional cell carcinoma of the bladder–comparison between UFT therapy alone and UFT therapy in combination with Bestatin. Int J Clin Pharmacol Ther. 1995;33(8):465–468.
36. Tsukamoto H, Shibata K, Kajiyama H, Terauchi M, Nawa A, Kikkawa F. Aminopeptidase N (APN)/CD13 inhibitor, Ubenimex, enhances radiation sensitivity in human cervical cancer. BMC Cancer. 2008;8:74. doi:10.1186/1471-2407-8-172
37. Grilo AL, Mantalaris A. Apoptosis: a mammalian cell bioprocessing perspective. Biotechnol Adv. 2019;37(3):459–475. doi:10.1016/j.biotechadv.2019.02.012
38. Bailey LJ, Cluett TJ, Reyes A, et al. Mice expressing an error-prone DNA polymerase in mitochondria display elevated replication pausing and chromosomal breakage at fragile sites of mitochondrial DNA. Nucleic Acids Res. 2009;37(7):2327–2335. doi:10.1093/nar/gkp091
39. Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477.
40. Wang W, Luo SM, Ma JY, Shen W, Yin S. Cytotoxicity and DNA Damage Caused from Diazinon Exposure by Inhibiting the PI3K-AKT Pathway in Porcine Ovarian Granulosa Cells. J Agric Food Chem. 2019;67(1):19–31. doi:10.1021/acs.jafc.8b05194
41. Mittal S, Sharma PK, Tiwari R, et al. Impaired lysosomal activity mediated autophagic flux disruption by graphite carbon nanofibers induce apoptosis in human lung epithelial cells through oxidative stress and energetic impairment. Part Fibre Toxicol. 2017;14(1):15. doi:10.1186/s12989-017-0194-4
42. Toshiyama R, Konno M, Eguchi H, et al. Poly(ethylene glycol)-poly(lysine) block copolymer-ubenimex conjugate targets aminopeptidase N and exerts an antitumor effect in hepatocellular carcinoma stem cells. Oncogene. 2019;38(2):244–260. doi:10.1038/s41388-018-0406-x
43. Qian X, He J, Zhao Y, Lin M. Inhibition of p38 MAPK Phosphorylation Is Critical for Bestatin to Enhance ATRA-Induced Cell Differentiation in Acute Promyelocytic Leukemia NB4 Cells. Am J Ther. 2016;23(3):e680–e689. doi:10.1097/01.mjt.0000433950.01406.b3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.