Back to Journals » Drug Design, Development and Therapy » Volume 11
Transcriptome analysis of endometrial tissues following GnRH agonist treatment in a mouse adenomyosis model
Authors Guo S, Lu X, Gu R, Zhang D, Sun Y, Feng Y
Received 16 November 2016
Accepted for publication 2 February 2017
Published 9 March 2017 Volume 2017:11 Pages 695—704
DOI https://doi.org/10.2147/DDDT.S127889
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Song Guo,1,* Xiaowei Lu,1,* Ruihuan Gu,2 Di Zhang,3 Yijuan Sun,2 Yun Feng1
1Department of Obstetrics and Gynecology, Reproductive Medicine Center, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China; 2Gynecology, Shanghai Ji Ai Genetics & In Vitro Fertilization Institute, Obstetrics and Gynecology Hospital, Fudan University, Shanghai, People’s Republic of China; 3Department of Gynecology and Obstetrics, Jinan Military General Hospital, Jinan, People’s Republic of China
*These authors contributed equally to this work
Purpose: Adenomyosis is a common, benign gynecological condition of the female reproductive tract characterized by heavy menstrual bleeding and dysmenorrhea. Gonadotropin-releasing hormone (GnRH) agonists are one of the medications used in adenomyosis treatment; however, their underlying mechanisms are poorly understood. Moreover, it is difficult to obtain endometrial samples from women undergoing such treatment. To overcome this, we generated an adenomyosis mouse model, which we treated with an GnRH agonist to determine its effect on pregnancy outcomes. We also analyzed endometrial gene expression following GnRH agonist treatment to determine the mechanisms that may affect pregnancy outcome in individuals with adenomyosis.
Methods: Neonatal female mice were divided into a control group, an untreated adenomyosis group, and an adenomyosis group treated with a GnRH agonist (n=6 each). The pregnancy outcome was observed and compared among the groups. Then, three randomly chosen transcriptomes from endometrial tissues from day 4 of pregnancy were analyzed between the adenomyosis group and the GnRH agonist treatment group by RNA sequencing and quantitative reverse transcription polymerase chain reaction (PCR).
Results: The litter size was significantly smaller in the adenomyosis group than in the control group (7±0.28 vs 11±0.26; P<0.05). However, the average live litter size was increased (10±0.28 vs 7±0.28; P<0.05) after GnRH agonist treatment. Three hundred and fifty-nine genes were differentially expressed in the GnRH agonist-treated group compared with the untreated group (218 were downregulated and 141 were upregulated). Differentially expressed genes were related to diverse biological processes, including estrogen metabolism, cell cycle, and metabolite biosynthesis.
Conclusion: GnRH agonist treatment appears to improve the pregnancy outcome of adenomyosis in a mouse model. Besides pituitary down-regulation, other possible mechanisms such as the regulation of cell proliferation may play a role in this. These new insights into GnRH agonist mechanisms will be useful for future adenomyosis treatment.
Keywords: adenomyosis, GnRH agonist, mouse, RNA-seq, pregnancy outcome
Introduction
Uterine adenomyosis is a benign gynecological disease that occurs in 19.5% of women of childbearing age.1 The pathological features of adenomyosis are invasion of the endometrium into the myometrium and ectopic endometrial glands surrounded by hypertrophic and hyperplastic myometrium.2 Menorrhagia, pelvic pain, dysmenorrhea, and infertility are the main clinical manifestations.3,4 Patients with adenomyosis have lower implantation, clinical pregnancy, and ongoing pregnancy rates, and an increased miscarriage rate compared with patients without adenomyosis.5 Thus, adenomyosis has a negative effect on female fertility. Several measures have been used to improve pregnancy outcomes, including a levonorgestrel-releasing intrauterine system, surgery, and gonadotropin-releasing hormone (GnRH) agonists.6–10
GnRH agonists are modeled after natural GnRH with chemical modifications of the sixth and tenth amino acids to improve efficacy.11 The actions of GnRH agonists are mediated through binding to and activation of the GnRH receptor in different tissues or organs.12 The GnRH receptor is predominantly distributed in the pituitary. When GnRH agonists bind to the pituitary GnRH receptor, the receptor initially induces increased secretion of gonadotropin, which is often referred to as the “flare-up effect” and lasts about 10 days. Then another effect, termed “pituitary down-regulation”, leads to inhibition of follicle-stimulating hormone and luteinizing hormone secretion and a further decrease in the secretion of ovarian hormones.13
The use of GnRH agonist as a noninvasive and effective therapy for adenomyosis is widespread in assisted reproductive technology.14,15 However, their mechanism of action is unclear. In the clinic, patients with adenomyosis who undergo GnRH agonist treatment are almost immediately ready for embryo transfer, so it is difficult to obtain endometrial samples for analysis during the window of implantation time. Therefore, mouse models of adenomyosis are a good alternative, but it has not yet been determined whether GnRH agonists improve pregnancy outcomes in mice with adenomyosis. To answer this, we evaluated pregnancy outcomes after GnRH agonist treatment in a mouse model of adenomyosis and used high-throughput RNA sequencing (RNA-Seq) data to compare the transcriptomes of endometrial tissues before and after treatment to investigate the effect of GnRH agonist therapy on endometrial gene expression with the aim of determining its mode of action.
Material and methods
Study design
Our experiments were divided into two parts. In the first part, the pregnancy outcomes were compared between the control group, the adenomyosis group, and the GnRH agonist treatment group. In the second part, RNA-seq-based transcriptome analysis was used to identify differentially expressed genes and affected pathways after GnRH agonist treatment.
Animals and treatment
In this study, a mouse adenomyosis model was generated using previously published methods.16–19 Five pregnant Institute of Cancer Research strain mice were purchased from Shanghai Laboratory Animal Corporation (Shanghai, People’s Republic of China), and each was housed in a single cage during the perinatal period. Female offspring were selected for use in this study. All mice were housed in the same temperature-controlled room, with alternating 12-h light/dark periods, and allowed free access to food and water.
For the first experiment, groups of 16 female neonatal mice were dosed orally with 2.7 μmol/kg tamoxifen (Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd., Shanghai, People’s Republic of China) suspended in a peanut oil/lecithin/condensed milk mixture (2:0.2:3 ratio, by volume) on days 2–5 after birth (day of birth = day 1). A control group of eight mice were fed the same amount of solvent without tamoxifen. After 75 days,16,20 the 16 tamoxifen-treated mice were split into two groups. The first group (n=8) received a single 8mg GnRH agonist trip (Ferring GmbH, Kiel, Germany) injected intraperitone.21 The second group (n=8) of control mice received the same amount of solvent with no GnRH agonist. Four weeks after the GnRH agonist injection, female mice were mated with male mice from 19:00–07:00 overnight. Females were mated only once, and male mice all had a history of fertility. The following morning, females were checked for the presence of a vaginal plug. The day of vaginal plug formation was considered to be day 1 of pregnancy. Pregnant mice were bred in a single cage and allowed free access to food and water. The average litter size at birth was determined 30 days after mating. The farrowing rate was defined as the total number of litters divided by the total number of matings.22
For the second experiment, 16 newborn female offspring were randomly divided into two equal groups: an untreated adenomyosis model group and an adenomyosis group treated with a GnRH agonist. The mice were then treated as described for the first experiment.
Tissue collection and application
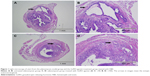
Mice were euthanized at 19:00–20:00 on day 4 of pregnancy.23,24 For histology, uteri were removed and fixed in 10% formalin overnight, followed by dehydration in 70% ethanol. The tissues were paraffin embedded, then sectioned and stained with hematoxylin and eosin (H&E) and smooth muscle actin. Three randomly selected sections were chosen for histopathology. These two testing methods were used to determine whether the mouse model of adenomyosis was successfully established. If the two tests were both positive, a diagnosis of adenomyosis was made and the endometrium was used for RNA-seq. The endometrial tissues were scraped off using a curved needle, then snap-frozen in liquid nitrogen and stored at −80°C for RNA-seq.
All experiments were performed under the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the Institutional Experimental Animals Review Board of Ruijin Hospital affiliated with Shanghai Jiaotong University School of Medicine.
Immunohistochemistry
After deparaffinization, sections were incubated in 3% H2O2 for 30 min to block endogenous peroxidase activity. Sections were rinsed and blocked with 10% normal goat serum (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, Fujian Province, People’s Republic of China) for 30 min, then incubated overnight at 4°C with mouse anti-a-SMA primary antibody (1:2000 dilution; GB13036, Wuhan Goodbio Technology Co., Ltd., Wuhan, Hubei Province, People’s Republic of China). After three washes in phosphate-buffered saline, the sections were incubated with the appropriate secondary antibodies (Proteintech Group, Inc., Chicago, IL, USA) for 1 h at room temperature. Mice uteri were then analyzed, focusing on the myometrium. If glandular tissue invading the myometrium was present, the adenomyosis model was considered to be successfully established.
RNA extraction and quality control
Total RNA was extracted from endometrial tissue from the two groups (each group contained three randomly chosen samples) using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The concentration of each sample was measured using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The raw sequencing data were evaluated using FastQC (Babraham Institute, Cambridge, UK). The data included the distribution of nucleotides, position-specific sequencing quality, GC base pairs content, proportion of any general-PCR duplications, and w-mer frequencies. These evaluation metrics were used to understand the nature of the data.
RNA-seq analysis
Raw reads of 50 base pairs or greater that passed filtering were used for mapping. MapSplice,25 an efficient splice junction mapper for RNA-seq reads, was used to align the reads and fix gapped alignments. Then, a mapping step was used to identify spliced alignments. We applied the DEseq algorithm26 to filter the genes differentially expressed between the two groups using a false discovery rate (FDR) threshold of 0.05.
Cluster analysis
Cluster analysis was used to identify the global trends and to model profiles of expression. Fisher’s exact test and the multiple comparison test27,28 were applied to identify the statistically significant model profiles.
Gene ontology (GO) analysis
GO analysis was used to analyze the biological implications of unique genes in the significant profiles.29 Fisher’s exact and chi squared tests were applied to classify significant GO categories, and the FDR30 was used to correct the P-value. Generally, when the FDR is set low, the P-value is more accurate.
Pathway analysis
Pathway analysis was applied to determine the significant pathways of differentially expressed genes according to Kyoto Encyclopedia of Genes and Genomes (KEGG), MapSplice, and Reactome databases.25,31,32 Fisher’s exact test was used to identify significantly enriched pathways, and the threshold of significance was defined as P<0.05 and FDR <0.05.33 The level of enrichment indicates the significance of the altered pathway.
Quantitative reverse transcription PCR (RT-qPCR)
RT-qPCR was performed to validate the gene expression data obtained from deep sequencing. Total mRNA was extracted using TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. The first strand of cDNA was synthesized using primers designed in our lab (Table 1). The RT product was amplified using SYBR Green on a 7500 Real-Time PCR System (Thermo Fisher Scientific Inc. Waltham, MA, USA). All samples were run in triplicate, and relative gene expression was analyzed according to the 2−ΔΔCt method. The housekeeping gene Actb was used for normalization of the expression data.
  | Table 1 RT–qPCR primers used in the study |
Statistical analysis
Statistical differences were analyzed using Statistical Package for the Social Sciences (SPSS) software (17.0) (SPSS Inc., Chicago, IL, USA). Expression levels among different groups were analyzed using an unpaired Student’s t-test. The results are represented as the mean ± standard deviation (SD), and any differences between means were considered statistically significant at P<0.05.
Results
Modeling results
The diagnosis of adenomyosis depends on pathological examination and immunohistochemistry. There was a concordance between H&E (Figure 1) and smooth muscle actin staining (Figure 2). During this experiment, two mice died in the GnRH agonist treatment group. Model establishment was unsuccessful in only one mouse, suggesting that the treatment of neonatal mice with tamoxifen is an effective method of modeling adenomyosis.
Pregnancy outcome
Compared with the control group, a significantly lower average litter size was found in the adenomyosis group (7±0.28 vs 11±0.26; P<0.05). However, after GnRH agonist treatment, the average litter size of mice with adenomyosis increased significantly (10±0.28 vs 7±0.28; P<0.05). No significant difference in the farrowing rate was found among these three groups (Table 2).
Gene expression changes
Compared with the adenomyosis group, 359 genes were at least twofold differentially expressed (P<0.05) in the endometrium of the GnRH agonist treatment group: 218 were downregulated and 141 were upregulated (Figure 3). The sequencing data of the present study are available in the Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo/info/linking.html) under accession numbers GSE89463.
  | Figure 3 Global expression profiles of the adenomyosis group and GnRH agonist-treated group analyzed by cluster analysis. |
Gene ontology analysis
Significantly enriched GO terms from the two groups were related to the cell cycle, including protein glycosylation, protein citrullination, the urea cycle, and transmembrane transport (Figure 4). GO terms related to inflammation were also enriched. This category included genes involved in the positive regulation of neutrophil chemotaxis.
Pathway analysis
Pathway analysis was used to identify significantly altered pathways according to the KEGG database. Twenty-eight pathways were significantly enriched with the identified differentially expressed genes: 20 were downregulated and eight were upregulated (P<0.05; Figure 4). These altered pathway terms were mainly metabolic pathways such as mucin type O-glycan biosynthesis, folate biosynthesis, and the metabolism of xenobiotics by cytochrome P450. They also included cell proliferation signal pathways such as the Rap1 signaling pathway and phosphatidylinositol 3-kinase-Akt signaling pathway.
Confirmation of RNA-Seq results by RT-qPCR
We analyzed the expression of 10 genes by RT-qPCR based on fold changes in expression shown in RNA-Seq and prospective therapeutic value to verify our data. These were Sprr2a1, Pla2g2e, Galnt4, Mgat4a, Muc13, Tff1, Aldh3b2, B4galnt3, Abca8b, and Cbr2. The expression ratios of these genes determined by RT-qPCR were consistent with those from RNA-seq analysis (Figure 5).
Discussion
Litter size is one important parameter in the assessment of female productivity.34 In our present study, litter size was smaller in the adenomyosis group than in the control group, suggesting that adenomyosis is harmful to pregnancy outcome in mice. These results are consistent with the clinical observations in humans that adenomyosis is associated with subfertility through reduced endometrial receptivity and impaired decidualization,35 while a separate study reported that adenomyosis is linked to infertility in humans.36 To our knowledge, our study is the first to analyze the effect of adenomyosis on pregnancy outcome in a mouse model. Previous research has focused on the relationship between uterine muscle contraction and adenomyosis in mice.19,37 The mechanisms behind these effects may be related to the induction of inflammatory factors,38 the increased production of oxygen free radicals,39 and abnormal uterine contraction.40 Importantly, we found that the average litter size was increased after GnRH agonist treatment, suggesting that GnRH agonist treatment may improve pregnancy outcome in mice as it does in humans. However, no significant difference in farrowing rate was found among these three groups, possibly because of the small number of female mice in each group.
GnRH agonists inhibit the secretion of follicle-stimulating hormone and luteinizing hormone, and decrease the secretion of ovarian hormones.13 These effects are considered to be the main mechanism underlying the treatment of adenomyosis. Like endometriosis, adenomyosis is an estrogen-dependent disorder. Moreover, a high local concentration of estradiol in endometriotic lesions contributes to the progression of endometriosis.41 GnRH agonists reduce the estradiol content in endometriotic lesions through pituitary down-regulation, leading to atrophy of ectopic endometrium. Through transcriptomic analysis in the present study, we found that the expression of estrogen-linked genes such as Tff1 and Sprr2a1 was significantly decreased in mice treated with a GnRH agonist. These results were verified with GO and pathway interaction analysis.
Many of the genes showing altered expression levels in our study, such as Aldh3b2, are involved in the metabolism of xenobiotics through the cytochrome P450 pathway. Cytochrome P450 aromatase is a key enzyme in estrogen biosynthesis that catalyzes the conversion of androgens to estrogens. Using pathway interaction analysis, we found that the cytochrome P450 pathway was significantly altered following GnRH agonist treatment in the adenomyosis mouse model. Our sequencing results are consistent with a previous analysis of cytochrome P450 aromatase expression in endometriotic and adenomyotic tissues.42 To our knowledge, Tff1, Sprr2a1, and Aldh3b2 have not previously been implicated in adenomyosis. Tff1 is an estrogen response gene that has been reported to be a potential tumor marker in the diagnosis of breast cancer.43–45 Sprr2a is an estrogen-responsive gene46 that encodes small proline-rich protein (SPRR)2a, which is a member of the SPRR family. This binds to and activates SH3 domain-containing proteins, resulting in physiological effects in a broad range of tissues.47 Apart from its important role as an estrogen moderator, Sprr2a has wound healing and inflammation regulatory functions48 We found that Tff1 and Sprr2a1 were downregulated in the adenomyosis mouse model, implying that an increased estrogen level is associated with adenomyosis, and that an impaired wound healing ability of the endometrium may be involved in the development of adenomyosis. These findings are consistent with a recent study that identified a “tissue injury and repair” mechanism in the development of adenomyosis, in which uterine peristalsis led to the invasion of endometrial tissue into the myometrium, resulting in adenomyosis49 In the present study, Tff1 and Sprr2a1 were upregulated following GnRH agonist treatment, suggesting that GnRH agonists may decrease the production of estrogen and improve the repair capacity of the endometrium. Few studies have been carried out into the role of Aldh3b2, and these are limited to neuroblastomas50 and keratinocytes,51 not adenomyosis. Nevertheless, our results, together with previous observations, increase our understanding of the pathogenesis of adenomyosis and the mechanism of action of GnRH agonists in the promotion of wound healing.
Recent studies have shown that the GnRH receptor is expressed in many extrapituitary organs, such as the ovary, endometrium, and prostate.52 Rather than using the classical GnRH receptor-signaling pathway, GnRH agonists might therefore act directly on the endometrium through locally expressed GnRH receptors.53 A previous study reported that gonadotropin down-regulation was achieved after injection of an GnRH agonist every 12 h for 5 consecutive days in mice.54 The mice in the current study were able to conceive up to 4 weeks after the GnRH agonist injection, suggesting that the pituitary down-regulation effect might have been attenuated or eliminated. It also indicates that other mechanisms or functions might underlie GnRH agonist action in adenomyosis therapy. Recent molecular studies have indicated a direct effect of GnRH agonists on endometrial function. By binding to specific receptors present on the endometrium, the GnRH agonists regulate several paracrine factors such as transforming growth factor, matrix metalloproteinase, and L-selectin, which play important roles in embryo implantation.55
In adenomyosis, the endometrium has been shown to invade hyperplastic myometrial fibers, causing alterations in the junctional zone.56 Khan et al12 investigated changes in tissue inflammation, angiogenesis, and apoptosis in tissues collected from women with endometriosis, adenomyosis, and uterine myomas. They showed that GnRH agonist therapy significantly reduced inflammatory reactions and induced a remarkable degree of apoptosis in eutopic endometrium and lesions. On the basis of these findings, we hypothesized that GnRH agonists might inhibit the proliferation and induce the apoptosis of eutopic endometrium cells. Our current observation that the expression of Galnt4, Pla2g2e, and Mgat4a decreased after the intraperitoneal injection of a GnRH agonist is in line with these findings because these genes are responsible for cellular protein biosynthesis and ATP metabolism in human endometrial glandular cells. Moreover, these results agree with the GO and pathway interaction analyses, which indicated that a GnRH agonist might play important roles in proliferation, apoptosis, and the cell cycle in endometrial glandular cells.
Our study has two main limitations. The first is the lack of confirmation of our results using human samples. Even though we have shown that mice are a good animal model for studying adenomyosis, they cannot reflect the natural course of the human disease. Hence, any extrapolation of our findings to humans should be done with caution. Therefore, the differentially expressed genes identified in our mouse model should be validated in patients with adenomyosis. Second, the transcriptomic analysis is only a preliminary step that does not reveal the full mechanism of action of GnRH agonists. Genes that are shortlisted by this analysis should be investigated using in vitro or in vivo models involving overexpression/knockdown studies, protein analyses, and functional assays. We plan to carry out these experiments in our future research.
Conclusion
In conclusion, adenomyosis can be detrimental to pregnancy; however, the pregnancy outcome may be improved by GnRH agonist treatment. In addition to the possible reduction of pituitary down-regulation by GnRH agonist therapy, the reduced proliferation and increased apoptosis of endometrial cells may contribute to the treatment of adenomyosis. Our research casts new light on the roles of GnRH agonists, but it is not possible to conclude that these mechanisms explain the improvement in fertility by GnRH agonists because this study is based on transcriptome analysis alone. Further study is needed to clarify the mechanisms through which GnRH agonists act in treating adenomyosis, which may involve the synergistic action of multiple factors.
Acknowledgments
This work was supported by the Shanghai Municipal Health and Family Planning Commission Fund (Grant number: 201640367) and the MerckSerono China Research Fund for Fertility Experts (Grant number: None).
Disclosure
The authors report no conflicts of interest in this work.
References
Devlieger R, D’Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9(2):139–147. | ||
Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98(3):572–579. | ||
Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4(4):312–322. | ||
Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18(4):374–392. | ||
Salim R, Riris S, Saab W, Abramov B, Khadum I, Serhal P. Adenomyosis reduces pregnancy rates in infertile women undergoing IVF. Reprod Biomed Online. 2012;25(3):273–277. | ||
Fedele L, Bianchi S, Raffaelli R, Portuese A, Dorta M. Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intrauterine device. Fertil Steril. 1997;68(3):426–429. | ||
Fujishita A, Masuzaki H, Khan KN, Kitajima M, Ishimaru T. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57(3):132–138. | ||
Grow DR, Filer RB. Treatment of adenomyosis with long-term GnRH analogues: a case report. Obstet Gynecol. 1991;78(3 Pt 2):538–539. | ||
Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79(3):189–193. | ||
Wang PH, Fuh JL, Chao HT, Liu WM, Cheng MH, Chao KC. Is the surgical approach beneficial to subfertile women with symptomatic extensive adenomyosis? J Obstet Gynaecol Res. 2009;35(3):495–502. | ||
Li X, Kang X, Deng Q, Cai J, Wang Z. Combination of a GnRH agonist with an antagonist prevents flare-up effects and protects primordial ovarian follicles in the rat ovary from cisplatin-induced toxicity: a controlled experimental animal study. Reprod Biol Endocrinol. 2013;11:16. | ||
Khan KN, Kitajima M, Hiraki K, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod. 2010;25(11):2878–2790. | ||
Hodgen GD. GnRH Analogs in reproductive medicine. Keio J Med. 1991;40(1):25–32. | ||
Lin J, Sun C, Zheng H. Gonadotropin-releasing hormone agonists and laparoscopy in the treatment of adenomyosis with infertility. Chin Med J (Engl). 2000;113(5):442–445. | ||
Silva PD, Perkins HE, Schauberger CW. Live birth after treatment of severe adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril. 1994;61(1):171–172. | ||
Parrott E, Butterworth M, Green A, White IN, Greaves P. Adenomyosis-a result of disordered stromal differentiation. Am J Pathol. 2001;159(2):623–630. | ||
Green AR, Edwards RE, Greaves P, White IN. Comparison of the effect of oestradiol, tamoxifen and raloxifene on nerve growth factor-alpha expression in specific neonatal mouse uterine cell types using laser capture microdissection. J Mol Endocrinol. 2003;30(1):1–11. | ||
Koike N, Tsunemi T, Uekuri C, et al. Pathogenesis and malignant transformation of adenomyosis (review). Oncol Rep. 2013;29(3):861–867. | ||
Zhu B, Chen Y, Zhang H, Liu X, Guo SW. Resveratrol reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice with induced adenomyosis. Reprod Sci. 2015;22(11):1336–1349. | ||
Greaves P, White IN. Experimental adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):503–510. | ||
Lotz L, Schneider H, Hackl J, et al. Does stimulation with human gonadotropins and gonadotropin-releasing hormone agonist enhance and accelerate the developmental capacity of oocytes in human ovarian tissue xenografted into severe combined immunodeficient mice? Fertil Steril. 2014;101(5):1477–1484. | ||
Baroncello E, Bernardi ML, Kummer AD, Wentz I, Bortolozzo FP. Fixed-time post-cervical artificial insemination in weaned sows following buserelin use combined with/without eCG. Reprod Domest Anim. 2017;51(1):76–82. | ||
Chu B, Zhong L, Dou S, et al. miRNA-181 regulates embryo implantation in mice through targeting leukemia inhibitory factor. J Mol Cell Biol. 2015;7(1):12–22. | ||
Guo C, Meng X, Bai J, et al. Expression and localization of transcription factors SNAIL and SLUG in mouse ovaries and pre-implantation embryos. Cell Tissue Res. 2014;358(2):585–595. | ||
Wang K, Singh D, Zeng Z, et al. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010;38(18):e178. | ||
Yao JQ, Yu F. DEB: a web interface for RNA-seq digital gene expression analysis. Bioinformation. 2011;7(1):44–45. | ||
Miller LD, Long PM, Wong L, Mukherjee S, McShane LM, Liu ET. Optimal gene expression analysis by microarrays. Cancer Cell. 2002;2(5):353–361. | ||
Ramoni MF, Sebastiani P, Kohane IS. Cluster analysis of gene expression dynamics. Proc Natl Acad Sci U S A. 2002;99(14):9121–9126. | ||
Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. | ||
Dupuy D, Bertin N, Hidalgo CA, et al. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25(6):663–668. | ||
Joshi-Tope G, Gillespie M, Vastrik I, et al. Reactome: a knowledge base of biological pathways. Nucleic Acids Res. 2005;33:D428–D432. | ||
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. | ||
Draghici S, Khatri P, Tarca AL, et al. A systems biology approach for pathway level analysis. Genome Res. 2007;17(10):1537–1545. | ||
Johnson RK, Nielsen MK, Casey DS. Responses in ovulation rate, embryonal survival, and litter traits in swine to 14 generations of selection to increase litter size. J Anim Sci. 1999;77(3):541–557. | ||
Jiang Y, Jiang R, Cheng X, et al. Decreased expression of NR4A nuclear receptors in adenomyosis impairs endometrial decidualization. Mol Hum Reprod. 2016;22(9):655–668. | ||
Puente JM, Fabris A, Patel J, et al. Adenomyosis in infertile women: prevalence and the role of 3D ultrasound as a marker of severity of the disease. Reprod Biol Endocrinol. 2016;14(1):60. | ||
Chen Y, Zhu B, Zhang H, Liu X, Guo SW. Epigallocatechin-3-Gallate reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice induced with adenomyosis. Reprod Sci. 2013;20(12):1478–1491. | ||
Yang JH, Wu MY, Chang DY, Chang CH, Yang YS, Ho HN. Increased interleukin-6 messenger RNA expression in macrophage-cocultured endometrial stromal cells in adenomyosis. Am J Reprod Immunol. 2006;55(3):181–187. | ||
Campo S, Campo V, Benagiano G. Adenomyosis and infertility. Reprod Biomed Online. 2012;24(1):35–46. | ||
Kissler S, Hamscho N, Zangos S, et al. Uterotubal transport disorder in adenomyosis and endometriosis – a cause for infertility. BJOG. 2006;113(8):902–908. | ||
Huhtinen K, Desai R, Ståhle M, et al. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J Clin Endocrinol Metab. 2012;97(11):4228–4235. | ||
Kitawaki J, Noguchi T, Amatsu T, et al. Expression of aromatase cytochrome p450 protain and messenger ribouncleic acid in human endometriotic and adenomyiotic tissues but not in normal endometrium. Biol Reprod. 1997;57(3):514–519. | ||
Corte MD, Tamargo F, Alvarez A, et al. Cytosolic levels of TFF1/pS2 in breast cancer: their relationship with clinical-pathological parameters and their prognostic significance. Breast Cancer Res Treat. 2006;96:63–72. | ||
Chen Y, Chen C, Yang B, et al. Estrogen receptor–related genes as an important panel of predictors for breast cancer response to neoadjuvant chemotherapy. Cancer Lett. 2011;302:63–68. | ||
Gatti-Mays ME, Venzon D, Galbo CE, et al. Exemestane use in postmenopausal women at high risk for invasive breast cancer: evaluating biomarkers of efficacy and safety. Cancer Prev Res (Phila). 2016;9(3):225–233. | ||
Burns KA, Zorrilla LM, Hamilton KJ, Reed CE, Birnbaum LS, Korach KS. A single gestational exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin disrupts the adult uterine response to estradiol in mice. Toxicol Sci. 2013;136(2):514–526. | ||
Mizuguchi Y, Isse K, Specht S, et al. Small proline rich protein 2a in benign and malignant liver disease. Hepatology. 2014;59(3):1130–1143. | ||
Mizuguchi Y, Specht S, Isse K, et al. Breast tumor kinase/protein tyrosine kinase 6 (Brk/PTK6) activity in normal and neoplastic biliary epithelia. J Hepatol. 2015;63(2):399–407. | ||
Shaked S, Jaffa AJ, Grisaru D, Elad D. Uterine peristalsis-induced stresses within the uterine wall may sprout adenomyosis. Biomech Model Mechanobiol. 2015;14(3):437–444. | ||
Hartomo TB, Van Huyen Pham T, Yamamoto N, et al. Involvement of aldehyde dehydrogenase 1A2 in the regulation of cancer stem cell properties in neuroblastoma. Int J Oncol. 2015;46(3):1089–1098. | ||
Naganuma T, Takagi S, Kanetake T, et al. Disruption of the Sjögren-Larsson syndrome gene Aldh3a2 in mice increases keratinocyte growth and retards skin barrier recovery. J Biol Chem. 2016;291(22):11676–11688. | ||
Aguilar R, Huerta-Reyes M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (Review). Oncol Rep. 2009;22(5):981–990. | ||
Yildiz GA, Sukur YE, Ates C, Aytac R. The addition of gonadotrophin releasing hormone agonist to routine luteal phase support in intracytoplasmic sperm injection and embryo transfer cycles: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2014;182:66–70. | ||
Hasky N, Uri-Belapolsky S, Goldberg K, et al. Gonadotrophin-releasing hormone agonists for fertility preservation: unraveling the enigma? Hum Reprod. 2015;30(5):1089–1101. | ||
Vlahos NF, Lipari CW, Bankowski B, et al. Effect of luteal-phase support on endometrial L-selectin ligand expression after recombinant follicle-stimulating hormone and ganirelix acetate for in vitro fertilization. J Clin Endocrinol Metab. 2006;91(10):4043–4049. | ||
Pontis A, D’Alterio MN, Pirarba S, de Angelis C, Tinelli R, Angioni S. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol. 2016;5:1–5. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.