Back to Journals » International Journal of Nanomedicine » Volume 14
Toxicity of Carbon Nanotubes as Anti-Tumor Drug Carriers
Authors Yan H, Xue Z, Xie J, Dong Y, Ma Z, Sun X, Kebebe Borga D, Liu Z, Li J
Received 19 June 2019
Accepted for publication 25 November 2019
Published 31 December 2019 Volume 2019:14 Pages 10179—10194
DOI https://doi.org/10.2147/IJN.S220087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Hongli Yan,1,2,* Zhifeng Xue,1,2,* Jiarong Xie,1,2 Yixiao Dong,1,2 Zhe Ma,1,2 Xinru Sun,1,2 Dereje Kebebe Borga,1–3 Zhidong Liu,1,2 Jiawei Li2,4
1Engineering Research Center of Modern Chinese Medicine Discovery and Preparation Technique, Ministry of Education, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, People’s Republic of China; 2Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, People’s Republic of China; 3School of Pharmacy, Institute of Health Sciences, Jimma University, Jimma, Ethiopia; 4Institute of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhidong Liu
Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, No. 10, Poyang Lake Road, Jinghai, Tianjin 301617, People’s Republic of China
Tel +86 22 5959 6163
Email [email protected]
Jiawei Li
Institute of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, No. 10, Poyang Lake Road, Jinghai, Tianjin 301617, People’s Republic of China
Tel +86 22 5959 6352
Email [email protected]
Abstract: Nanoparticle drug formulations have enormous application prospects owing to achievement of targeted and sustained release drug delivery, improvement in drug solubility and reduction of adverse drug reactions. Recently, a variety of efficient drug nanometer carriers have been developed, among which carbon nanotubes (CNT) have been increasingly utilized in the field of cancer therapy. However, these nanotubes exert various toxic effects on the body due to their unique physical and chemical properties. CNT-induced toxicity is related to surface modification, degree of aggregation in vivo, and nanoparticle concentration. This review has focused on the potential toxic effects of CNTs utilized as anti-tumor drug carriers. The main modes by which CNTs enter target sites, the toxicity expressive types and the factors affecting toxicity are discussed.
Keywords: anti-tumor, cancer, CNTs, nanometer preparation, nanometer carrier, toxicity
Introduction
Malignant tumors are one of the leading causes of human disease and death, contributing to increasing mortality rates over the years.1 All clinically available anti-cancer drugs have several limitations, such as poor stability, low bioavailability,2 restricted targeting ability, degradation and potential drug resistance.3–5 Although breakthroughs have been achieved in the clinical field of oncology, various treatment options have been shown to cause damage to normal cells along with eliminating tumor cells, resulting in local or systemic toxicity. Therefore, development of a targeting drug system (TDS) that allows delivery of drugs to tumors while avoiding injury to normal tissue is essential to improve therapeutic efficacy. Nanometer TDS based on the advantages of nanotechnology has developed rapidly in recent years. Nanometer-targeted preparations have successfully achieved improved drug solubility and bioavailability and specific targeting of drugs to organs or cells, allowing sustained or controlled release, prolongation of drug retention times, and more rapid and efficient drug entry through physiological barriers. These preparations have enriched the selection range of pharmaceutical dosage forms and thus attracted considerable research attention. Nanometer materials are ubiquitous in biomedical fields, including in vivo imaging,6 cancer treatment,7 targeted transport8,9 and drug discovery.10
CNTs are a type of highly efficient nanometer TDS displaying adequate adsorption activity that have considerable potential as anticancer drugs with high selectivity for tumor sites.11,12 Recent in vitro studies have shown that CNTs internalize into mammalian cells easily, effectively transporting molecular cargo into the cytoplasm and potentially nucleus.13–16 CNTs mainly comprise multiple coaxial tubes composed of hexagonal carbon atoms. A seamless, hollow tubular novel nanometer material is rolled into a graphite sheet constituting carbon atom bonds (sp2 hybridization).17,18 According to the number of sp2 hybrid carbon atoms, CNTs are subdivided into single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT). Due to their unique structures, SWCNTs and MWCNTs display excellent physical, chemical, electrical and thermodynamic properties, such as ultra-high specific surface area, good adsorption ability, unique fluorescence, and Raman spectroscopy in the near-infrared region.19,20 CNTs can convert infrared light to heat and effectively utilize the property of poor heat resistance of tumor cells. At a tumor site temperature of >42°C, cell killing phenomena are evident, such as destruction of cell membrane, denaturation of proteins and irreversible damage of tumor cells,21,22 while normal cells remain intact. These anti-tumor effects are greatly enhanced upon coupling with anti-tumor drugs.23,24 Combination of CNTs with inorganic materials, polymers, can be utilized as a strategy to simultaneously diagnose and treat cancers.25–27 As a nanometer carrier type displaying high drug loading, strong targeting and easily penetrable cell membranes,28,29 CNTs are commonly employed in multiple biomedical fields, in particular, drug delivery30–33 and cancer treatment.34–36
On the other hand, the safety of clinical application of CNTs as anti-tumor drug carriers has been a subject of concern in recent years.37 Toxic effects exerted by CNTs mainly stems from their similarities in structure to asbestos fibers.38–40 Commonly reported toxicities include inflammatory response,41,42 malignant mesothelioma43,44 and biological persistence.45,46 A recent study by Ursini et al47 clearly demonstrated the toxicity of original MWCNTs. Moreover, functionalized MWCNTs (MWCNT-OH and MWCNT-COOH) exerted toxicity to specific cell types (e.g., human alveolar (A549) epithelial cells and normal bronchial (BEAS-2B) cells) through multiple mechanisms. However, inconsistent findings on the potential toxicity of CNTs have been obtained to date. A number of other studies have reported no damage or toxicity to normal tissue by CNTs.48,49 Induction of toxicity by anti-tumor nanometer preparations of carrier CNTs may be attributable to the specific methods used for the experiment and related to surface modification, degree of aggregation, and nanotube concentration.
In an earlier study, Jabr-Milane and co-workers bound doxorubicin (DOX) to the SWCNT complex for targeting WiDr colon cancer cells. Upon separation of the DOX-SWCNT complex, DOX was released into the nucleus while SWCNTs remained in the cytoplasm.50 Following injection of SWCNT into tumor-bearing mice, transmission electron microscopy (TEM) observation disclosed a large quantity of SWNT in urine of mice after 30 min. After 2 h, SWCNTs were collected from blood-rich tissues, such as liver and heart, and showed accumulation in the tumor area after 20 h.51 Free CNTs were preferentially distributed in normal tissues, giving rise to the concern that these nanomolecules may be more toxic to normal than tumor cells.52 This review has focused on the toxicity of CNTs used as anti-tumor carriers in terms of: (1) main routes used by CNTs to enter the target site; (2) toxicity expressive types; and (3) the factors affecting toxicity.
Entry Mechanisms of Anti-Tumor Carriers CNTs into Target Sites
Relative to the low cell permeability of macromolecules and small-molecule anticancer drugs, CNTs are considered a highly efficient novel material carrier for the delivery of anticancer drugs and diagnostic molecules.53,54 CNTs containing antitumor drugs should be delivered to cancer cells from the site of administration. Subsequently, free CNTs are dispersed from the target site to the excretory organ.55,56 The ability of CNTs to move forward in vivo may depend on their chemical reactivity, surface characteristics, and ability to combine with body proteins.57,58 CNTs transport anti-tumor drugs into target cells through two principal pathways: non-energy-dependent diffusion and energy-dependent endocytic pathways59,60 (Figure 1). The differences in access routes are related to CNT size.61 Kan and colleagues co-cultured SWNTs of different lengths with Hep G2 cells for 5 h. Confocal imaging and flow cytometry data disclosed that the internalization of L-SWNTs into cells mainly occurred through energy-dependent endocytosis. S-SWNTs partly utilized energy-independent pathways such as diffusion across the cell membranes for cell entry.62 The group of Imaninezhad further highlighted that integrins promote CNT entry into cells. In their experiments, CNTs were co-cultured with NIH 3T3 fibroblasts and PC12 neuron-like cells and randomly divided into two groups (one treated with an integrin inhibitor CWHM-96). Cells treated with CWHM-96 spread further and showed elongated shape while control cells with no CWHM-96 displayed round morphology. These findings confirmed that integrins promote CNT entry into cells, leading to more significant effects.
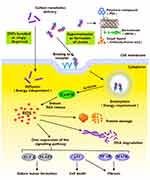 |
Figure 1 Schematic diagram illustrating the cells entering process of anti-tumor nanometer preparation with CNTs as a carrier. |
Non-Energy-Dependent Diffusion Pathways
The length and chemical nature of CNTs are the main factors affecting entry into target cells.63 Since the cell membrane consists of a phospholipid bilayer, CNTs in the non-energy diffusion pathway enter the cell based on their diminutive sizes (submicron-sized) and hydrophobicity. Pantarotto and co-workers64 incubated HeLa cells with CNTs containing sodium azide (an inhibitor of energy-dependent cellular processes). Molecular dynamics simulation revealed that cationic functional groups on the CNT surface bind the HeLa cell membrane surface, allowing spontaneous entry through cell membrane diffusion with no energy dependence. Raffa et al65 reached a similar conclusion that functionalized CNTs can effectively enter cells through non-energy-dependent internalization pathways.
Energy-Dependent Endocytic Pathways
Supramolecular CNTs complexes enter cells in a manner dependent on energy. The CNT surface is usually loaded with bio-macromolecules, such as antibodies, amino acids and siRNAs, which are often involved in energy-dependent endocytic pathways.66 Several studies have confirmed that CNTs enter the cell with the aid of endocytosis.67–71 In a study by Kayo et al, endocytosis was shown to incorporate a combination of three pathways: (1) clathrin-mediated endocytosis, (2) caveolae-mediated endocytosis, and (3) macropinocytosis.72 Lima and co-workers suggested that the endocytic pathway is divided into three stages: (1) cell membrane contact, (2) penetration of lipid head groups, and (3) entry of lipid tails. Moreover, entry of CNTs into cells depends on physicochemical characteristics, such as size and shape. CNTs with sizes ranging from 100 to 200 nm undergo clathrin-mediated endocytosis while CNTs <50 nm enter the cells through energy-independent passive diffusion.95 In a study conducted by Kam et al73 green fluorescence-labeled SWCNT (a) and SWCNT-biotin-green fluorescently streptavidin (b) were incubated with HL60 cells for 1 h at 37°C. Confocal microscopy revealed green fluorescence in group A and yellow fluorescence in group B (SWCNT showed green fluorescence and endosomes of red dots overlapped to produce yellow fluorescence). Their findings indicate that SWCNTs use the endocytotic pathway as a cellular uptake mechanism and accumulate in the cytoplasm after internalization. The group of Ke74 cultured HeLa cells with AO-SWCNTs (20 mg/mL) for 30 min at 37°C (AO binds DNA to emit green fluorescence and RNA to emit red fluorescence). TEM observations revealed green fluorescence in the cytoplasm of HeLa cells. After treatment with chlorpromazine (an endocytosis inhibitor), green fluorescence was attenuated. The authors concluded that CNTs enter cells through the endocytic pathway after transporting the anticancer drug to the target site.
When CNTs enter cells into the blood circulation, phagocytic cells of the immune system (neutrophils, eosinophils and macrophages) internalize nanoparticles and NADPH oxidase is activated for accumulation on the phagolysosomal membrane. Electrons are transferred to oxygen to form superoxide (ROS).75,76 The degradation of CNTs by neutrophils,77 macrophages78 and primary microglia79 has been further investigated. CNTs are removed mainly via enzymatic degradation. Hydrogen peroxide is converted to different acids that eliminates CNTs by peroxidases, such as human myeloperoxidase,80 the horseradish peroxidase system,81 and catalase.82,83 In addition, degradation of CNTs is associated with proteins, whereby binding to proteins enhances their ability to enter cells, thus promoting degradation.84–86 The group of Karimi87 conjugated actin to CNTs via covalent bonding, which was subsequently incubated with HeLa cells (immunofluorescence labeling) for 4 h. CNTs were indirectly modified by actin, as determined based on fluorescence intensity. Actin can generate mechanical forces to drive CNTs into the nucleus. Therefore, future studies should focus on utilization of the properties of enzymes and proteins in cells to enhance degradation of CNTs.
Oxidative stress is one of the leading causes of cytotoxicity.88,89 During the process of CNT entry into cells for degradation, high levels of ROS are induced, leading to destruction of cellular structure and enhanced lethality against cancer cells. Homeostasis may be disrupted upon significant elevation of intracellular ROS levels. However, during the process of blood transport, CNTs are also in contact with normal cells. Excessive ROS levels can trigger DNA strand breakage,90 protein-peptide chain disruption,91 lipid peroxidation92 and other macromolecular damage, eventually leading to cell death.93,94 The main targets of CNT cytotoxicity are cell membrane, lysosome, mitochondria, nucleus and cytoskeleton (such as actin). Damage to these structures induces loss of phagocytic ability, release of ROS, and injury to normal tissues.95,96 At the molecular level, many of the mechanisms underlying CNT toxicity involve specific cellular signaling pathways and programs. Signaling pathways activated by CNTs include NF-kB, NLRP3 inflammasome, p53, TGF-β, and MAPK.93 However, these pathways may be involved in the development of fibrosis, leading to cytotoxicity and apoptosis of normal cells. Huaux et al97 proposed immunosuppression as another mechanism of CNT-induced cytotoxicity. In their experiments, a specific type of CNT, Mitsui-7 CNT, was injected into the peritoneal cavity of Wistar rats and C57BL/6 mice for 12 months, and development of mesothelioma and monocytic myeloid-derived suppressor cells (M-Changes in MDSC) were examined. In the early stages of CNT-induced mesothelioma formation, M-MDSC rapidly and continuously accumulated in the peritoneal cavity of rats, preventing tumor cell monitoring by immune cells and thereby inducing mesothelioma. The group of Shvedova additionally reported that CNT-induced lung tumor formation is associated with the upregulation of MDSC.98 Therefore, immunosuppression presents another key mechanism underlying cytotoxicity, mainly through accumulation of MDSC and upregulation of TGF-β toxicity, promoting tumor emergence.99
Toxic Manifestations of Anti-Tumor Drug Carrier CNTs
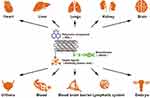
Following delivery of anticancer drugs to target cells, CNTs are transported by blood to the heart, liver, lungs, kidneys,100 brain,101 embryo102 and other organs (Figure 2), producing oxidative stress and causing cellular damage.103,104 Due to their specific surface properties and small size, even purified CNTs can cause toxicity to tissues or organs. For example, after 48 h co-culture of HeLa cells with 100 μg/mL untreated and purified SWCNTs by Tsuji and colleagues, 70% HeLa cells in the untreated group displayed apoptosis relative to 40% HeLa cells in the purified group, supporting the theory that CNTs are inherently toxic irrespective of the purity of the preparation.105 Therefore, it is necessary to consider the potential types of toxicity to body organs induced by anti-tumor nanoformulation prepared with CNTs. CNT-induced associated toxicities include hepatotoxicity,106 lung toxicity,107 and cardiovascular toxicity.108
Hepatotoxicity
Since most chemicals are metabolized by the liver, the problem of potential CNT-triggered liver toxicity should not be underestimated. Recent studies have indicated that CNTs intercepted by the reticuloendothelial system are primarily concentrated in the liver of mice.109,110 Pathological changes caused by CNTs accumulating in the liver mainly include macrophage damage, cell swelling, non-specific inflammation, spot necrosis and blood coagulation.111
An earlier study by Ji et al112 reported on the hepatotoxicity of MWCNTs. In their experiments, Kunming mice were injected with phosphate buffer saline (PBS) (10 and 60 mg/kg) and MWCNT (10 and 60 mg/kg), respectively, and changes evaluated after 15 and 60 days. Compared with the PBS group, total bilirubin and aspartate aminotransferase levels of the MWCNT group were increased in a dose-dependent manner and hepatocyte mitochondria showed swelling and dissolving. Meanwhile, partial gene expression patterns of mouse liver in the MWCNT group changed, including those associated with G protein coupled receptor, cholesterol biosynthesis, cytochrome P450 metabolism, along with Gsta2 downregulation, Cyp2B19 upregulation and Cyp2C50 downregulation. These results clearly indicated that MWCNTs cause hepatotoxicity in mice. Patlolla et al113 intraperitoneally administered varying doses of functionalized MWCNTs (carboxyl groups) (0.25, 0.5, and 0.75 mg/kg) to mice for 5 days and examined consequent hepatotoxicity produced based on pathological features of the liver. Compared to the control group, mice exposed to functional MWCNTs showed significantly increased liver weight, hepatocyte vacuolization mucus or nuclear cohesion, hepatocyte rupture and atrophy of hepatocytes. Moreover, activities of liver enzymes (ALT/GPT and AST/GOT) in various types of serum were enhanced with functionalized MWCNT concentration, leading to the conclusion that functionalized MWCNTs induce hepatotoxicity and oxidative stress as the main toxicological mechanisms. Isaac and co-workers administered a suspension of carboxylate MWCNTs at concentrations of 0.25, 0.5, 0.75, and 1.0 mg/kg in rats for 5 consecutive days. Rats in the control group were administered normal saline plus 1% Tween-80 in a similar manner to the treatment group. Venous blood was obtained from the iliac crest and the liver function index analyzed. Notably, serum activities of aspartate AST, alanine aminotransferase (ALT), alkaline phosphatase and gamma glutamyltransferase were significantly higher in the treatment than the control group. Conversely, super superoxide dismutase and glutathione S-transferase activities as well as glutathione levels were significantly reduced. The collective results clearly suggest that carboxylate MWCNTs cause damage to the liver by destroying the antioxidant defense system.113
Pulmonary Toxicity
The lung is the target organ of nanoparticles and one of the pathways of nanoparticle entry into the body. Some nanoparticles are engulfed by pulmonary macrophages or absorbed by epithelial cells and finally deposited in the lungs.114,115 A proportion of nanoparticles can also be transferred to liver, embryo, kidney and lymph nodes, causing toxic effects in other organs.116 Nanoparticles induce significant production of ROS, which have an oxidative stress effect. Pulmonary toxicity of CNTs is mainly attributable to their similar structures to asbestos. Inhalation of asbestos fibers is known to trigger asbestosis, lung cancer and pleural malignant mesothelioma.40 Epithelioid granulomas and small nodules have been reported in the lungs of rodents as a dose-dependent inflammation. Even purified CNTs are known to induce granuloma in the lung.114,117–119 At the same time, even purified CNTs are known to induce granuloma in the lung.41,120 Anti-tumor nanoparticles used as therapy for lung cancer reach the organ and effectively act on cancer cells but can additionally exert toxic effects on normal cells. The group of Chou divided ICR mice into two groups (untreated control and treatment group administered a single dose of 0.5 mg/kg SWCNTs into the trachea). On day 3, the condition of mice was assessed. Foamy macrophages with SWCNTs in the injection group had accumulated in the alveolus. After day 14, granuloma containing multifocal macrophages was produced around the SWCNT aggregation site and chronic lung inflammation observed. In these in vivo experiments, production of granulomas promoted SWCNT cytotoxicity characterized by abnormal lung inflammation.121 In a study by Ming et al,122 0, 0.1 and 0.5 mg CNTs were instilled into trachea of mice (using carbon black as a negative control). Animals were euthanized 7 and 90 days after a single treatment and the lungs isolated for histopathological analysis. In terms of changes, CNT aggregation in alveolar macrophages and concentration-dependent cytotoxicity were observed. Inflammation around bronchi was evident 7 days after treatment, with a more pronounced degree of inflammation at 90 days after administration. In contrast, the lungs of mice remained unaffected in the control group. These experiments clearly demonstrated serious damage induced by CNTs to lung. In another study, Qin and co-workers injected SWCNT into the tail vein of experimental mice, which were assigned to six groups (1, 7, 30, 60, 90 and 120 days). At the end of each time-period, 8 mice were randomly selected and lungs removed for follow-up studies. The results showed that the total amount of carbon in lung was positively correlated with length of time. Pulmonary capillary continuous embolization, granuloma formation, pulmonary fibrosis, and numerous pro-inflammatory factors were stimulated.123 Therefore, consideration of the chronic toxicity and cumulative toxicity of free CNTs distributed in the body is essential before clinical application of CNTs as anti-tumor drug carriers.
Cardiovascular Toxicity
CNTs released in the body are strongly dependent on the blood vessel wall. These nanomolecules have a significant killing effect on tumor blood vessels and can also cause cardiovascular damage during circulation in the body. CNTs possess high hardness and mechanical strength, which may cause mechanical damage upon contact with the vessel wall.124 Furthermore, CNTs can trigger substantial release of ROS or inflammatory factors, leading to cellular damage and inhibition of growth. At the same time, these nanotubes could affect the reconstruction of new blood vessel walls and cause myocardial ischemia, leading to cardiovascular toxicity, such as atherosclerosis.125
Ge et al126 administered a solution containing SWCNTs to male spontaneous hypertensive rats once a day for two continuous days, followed by examination of mouse hearts. Compared with the control group, arterial vascular thickening and myocardial fibrosis were evident in treated mice. Capillary congestion and spongy appearance were obvious upon microstructural analysis, along with thrombosis and oozing of blood vessels as well as mitochondrial swelling. The results clearly suggest that that SWCNTs cause damage to the cardiovascular system and are therefore a high risk for patients with cardiovascular disease. Similar findings were reported by Chen et al127 who highlighted the risk of long-term toxicity of SWCNTs. The effects of CNTs on important monocyte adhesion during atherogenesis and endothelial progenitor cells (EPCs) isolated from human atherosclerotic model ApoE/mouse bone marrow were ascertained by Suzuki and co-workers.128 To this end, normal human aortic endothelial cells (HAECs) were cultured and exposed to SWCNTs for 16 h. ApoE/mice were exposed to SWCNTs or DWCNTs (10 or 40 μg/mouse) once every other week for 10 weeks via pharyngeal aspiration. As a result, adhesion molecule (ICAM-1) was upregulated and THP-1 monocyte adhesion with HAEC enhanced. Compared with the blank group, the ApoE/mouse plaque area was increased, as observed from aortic oil red O staining, and ICAM-1 expression upregulated. The study concluded that SWCNTs and DWCNTs enhance atherogenesis by promoting adhesion of monocytes to endothelial cells and inducing EPC dysfunction. Cell morphology of human umbilical vein endothelial cells (HUVEC) co-cultured with MWCNTs (0.5, 5 and 20 μg/mL) for 24 h was examined by the group of Guo. Microscopic examination revealed formation of cell solute vacuoles, disordering of cellular orientation and decreased cell densities. TEM analysis showed that compared with the control group, the MWCNT group (20 μg/mL, 24 h) displayed significant vacuolization and internalization of HUVECs, with vacuoles containing several MWCNTs. Moreover, MWCNT-induced cytotoxic effects were dose-dependent. Flow cytometry using Annexin V-FITC and PI staining was used to examine the extent of HUVEC apoptosis in the MWCNT-treated groups. Cells in the MWCNT group showed a greater decrease in viability relative to control cells, indicating that the decrease in HUVEC activity may be at least partially attributed to MWCNT-induced apoptosis.129
Other Toxicities
Upon administration of CNT anti-tumor drug carriers, free CNTs not only accumulate in normal cell but are also distributed through the blood to other organs and exert toxic effects. The studies below represent recent investigations on the toxicity of CNTs to various tissues and organs (Table 1).
 |
Table 1 Toxicities of CNTs to Different Organs |
Factors Influencing CNT-Induced Toxicity
Unlike traditional chemical materials, surface modification, aggregation, concentration, size and shape of CNTs are associated with biological effects. To optimize the therapeutic efficacy of CNTs in the medical field, elucidation of the factors and mechanisms underlying CNT-mediated toxicity is critical.
Surface Modification
Surface modification is performed to improve the dispersion, excretion, and biocompatibility of CNTs.145,146 Poor water solubility of CNTs carrying anti-tumor drugs is a major contributory factor to their toxic effects on the body, which may be addressed by surface modification.147–150 The addition of proteins and surfactants on the CNT surface has been shown to not only facilitate effective targeting of cancer cells but also reduce toxicity83 and improve therapeutic effects.151–154 Among these, folate receptors are expressed on a variety of tumor cells, and binding of folate to CNTs improves both tumor targeting and toxicity in vivo.155–157
Ji and co-workers158 designed a novel drug delivery system involving modification of chitosan (CHI) on the surface of SWNTs to control the loading and release of the anticancer agent DOX, which led to simultaneous improvement of the water solubility and biocompatibility of SWNTs. Loading of folic acid (FA) on SWCNTs was shown to achieve targeted killing of tumor cells. In these experiments, the liver cancer cell line, HCC SMMC-7721, was treated with DOX and DOX/FA/CHI/SWNT (100 μg/mL), and cell viability recorded at 24, 48 and 72 h. The results showed lower cell viability of the DOX/FA/CHI/SWNT than the DOX group, indicative of time-dependent inhibition of liver cancer growth in nude mice.
Wu et al159 performed surface functionalization of CNTs via enrichment of carboxylic groups with optimized oxidization treatment, followed by covalent linking of hydrophilic diaminotriethylene glycol via amidation reaction. Finally, hydroxylcamptothecin (HCPT) was chemically attached to CNTs through a cleavable ester linkage to successfully generate a novel MWCNT drug delivery system. Subcutaneous liver H22 tumor-bearing mice were used as model animals for injection of MWCNT-HCPT (5 mg/kg). After 15 days, compared with the currently used HCPT preparations, tumors treated with the MWCNT-HCPT complex were extensively damaged while normal tissue sites remained relatively unaffected. Overall, the newly generated MWCNT-HCPT complex showed excellent antitumor activity and low toxicity. Furthermore, the complexity of MWCNT-HCPT led to longer blood circulation times and higher tumor-specific drug accumulation. Therefore, reasonable surface modification of CNTs should enhance the anti-tumor effect and decrease toxicity to normal tissues of the body. The group of Patlolla additionally evaluated the toxicity of primary and oxidized MWNTs on normal human dermal fibroblasts (NHDF). To this end, NHDFs were cultured with three different concentrations (40, 200, 400 g/mL) of raw and oxidized MWCNTs. The results showed dose-dependent toxicity of both MWNT types. Compared to the control group, 400 g/mL oxidized MWNTs inactivated NHDF via DNA damage.111 Therefore, for effective utilization of CNTs as drug carriers, both the concentrations of CNTs accumulating in the body and modifying groups on the CNT surface need to be considered.
Degree of Aggregation
Nanoparticles with small particle size and large specific surface area have intense aggregation tendency owing to van der Waals attractions in solution.160 A number of studies have indicated that SWCNT toxicity in vivo is caused by aggregates rather than individual molecules.161,162 Highly aggregated CNTs can become bulky and strong,163 consequently exerting more harmful effect on cells.
To investigate the potential lung toxicity of dispersed SWCNTs, Mutlu et al107 administered equivalent doses of dispersed and aggregated SWCNTs for 30 days after intratracheal administration to mice. Dispersed SWCNTs were taken up by alveolar macrophages through cilia via mucosal clearance or other mechanisms that gradually cleared over time. Aggregated SWCNTs displayed a granulomatous structure with mild fibrosis in mouse trachea. Accordingly, it was concluded that the toxicity caused by SWCNTs in vivo is mainly attributable to aggregates rather than SWCNTs with a large aspect ratio.164 A number of studies have highlighted that dispersed MWCNTs with extreme aspect ratios induce higher cytotoxicity than those with low aspect ratios.165 In a study by Wick et al166 the mesothelioma cell line, MSTO-211H, was exposed to disperse CNT bundles and three different concentrations of CNT agglomerates (7.5, 15 and 30 g/mL). After three days, significant cellular morphological changes and decreased cell activity were observed in the CNT aggregation groups. Toxicity was increased in a concentration-dependent manner. CNTs exert toxic effects on cancer cells, and therefore, damage to normal cells is not unexpected. Belyanskaya and co-workers studied the effects of SWCNTs with varying degrees of aggregation on chicken embryonic spinal cord and dorsal root ganglia. Two dissimilar degrees of agglomerates were utilized, specifically, SWCNT agglomerates (SWCNT-a) and better dispersed SWCNT bundles (SWCNT-b). The overall DNA content of mixed glial cells in SWCNT-a and SWCNT-b groups at a concentration of 30 μg/mL was determined with the Hoechst assay, which revealed a marked decrease in the DNA content in the SWCNT-a group. SWCNTs induced acute toxicity in the central and peripheral nervous systems after entry into the body.167 Moreover, the level of toxicity was dependent, in part, on the agglomeration state of SWCNTs. Dispersed SWCNTs showed an increased aspect ratio relative to the resulting aggregates. Phagocytic cells were able to eliminate SWCNTs and reduce toxicity to the body to a greater extent.168,169
Concentration
After anti-tumor nanometer preparations with CNTs are separated from target organs and drugs, a proportion is removed from the body while the remainder translocates to different parts of the body via the blood circulation and exerts toxic effects. The magnitude of toxicity is significantly correlated with the concentration of aggregated CNTs.170 Bottini et al137 incubated T lymphocyte cells with 40 μg/mL and 400 μg/mL CNTs and collected them for examination at different time-periods. The trypan blue exclusion assay was employed to assess the effects of CNTs on T cell viability and annexinV binding assay used to determine cell apoptosis. Cells lost 80% viability within 5 days in the presence of 400 μg/mL CNTs. Further microscopic examination was performed for chromatin condensation and membrane vesicles, which are markers of apoptosis. At a concentration of 40 μg/mL, CNTs did not appear to have toxic effects on T cells, leading to the conclusion that CNTs do not cause measurable damage to cells at this concentration and toxicity is positively correlated with dose. Fanizza and co-workers evaluated MWCNT toxicity to human bronchial normal cells (BEAS-2B) by exposing cells to 10, 40 and 100 μg/mL MWCNTs. Cellular microvilli structural changes, microvilli reduction and mild herpes development were observed after 24 h. At the same time, cell DNA damage in the 40 and 100 μg/mL MWCNT-treated groups was evident after 4 h using the comet assay. Data from these experiments confirmed the cytotoxicity of MWCNTs in normal cells.171
CNT Size
Different sizes of CNTs may induce various degrees of toxicity.172–177 Diminutive CNTs have a large specific surface area and strong ability to cross cell membranes.153 These molecules can damage cellular components and proteins, causing dysfunction or even death of macrophages.132 In a study by Sohaebuddin et al178 3T3 fibroblasts were positioned in the environment of MWCNTs with diameters <8 nm and morphology recorded after 12 h. MWCNTs with small diameters could induce membrane instability of lysosomes and promote release of components while those with large diameters caused little damage to lysosomes. The toxicities of different lengths of SWCNTs on HepG2 cells were further reported by the group of Shen.180 Measurements of cell viability and oxidative stress revealed that SWCNTs of different lengths induced a decrease in HepG2 viability and increase in intracellular ROS. Martinez et al179 used a zebrafish model to evaluate the effects of different sizes of MWCNTs on juveniles. The physiological and behavioral responses of juvenile fish indicated that short MWCNTs are neurotoxic and immunotoxic to larvae while long MWCNTs induce developmental malformations, cardiotoxicity and immunotoxicity, indicating that different-sized CNTs of the same material exert different toxicities. However, long SWCNTs exerted stronger cytotoxic effects than their shorter counterparts, supporting the theory that cytotoxicity may be effectively reduced by controlling CNT size.
Shape
CNTs are needle-like structures similar to fibers that form various shapes depending on the number of layers and length, such as single-walled carbon nanotubes (SWCNT), multi-walled carbon nanotubes (MWCNT), high aspect ratio nanotubes, short nanotubes, straight carbon nanotubes, and curved carbon nanotubes. Interestingly, CNTs of different shapes may exert differential toxic effects.177,181–184 A number of reports suggest that more profound toxicity stems from SWCNTs than MWCNTs185 and SWCNTs inhibit phagocytosis more intensely than equivalent doses of MWCNTs.181 In an earlier investigation, El-Gazzar and co-workers divided rats into DWCNT and MWCNT-7 treatment groups. Equivalent doses of DWCNT and MWCNT-7 were administered via intratracheal intrapulmonary spray every other day for 15 days and rats sacrificed after six weeks. Notably, the PCNA index of lung cells of the MWCNT-7 group was increased, compared with the DWCNT group.186 The results indicate that it is preferable to use CNTs with a small number of layers as carriers for antitumor nanometer preparations. In another study, Fenoglio and colleagues incubated MWCNTs of different thicknesses with murine alveolar macrophages (MH-S) and assessed cytotoxicity based on changes in ROS and glutathione. In these experiments, thin MWCNTs appeared significantly more toxic than the thicker counterparts.187 MWCNTs with four different shapes were injected into mice by the group of Rittinghausen and the effects on mesothelioma examined. With increased curvature of different MWCNT types, the probability of inducing mesothelioma was decreased. In other words, straight needle-shaped MWCNTs induced the greatest toxicity and carcinogenicity.188 Luisana et al189 incubated needle-like and tangled MWCNTs with the mouse macrophage cell line, Raw 264.7, and alveolar macrophages, MH-S. The cell viability value of the needle-like MWCNT-treated group was significantly decreased with high nitrite accumulation in the medium, clearly supporting the theory that MWCNT shape is related to cytotoxicity. Sakamoto and co-workers further compared the carcinogenicity of seven MWCNTs showing differences in size and shape. The carcinogenic rates of four needle-shaped MWCNTs were as high as 100% while tangled MWCNTs did not induce mesothelioma.190 Comprehensive findings from multiple studies thus suggest that different shapes of CNTs exert differing levels of cytotoxicity, with the greatest toxicity induced by needle-shaped CNTs.
Conclusion
With the extensive development and utilization of nanotechnology in the field of oncology, CNTs have been generated that play an irreplaceable role as anticancer drug carriers. However, these nanomolecules have a number of drawbacks in the clinic. During CNT-mediated delivery of anticancer drugs to target organs, free CNTs are retained in the body after dissociation of the drug, causing secondary damage. The mechanisms of action of CNTs on normal cell tissues are not fully understood at present, thus limiting their clinical application. To resolve this issue, specific proteins could be loaded on the surface of CNTs, which stimulate MPO release by neutrophils so that CNTs themselves degrade and eventually achieve attenuation effects. Simultaneously, dual drug-loading methods may be effective in protecting normal tissues.
In recent years, the changes and mechanisms of gene expression associated with CNT toxicity have been a considerable focus of research interest. The development of CNTs as anti-tumor nanometer carriers in the future will depend on the consequences of effective treatment. Due to the special nanostructural properties of CNTs, potential toxic effects and improved biocompatibility may be avoided to ensure clinical drug safety. To achieve these positive effects, we need to clarify the mechanisms underlying CNT-induced toxicity to eliminate toxic nanometer formulations. Furthermore, determination of the absorption, distribution, metabolism and excretion properties of CNTs in the body is essential. In summary, comprehensive evaluation of the safety of nanoformulations, optimization of drug payload and reduction of potential toxicity are essential steps to maximize their anti-tumor effects.
Acknowledgements
We thank International Science Editing for editing this manuscript. Zhidong Liu and Jiawei Li are co-correspondence authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li H, Shi L, Wei J, et al. Cellular uptake and anticancer activity of salvianolic acid B phospholipid complex loaded nanoparticles in head and neck cancer and precancer cells. Colloids Surf B Biointerfaces. 2016;147:65–72. doi:10.1016/j.colsurfb.2016.07.053
2. Shanmugam MK, Warrier S, Kumar AP, et al. Potential role of natural compounds as anti-angiogenic agents in cancer. Curr Vasc Pharmacol. 2017;15:503–519. doi:10.2174/1570161115666170713094319
3. Yallapu MM, Gupta BK, Jaggi M, et al. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351:19–29. doi:10.1016/j.jcis.2010.05.022
4. Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. doi:10.1093/eurheartj/ehw211
5. Yitzhak R, Elman NM. Carbon nanotubes in drug delivery: focus on infectious diseases. Expert Opin Drug Del. 2009;6:517–530. doi:10.1517/17425240902865579
6. Eroglu MS, Oner ET, Mutlu EC, et al. Sugar based biopolymers in nanomedicine; new emerging era for cancer imaging and therapy. Curr Top Med Chem. 2016;17:1507–1520. doi:10.2174/1568026616666161222101703
7. Jain KK. Role of nanodiagnostics in personalized cancer therapy. Clin Lab Med. 2012;32:15–31. doi:10.1016/j.cll.2011.10.001
8. Nagesetti A, Srinivasan S, McGoron AJ. Polyethylene glycol modified ORMOSIL theranostic nanoparticles for triggered doxorubicin release and deep drug delivery into ovarian cancer spheroids. J Photochem Photobiol B. 2017;174:209–216. doi:10.1016/j.jphotobiol.2017.07.020
9. Watermann A, Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials (Basel). 2017;7:189.
10. Jain KK. Advances in the field of nanooncology. BMC Med. 2010;8:83. doi:10.1186/1741-7015-8-83
11. Liu Z, Robinson JT, Tabakman SM, et al. Carbon materials for drug delivery & cancer therapy. Mater Today. 2011;14:316–323. doi:10.1016/S1369-7021(11)70161-4
12. Gately RD, In Het Panhuis M. Filling of carbon nanotubes and nanofibres. Beilstein J Nanotechnol. 2015;6:508–516. doi:10.3762/bjnano.6.53
13. Jin H, Heller DA, Sharma R, et al. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano. 2009;3:149–158. doi:10.1021/nn800532m
14. Lacerda L, Bianco A, Prato M, et al. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev. 2006;58:1460–1470. doi:10.1016/j.addr.2006.09.015
15. Lamprecht C, Plochberger B, Ruprecht V, et al. A single-molecule approach to explore binding, uptake and transport of cancer cell targeting nanotubes. Nanotechnology. 2014;25:125704. doi:10.1088/0957-4484/25/12/125704
16. Lamprecht C, Gierlinger N, Heister E, et al. Mapping the intracellular distribution of carbon nanotubes after targeted delivery to carcinoma cells using confocal Raman imaging as a label-free technique. J Phys Condens Matter. 2012;24:164206. doi:10.1088/0953-8984/24/16/164206
17. Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. doi:10.1038/354056a0
18. Ezzati Nazhad Dolatabadi J, Omidi Y, Losic D. Carbon nanotubes as an advanced drug and gene delivery nanosystem. Curr Nanosci. 2011;7:297–314. doi:10.2174/157341311795542444
19. Liu X, Tao H, Yang K, et al. Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials. 2011;32:144–151. doi:10.1016/j.biomaterials.2010.08.096
20. Wang L, Shi J, Jia X, et al. NIR-/pH-responsive drug delivery of functionalized single-walled carbon nanotubes for potential application in cancer chemo-photothermal therapy. Pharm Res. 2013;30:2757–2771. doi:10.1007/s11095-013-1095-3
21. Zhang B, Wang H, Shen S, et al. Fibrin-targeting peptide CREKA-conjugated multi-walled carbon nanotubes for self-amplified photothermal therapy of tumor. Biomaterials. 2016;79:46–55. doi:10.1016/j.biomaterials.2015.11.061
22. Marches R, Chakravarty P, Musselman IH, et al. Specific thermal ablation of tumor cells using single-walled carbon nanotubes targeted by covalently-coupled monoclonal antibodies. Int J Cancer. 2009;125:2970–2977. doi:10.1002/(ISSN)1097-0215
23. Jeyamohan P, Hasumura T, Nagaoka Y, et al. Accelerated killing of cancer cells using a multifunctional single-walled carbon nanotube-based system for targeted drug delivery in combination with photothermal therapy. Int J Nanomed. 2013;8:2653–2667.
24. Sobhani Z, Behnam MA, Emami F, et al. Photothermal therapy of melanoma tumor using multiwalled carbon nanotubes. Int J Nanomed. 2017;12:4509–4517. doi:10.2147/IJN
25. Roy E, Patra S, Madhuri R, et al. Carbon dot/TAT peptide co-conjugated bubble nanoliposome for multicolor cell imaging, nuclear-targeted delivery, and chemo/photothermal synergistic therapy. Chem Eng J. 2017;312:144–157. doi:10.1016/j.cej.2016.11.122
26. Sheikhpour M, Golbabaie A, Kasaeian A. Carbon nanotubes: a review of novel strategies for cancer diagnosis and treatment. Mater Sci Eng C Mater Biol Appl. 2017;76:1289–1304. doi:10.1016/j.msec.2017.02.132
27. Chakrabarti M, Kiseleva R, Vertegel A, et al. Carbon nanomaterials for drug delivery and cancer therapy. J Nanosci Nanotechno. 2015;15:5501–5511. doi:10.1166/jnn.2015.10614
28. Caoduro C, Hervouet E, Girard-Thernier C, et al. Carbon nanotubes as gene carriers: focus on internalization pathways related to functionalization and properties. Acta Biomater. 2017;49:36–44. doi:10.1016/j.actbio.2016.11.013
29. Battigelli A, Menard-Moyon C, Da Ros T, et al. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv Drug Deliv Rev. 2013;65:1899–1920. doi:10.1016/j.addr.2013.07.006
30. Elhissi AM, Ahmed W, Hassan IU, et al. Carbon nanotubes in cancer therapy and drug delivery. J Drug Deliv. 2012;2012:837327. doi:10.1155/2012/837327
31. Kushwaha SKS, Ghoshal S, Rai AK, et al. Carbon nanotubes as a novel drug delivery system for anticancer therapy: a review. Braz J Pharm Sci. 2013;49:629–643. doi:10.1590/S1984-82502013000400002
32. Wan L, Jiao J, Cui Y, et al. Hyaluronic acid modified mesoporous carbon nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanotechnology. 2016;27:135102. doi:10.1088/0957-4484/27/13/135102
33. Karimi M, Solati N, Ghasemi A, et al. Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert Opin Drug Deliv. 2015;12:1089–1105. doi:10.1517/17425247.2015.1004309
34. Augustine S, Singh J, Srivastava M, et al. Recent advances in carbon based nanosystems for cancer theranostics. Biomater Sci. 2017;5:901–952. doi:10.1039/C7BM00008A
35. Chen W, Zheng X, Li S, et al. One-pot synthesis of FePt/CNTs nanocomposites for efficient cellular imaging and cancer therapy. J Nanopart Res. 2015;17:444.
36. Saeednia L, Yao L, Cluff K, et al. Sustained releasing of methotrexate from injectable and thermosensitive chitosan-carbon nanotube hybrid hydrogels effectively controls tumor cell growth. ACS Omega. 2019;4:4040–4048. doi:10.1021/acsomega.8b03212
37. Palmer BC, Phelan-Dickenson SJ, DeLouise LA. Multi-walled carbon nanotube oxidation dependent keratinocyte cytotoxicity and skin inflammation. Part Fibre Toxicol. 2019;16:3. doi:10.1186/s12989-018-0285-x
38. Kasai T, Umeda Y, Ohnishi M, et al. Thirteen-week study of toxicity of fiber-like multi-walled carbon nanotubes with whole-body inhalation exposure in rats. Nanotoxicology. 2015;9:413–422. doi:10.3109/17435390.2014.933903
39. Emerce E, Ghosh M, Oner D, et al. Carbon nanotube- and asbestos-induced DNA and RNA methylation changes in bronchial epithelial cells. Chem Res Toxicol. 2019;32:850–860. doi:10.1021/acs.chemrestox.8b00406
40. Chernova T, Murphy FA, Galavotti S, et al. Long-fiber carbon nanotubes replicate asbestos-induced mesothelioma with disruption of the tumor suppressor gene Cdkn2a (Ink4a/Arf). Curr Biol. 2017;27:3302–3314 e3306. doi:10.1016/j.cub.2017.09.007
41. Roda E, Coccini T, Barni S, et al. Comparative pulmonary toxicity assessment of pristine and functionalized multi-walled carbon nanotubes intratracheally instilled in rats. Toxicol Lett. 2010;196:S277. doi:10.1016/j.toxlet.2010.03.1137
42. Morimoto Y, Hirohashi M, Ogami A, et al. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology. 2012;6:587–599. doi:10.3109/17435390.2011.594912
43. Shen CX, Zhang QF, Li J, et al. Induction of programmed cell death in arabidopsis and rice by single-wall carbon nanotubes. Am J Bot. 2010;97:1602–1609. doi:10.3732/ajb.1000073
44. Oberdorster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med. 2010;267:89–105. doi:10.1111/j.1365-2796.2009.02187.x
45. Thurnherr T, Brandenberger C, Fischer K, et al. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol Lett. 2011;200:176–186. doi:10.1016/j.toxlet.2010.11.012
46. Osmond-McLeod MJ, Poland CA, Murphy F, et al. Durability and inflammogenic impact of carbon nanotubes compared with asbestos fibres. Part Fibre Toxicol. 2011;8:15.
47. Ursini CL, Maiello R, Ciervo A, et al. Evaluation of uptake, cytotoxicity and inflammatory effects in respiratory cells exposed to pristine and -OH and -COOH functionalized multi-wall carbon nanotubes. J Appl Toxicol. 2016;36:394–403. doi:10.1002/jat.v36.3
48. Santos T, Fang X, Chen MT, et al. Sequential administration of carbon nanotubes and near-infrared radiation for the treatment of gliomas. Front Oncol. 2014;4:180. doi:10.3389/fonc.2014.00180
49. Wang L, Shi J, Zhang H, et al. Synergistic anticancer effect of RNAi and photothermal therapy mediated by functionalized single-walled carbon nanotubes. Biomaterials. 2013;34:262–274. doi:10.1016/j.biomaterials.2012.09.037
50. Jabr-Milane LS, van Vlerken LE, Yadav S, et al. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev. 2008;34:592–602. doi:10.1016/j.ctrv.2008.04.003
51. Meng L, Zhang X, Lu Q, et al. Single walled carbon nanotubes as drug delivery vehicles: targeting doxorubicin to tumors. Biomaterials. 2012;33:1689–1698. doi:10.1016/j.biomaterials.2011.11.004
52. Pacurari M, Yin XJ, Ding M, et al. Oxidative and molecular interactions of multi-wall carbon nanotubes (MWCNT) in normal and malignant human mesothelial cells. Nanotoxicology. 2009;2:155–170. doi:10.1080/17435390802318356
53. Hong SY, Tobias G, Al-Jamal KT, et al. Filled and glycosylated carbon nanotubes for in vivo radioemitter localization and imaging. Nat Mater. 2010;9:485–490. doi:10.1038/nmat2766
54. Ménard-Moyon, Cécilia, Venturelli E, Fabbro C, et al. The alluring potential of functionalized carbon nanotubes in drug discovery. Expert Opin Drug Dis. 2010;7:691–707. doi:10.1517/17460441.2010.490552
55. Zhang W, Zhang Z, Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett. 2011;6:555. doi:10.1186/1556-276X-6-555
56. Lia Z, ALBd B, Soaresc DCF, et al. Functionalized single-walled carbon nanotubes: cellular uptake, biodistribution and applications in drug delivery. Int J Pharm. 2017;524:41–54. doi:10.1016/j.ijpharm.2017.03.017
57. Kayat J, Gajbhiye V, Tekade RK, et al. Pulmonary toxicity of carbon nanotubes: a systematic report. Nanomedicine. 2011;7:40–49. doi:10.1016/j.nano.2010.06.008
58. Shams H, Holt BD, Mahboobi SH, et al. Actin reorganization through dynamic interactions with single-wall carbon nanotubes. ACS Nano. 2014;8:188–197. doi:10.1021/nn402865e
59. Al-Jamal KT, Nerl H, Müller KH, et al. Cellular uptake mechanisms of functionalised multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale. 2011;3:2627–2635. doi:10.1039/c1nr10080g
60. Kostarelos K, Lacerda L, Pastorin G, et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol. 2007;2:108–113. doi:10.1038/nnano.2006.209
61. Dan E, Walid D, Cécilia MM, et al. Carbon nanotube degradation in macrophages: live nanoscale monitoring and understanding of biological pathway. ACS Nano. 2015;9:10113–10124. doi:10.1021/acsnano.5b03708
62. Kang B, Chang S, Dai Y, et al. Cell response to carbon nanotubes: size-dependent intracellular uptake mechanism and subcellular fate. Small. 2010;6:2362–2366. doi:10.1002/smll.201001260
63. Kang B, Dai Y, Chang S, et al. Cell response to carbon nanotubes: size-dependent intracellular uptake mechanism and subcellular fate. Small. 2010;6:2362–2366. doi:10.1002/smll.201001260
64. Pantarotto D, Briand J-P, Prato M, et al. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun. 2003;10:16–17.
65. Raffa V, Ciofani G, Vittorio O, et al. Physicochemical properties affecting cellular uptake of carbon nanotubes. Nanomedicine. 2010;5:89–97. doi:10.2217/nnm.09.95
66. Lacerda L, Russier J, Pastorin G, et al. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials. 2012;33:3334–3343. doi:10.1016/j.biomaterials.2012.01.024
67. Serag MF, Kaji N, Okamoto CGY, et al. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano. 2011;5:493–499. doi:10.1021/nn102344t
68. Caoduro C, Kacem R, Boukari K, et al. Carbon nanotube – protamine hybrid: evaluation of DNA cell penetration. Carbon. 2016;96:742–752. doi:10.1016/j.carbon.2015.09.098
69. Yaron PN, Holt BD, Short PA, et al. Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration. J Nanobiotechnol. 2011;9:45. doi:10.1186/1477-3155-9-45
70. HalamodaKenzaoui B, Ceridono M, Urbán P, et al. The agglomeration state of nanoparticles can influence the mechanism of their cellular internalisation. J Nanobiotechnol. 2017;15:48. doi:10.1186/s12951-017-0281-6
71. Mousavi SZ, Nademi Y, Amjad-Iranagh S. et al. Carbon nanotube-encapsulated drug penetration through the cell membrane: an investigation based on steered molecular dynamics simulation. J Membrane Biol;2013. 697–704. doi:10.1007/s00232-013-9587-y
72. Maruyama K, Haniu H, Saito N, et al. Endocytosis of multiwalled carbon nanotubes in bronchial epithelial and mesothelial cells. Biomed Res Int. 2015;2015:793186. doi:10.1155/2015/793186
73. Kam NWS, Jessop TC, Wender PA, et al. Nanotube molecular transporters? Internalization of carbon nanotube? Protein conjugates into mammalian cells. J Am Chem Soc. 2004;126:6850–6851. doi:10.1021/ja0486059
74. Zhang X, Meng L, Wang X, et al. Preparation and cellular uptake of pH-dependent fluorescent single-wall carbon nanotubes. Chemistry. 2010;16:556–561. doi:10.1002/chem.200901168
75. Kotchey GP, Zhao Y, Kagan VE, et al. Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo. Adv Drug Deliv Rev. 2013;65:1921–1932. doi:10.1016/j.addr.2013.07.007
76. Ding Y, Tian R, Yang Z, et al. NADPH oxidase-dependent degradation of single-walled carbon nanotubes in macrophages. J Mater Sci Mater Med. 2017;28:7. doi:10.1007/s10856-016-5817-z
77. Kagan VE, Konduru NV, Feng W, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354–359. doi:10.1038/nnano.2010.44
78. Hou J, Wan B, Yang Y, et al. Biodegradation of single-walled carbon nanotubes in macrophages through respiratory burst modulation. Int J Mol Sci. 2016;17:409. doi:10.3390/ijms17030409
79. Bussy C, Hadad C, Prato M, et al. Intracellular degradation of chemically functionalized carbon nanotubes using a long-term primary microglial culture model. Nanoscale. 2016;8:590–601. doi:10.1039/C5NR06625E
80. Ding Y, Tian R, Yang Z, et al. Binding of human IgG to single-walled carbon nanotubes accelerated myeloperoxidase-mediated degradation in activated neutrophils. Biophys Chem. 2016;218:36–41.
81. Xanat D, HM M, SF A, et al. Slow biotransformation of carbon nanotubes by horseradish peroxidase. Environ Sci Technol. 2014;48:4826–4834. doi:10.1021/es4053279
82. Allen BL, Kichambare PD, Gou P, et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–3903. doi:10.1021/nl802315h
83. Liu Y, Zhao YL, Sun BY, et al. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46:702–713. doi:10.1021/ar300028m
84. Ding Y, Tian R, Yang Z, et al. Effects of serum albumin on the degradation and cytotoxicity of single-walled carbon nanotubes. Biophys Chem. 2017;222:1–6. doi:10.1016/j.bpc.2016.12.002
85. Lu N, Sui Y, Ding Y, et al. Fibrinogen binding-dependent cytotoxicity and degradation of single-walled carbon nanotubes. J Mater Sci Mater Med. 2018;29:115. doi:10.1007/s10856-018-6123-8
86. Zhang T, Tang M, Yao Y, et al. MWCNT interactions with protein: surface-induced changes in protein adsorption and the impact of protein corona on cellular uptake and cytotoxicity. Int J Nanomedicine. 2019;14:993–1009. doi:10.2147/IJN.S191689
87. Sadr Karimi S, Pante N. Carbon nanotubes as molecular transporters to study a new mechanism for molecular entry into the cell nucleus using actin polymerization force. PLoS One. 2019;14:e0221562. doi:10.1371/journal.pone.0221562
88. Shvedova AA, Pietroiusti A, Fadeel B, et al. Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharmacol. 2012;261:121–133. doi:10.1016/j.taap.2012.03.023
89. Ying L, Yuliang Z, Baoyun S, et al. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46:702–713. doi:10.1021/ar300028m
90. Chestkov IV, Jestkova EM, Ershova ES, et al. ROS-induced DNA damage associates with abundance of mitochondrial DNA in white blood cells of the untreated schizophrenic patients. Oxid Med Cell Longev. 2018;2018:8587475. doi:10.1155/2018/8587475
91. Luceri C, Bigagli E, Femia AP, et al. Aging related changes in circulating reactive oxygen species (ROS) and protein carbonyls are indicative of liver oxidative injury. Toxicol Rep. 2018;5:141–145. doi:10.1016/j.toxrep.2017.12.017
92. Dey U, Ghosh A, Abbas S, et al. Cell damage and mitigation in Swiss albino mice: experiment and modelling. Quantitative Biology. 2019;1904:10394.
93. Dong J, Ma Q. Advances in mechanisms and signaling pathways of carbon nanotube toxicity. Nanotoxicology. 2015;9:658–676. doi:10.3109/17435390.2015.1009187
94. Hiraku Y, Guo F, Ma N, et al. Multi-walled carbon nanotube induces nitrative DNA damage in human lung epithelial cells via HMGB1-RAGE interaction and toll-like receptor 9 activation. Part Fibre Toxicol. 2016;13:16. doi:10.1186/s12989-016-0127-7
95. de Carvalho Lima EN, Piqueira JRC, Maria DA. Advances in carbon nanotubes for malignant melanoma: a chance for treatment. Mol Diagn Ther. 2018;22:703–715. doi:10.1007/s40291-018-0363-7
96. Chen M, Sun Y, Liang J, et al. Understanding the influence of carbon nanomaterials on microbial communities. Environ Int. 2019;126:690–698. doi:10.1016/j.envint.2019.02.005
97. Huaux F, d’Ursel de Bousies V, Parent MA, et al. Mesothelioma response to carbon nanotubes is associated with an early and selective accumulation of immunosuppressive monocytic cells. Part Fibre Toxicol. 2016;13:46. doi:10.1186/s12989-016-0158-0
98. Shvedova AA, Tkach AV, Kisin ER, et al. Carbon nanotubes enhance metastatic growth of lung carcinoma via up-regulation of myeloid-derived suppressor cells. Small. 2013;9:1691–1695. doi:10.1002/smll.201201470
99. Shvedova AA, Kisin ER, Yanamala N, et al. MDSC and TGFbeta are required for facilitation of tumor growth in the lungs of mice exposed to carbon nanotubes. Cancer Res. 2015;75:1615–1623. doi:10.1158/0008-5472.CAN-14-2376
100. Dong X, Liu L, Zhu D, et al. Transactivator of transcription (TAT) peptide-chitosan functionalized multiwalled carbon nanotubes as a potential drug delivery vehicle for cancer therapy. Int J Nanomedicine. 2015;10:3829–3840. doi:10.2147/IJN.S81762
101. Liu X, Zhang Y, Li J, et al. Cognitive deficits and decreased locomotor activity induced by single-walled carbon nanotubes and neuroprotective effects of ascorbic acid. Int J Nanomed. 2014;9:823–839.
102. Campagnolo L, Massimiani M, Palmieri G, et al. Biodistribution and toxicity of pegylated single wall carbon nanotubes in pregnant mice. Part Fibre Toxicol. 2013;10:21. doi:10.1186/1743-8977-10-21
103. Simon A, Maletz SX, Hollert H, et al. Effects of multiwalled carbon nanotubes and triclocarban on several eukaryotic cell lines: elucidating cytotoxicity, endocrine disruption, and reactive oxygen species generation. Nanoscale Res Lett. 2014;9:396–410. doi:10.1186/1556-276X-9-396
104. Jiang Y, Zhang H, Wang Y, et al. Modulation of apoptotic pathways of macrophages by surface-functionalized multi-walled carbon nanotubes. PLoS One. 2013;8:e65756. doi:10.1371/journal.pone.0065756
105. TSUJI T, USUKURA J. Assessment on a biological toxicity caused by single-walled carbon nanotubes. Nano Biomed. 2012;4:125–132.
106. Adedara IA, Anao OO, Forcados GE, et al. Low doses of multi-walled carbon nanotubes elicit hepatotoxicity in rats with markers of oxidative stress and induction of pro-inflammatory cytokines. Biochem Biophys Res Commun. 2018;503:3167–3173. doi:10.1016/j.bbrc.2018.08.112
107. Mutlu GM, Budinger GR, Green AA, et al. Biocompatible nanoscale dispersion of single-walled carbon nanotubes minimizes in vivo pulmonary toxicity. Nano Lett. 2010;10:1664–1670. doi:10.1021/nl9042483
108. Mostovenko E, Young T, Muldoon PP, et al. Nanoparticle exposure driven circulating bioactive peptidome causes systemic inflammation and vascular dysfunction. Part Fibre Toxicol. 2019;16:20. doi:10.1186/s12989-019-0304-6
109. Liu Z, Cai W, He L, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi:10.1038/nnano.2006.170
110. Deng X, Yang S, Nie H, et al. A generally adoptable radiotracing method for tracking carbon nanotubes in animals. Nanotechnol. 2008;19:075101. doi:10.1088/0957-4484/19/7/075101
111. Awasthi KK, John PJ, Awasthi A, et al. Multi walled carbon nano tubes induced hepatotoxicity in Swiss albino mice. Micron. 2013;44:359–364. doi:10.1016/j.micron.2012.08.008
112. Ji Z, Zhang D, Li L, et al. The hepatotoxicity of multi-walled carbon nanotubes in mice. Nanotechnol. 2009;20:445101. doi:10.1088/0957-4484/20/44/445101
113. Patlolla AK, Berry A, Tchounwou PB. Study of hepatotoxicity and oxidative stress in male Swiss-Webster mice exposed to functionalized multi-walled carbon nanotubes. Mol Cell Biochem. 2011;358:189–199. doi:10.1007/s11010-011-0934-y
114. Otsuka K, Yamada K, Taquahashi Y, et al. Long-term polarization of alveolar macrophages to a profibrotic phenotype after inhalation exposure to multi-wall carbon nanotubes. PLoS One. 2018;13:e0205702. doi:10.1371/journal.pone.0205702
115. Jacobsen NR, Møller P, Clausen PA, et al. Biodistribution of carbon nanotubes in animal models. Basic Clin Pharmacol Toxicol. 2016;121(Suppl 3):30. doi:10.1111/bcpt.12705
116. Puisney C, Baeza-Squiban A, Boland S. Mechanisms of uptake and translocation of nanomaterials in the lung. Adv Exp Med Biol. 2018;1048:21–36.
117. Poulsen SS, Jackson P, Kling K, et al. Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology. 2016;10:1263–1275. doi:10.1080/17435390.2016.1202351
118. Duke KS, Bonner JC. Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10:e1498. doi:10.1002/wnan.2018.10.issue-3
119. Ema M, Gamo M, Honda K. A review of toxicity studies of single-walled carbon nanotubes in laboratory animals. Regul Toxicol Pharmacol. 2016;74:42–63. doi:10.1016/j.yrtph.2015.11.015
120. Francis AP, Devasena T. Toxicity of carbon nanotubes: a review. Toxicol Ind Health. 2018;34:200–210. doi:10.1177/0748233717747472
121. Chou CC, Hsiao HY, Hong QS, et al. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8:437–445. doi:10.1021/nl0723634
122. Lam CW, James JT, McCluskey R, et al. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi:10.1093/toxsci/kfg243
123. Qin Y, Li S, Zhao G, et al. Long-term intravenous administration of carboxylated single-walled carbon nanotubes induces persistent accumulation in the lungs and pulmonary fibrosis via the nuclear factor-kappa B pathway. Int J Nanomed. 2017;12:263–277. doi:10.2147/IJN.S123839
124. Johnson BB, Santare MH, Novotny JE, et al. Wear behavior of carbon nanotube/high density polyethylene composites. Mech Mater. 2009;41:1108–1115. doi:10.1016/j.mechmat.2009.04.003
125. Erdely A, Hulderman T, Salmen R, et al. Cross-talk between lung and systemic circulation during carbon nanotube respiratory exposure. Potential biomarkers. Nano Lett. 2009;9:36–43. doi:10.1021/nl801828z
126. Ge C, Meng L, Xu L, et al. Acute pulmonary and moderate cardiovascular responses of spontaneously hypertensive rats after exposure to single-wall carbon nanotubes. Nanotoxicol. 2012;6:526–542. doi:10.3109/17435390.2011.587905
127. Chen R, Zhang L, Ge C, et al. Subchronic toxicity and cardiovascular responses in spontaneously hypertensive rats after exposure to multiwalled carbon nanotubes by intratracheal instillation. Chem Res Toxicol. 2015;28:440–450. doi:10.1021/tx5004003
128. Suzuki Y, Tada-Oikawa S, Hayashi Y, et al. Single- and double-walled carbon nanotubes enhance atherosclerogenesis by promoting monocyte adhesion to endothelial cells and endothelial progenitor cell dysfunction. Part Fibre Toxicol. 2016;13:54. doi:10.1186/s12989-016-0166-0
129. Guo YY, Zhang J, Zheng YF, et al. Cytotoxic and genotoxic effects of multi-wall carbon nanotubes on human umbilical vein endothelial cells in vitro. Mutat Res. 2011;721:184–191. doi:10.1016/j.mrgentox.2011.01.014
130. Zheng W, McKinney W, Kashon M, et al. The influence of inhaled multi-walled carbon nanotubes on the autonomic nervous system. Part Fibre Toxicol. 2016;13:8. doi:10.1186/s12989-016-0119-7
131. Aragon MJ, Topper L, Tyler CR, et al. Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment. Proc Natl Acad Sci U S A. 2017;114:E1968–E1976. doi:10.1073/pnas.1616070114
132. Gholamine B, Karimi I, Salimi A, et al. Neurobehavioral toxicity of carbon nanotubes in mice. Toxicol Ind Health. 2017;33:340–350. doi:10.1177/0748233716644381
133. Larner SF, Wang J, Goodman J, et al. In vitro neurotoxicity resulting from exposure of cultured neural cells to several types of nanoparticles. J Cell Death. 2017;10:1179670717694523. doi:10.1177/1179670717694523
134. Chen H, Zheng X, Nicholas J, et al. Single-walled carbon nanotubes modulate pulmonary immune responses and increase pandemic influenza a virus titers in mice. Virol J. 2017;14:242. doi:10.1186/s12985-017-0909-z
135. Park EJ, Choi J, Kim JH, et al. Subchronic immunotoxicity and screening of reproductive toxicity and developmental immunotoxicity following single instillation of HIPCO-single-walled carbon nanotubes: purity-based comparison. Nanotoxicology. 2016;10:1188–1202. doi:10.1080/17435390.2016.1202348
136. Lee S, Khang D, Kim S-H. High dispersity of carbon nanotubes diminishes immunotoxicity in spleen. Int J Nanomed. 2015;10:2697–2710.
137. Bottini M, Bruckner S, Nika K, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–126. doi:10.1016/j.toxlet.2005.06.020
138. Pietroiusti A, Massimiani M, Fenoglio I, et al. Low doses of pristine and oxidized single-wall carbon nanotubes affect mammalian embryonic development. ACS Nano. 2011;5:4624–4633. doi:10.1021/nn200372g
139. Qi W, Bi J, Zhang X, et al. Damaging effects of multi-walled carbon nanotubes on pregnant mice with different pregnancy times. Sci Rep. 2014;4:4352. doi:10.1038/srep04352
140. Cheng J, Cheng SH. Influence of carbon nanotube length on toxicity to zebrafish embryos. Int J Nanomed. 2012;7:3731–3739. doi:10.2147/IJN
141. Huang X, Zhang F, Sun X, et al. The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials. 2014;35:856–865. doi:10.1016/j.biomaterials.2013.10.027
142. Zhu B, Song Z, Jian L, et al. Developmental toxicity, bioaccumulation and distribution of oxidized single walled carbon nanotubes in Artemia salina. Toxicol Res (Camb). 2018;7:897–906.
143. Bai Y, Zhang Y, Zhang J, et al. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat Nanotechnol. 2010;5:683–689. doi:10.1038/nnano.2010.153
144. Lindberg HK, Falck GC, Singh R, et al. Genotoxicity of short single-wall and multi-wall carbon nanotubes in human bronchial epithelial and mesothelial cells in vitro. Toxicology. 2013;313:24–37. doi:10.1016/j.tox.2012.12.008
145. Zhang D, Deng X, Ji Z, et al. Long-term hepatotoxicity of polyethylene-glycol functionalized multi-walled carbon nanotubes in mice. Nanotechnology. 2010;21:175101. doi:10.1088/0957-4484/21/17/175101
146. Perez-Luna V, Moreno-Aguilar C, Arauz-Lara JL, et al. Interactions of functionalized multi-wall carbon nanotubes with giant phospholipid vesicles as model cellular membrane system. Sci Rep. 2018;8:17998. doi:10.1038/s41598-018-36531-9
147. Adenuga AA, Truong L, Tanguay RL, et al. Preparation of water soluble carbon nanotubes and assessment of their biological activity in embryonic zebrafish. Int J Biomed Nanosci Nanotechnol. 2013;3:38–51. doi:10.1504/IJBNN.2013.054514
148. Au - Lin J, Au - Hu Y, Au - Zhao -J-J. Repression of multiple myeloma cell growth in vivo by Single-wall Carbon Nanotube (SWCNT)-delivered MALAT1 antisense oligos. JoVE. 2018;13:e58598.
149. Suo N, Wang M, Jin Y, et al. Magnetic multiwalled carbon nanotubes with controlled release of epirubicin: an intravesical instillation system for bladder cancer. Int J Nanomedicine. 2019;14:1241–1254. doi:10.2147/IJN.S189688
150. Lay CL, Liu HQ, Tan HR, et al. Delivery of paclitaxel by physically loading onto poly(ethylene glycol) (PEG)-graft-carbon nanotubes for potent cancer therapeutics. Nanotechnology. 2010;21:065101. doi:10.1088/0957-4484/21/6/065101
151. Zhu Y, Li W, Li Q, et al. Effects of serum proteins on intracellular uptake and cytotoxicity of carbon nanoparticles. Carbon. 2009;47:1351–1358. doi:10.1016/j.carbon.2009.01.026
152. Schipper ML, Nakayama-Ratchford N, Davis CR, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–221. doi:10.1038/nnano.2008.68
153. Costa PM, Bourgognon M, Wang JT, et al. Functionalised carbon nanotubes: from intracellular uptake and cell-related toxicity to systemic brain delivery. J Control Release. 2016;241:200–219. doi:10.1016/j.jconrel.2016.09.033
154. Dong X, Sun Z, Wang X, et al. An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer. Nanomedicine. 2017;13:2271–2280. doi:10.1016/j.nano.2017.07.002
155. Pardo J, Peng Z, Leblanc RM. Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Molecules. 2018;23:378.
156. Huang H, Yuan Q, Shah JS, et al. A new family of folate-decorated and carbon nanotube-mediated drug delivery system: synthesis and drug delivery response. Adv Drug Deliv Rev. 2011;63:1332–1339. doi:10.1016/j.addr.2011.04.001
157. Yan Y, Wang R, Hu Y, et al. Stacking of doxorubicin on folic acid-targeted multiwalled carbon nanotubes for in vivo chemotherapy of tumors. Drug Deliv. 2018;25:1607–1616. doi:10.1080/10717544.2018.1501120
158. Ji Z, Lin G, Lu Q, et al. Targeted therapy of SMMC-7721 liver cancer in vitro and in vivo with carbon nanotubes based drug delivery system. J Colloid Interface Sci. 2012;365:143–149. doi:10.1016/j.jcis.2011.09.013
159. Wu W, Li R, Bian X, et al. Covalently combining carbon nanotubes with anticancer agent: preparation and antitumor activity. ACS Nano. 2009;3:2740–2750. doi:10.1021/nn9005686
160. Ma X, Zare Y, Rhee KY. A two-step methodology to study the influence of aggregation/agglomeration of nanoparticles on Young’s modulus of polymer nanocomposites. Nanoscale Res Lett. 2017;12:621. doi:10.1186/s11671-017-2386-0
161. Demming A. Nanotechnology under the skin. Nanotechnol. 2011;22:260201. doi:10.1088/0957-4484/22/26/260201
162. Medepalli K, Alphenaar B, Raj A, et al. Evaluation of the direct and indirect response of blood leukocytes to carbon nanotubes (CNTs). Nanomedicine. 2011;7:983–991. doi:10.1016/j.nano.2011.04.002
163. Alshehri R, Ilyas AM, Hasan A, et al. Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J Med Chem. 2016;59:8149–8167. doi:10.1021/acs.jmedchem.5b01770
164. Niezabitowska E, Smith J, Prestly MR, et al. Facile production of nanocomposites of carbon nanotubes and polycaprolactone with high aspect ratios with potential applications in drug delivery. RSC Adv. 2018;8:16444–16454. doi:10.1039/C7RA13553J
165. Kim JS, Song KS, Joo HJ, et al. Determination of cytotoxicity attributed to multiwall carbon nanotubes (MWCNT) in normal human embryonic lung cell (WI-38) line. J Toxicol Environ Health A. 2010;73:1521–1529. doi:10.1080/15287394.2010.511577
166. Wick P, Manser P, Limbach LK, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–131. doi:10.1016/j.toxlet.2006.08.019
167. Belyanskaya L, Weigel S, Hirsch C, et al. Effects of carbon nanotubes on primary neurons and glial cells. Neurotoxicology. 2009;30:702–711. doi:10.1016/j.neuro.2009.05.005
168. Ryman-Rasmussen JP, Cesta MF, Brody AR, et al. Inhaled carbon nanotubes reach the sub-pleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi:10.1038/nnano.2009.305
169. Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi:10.1038/nnano.2008.111
170. Girardi FA, Bruch GE, Peixoto CS, et al. Toxicity of single-wall carbon nanotubes functionalized with polyethylene glycol in zebrafish (Danio rerio) embryos. J Appl Toxicol. 2017;37:214–221. doi:10.1002/jat.v37.2
171. Fanizza C, Paba E, Casciardi S, et al. Cytotoxic and genotoxic effects of multi-walled carbon nanotubes on human bronchial normal cells (BEAS-2B). Toxicol Lett. 2009;189:S186. doi:10.1016/j.toxlet.2009.06.648
172. Sweeney S, Berhanu D, Misra SK, et al. Multi-walled carbon nanotube length as a critical determinant of bioreactivity with primary human pulmonary alveolar cells. Carbon N Y. 2014;78:26–37. doi:10.1016/j.carbon.2014.06.033
173. Liu D, Wang L, Wang Z, et al. Different cellular response mechanisms contribute to the length-dependent cytotoxicity of multi-walled carbon nanotubes. Nanoscale Res Lett. 2012;7:361. doi:10.1186/1556-276X-7-361
174. Fujita K, Fukuda M, Endoh S, et al. Size effects of single-walled carbon nanotubes on in vivo and in vitro pulmonary toxicity. Inhal Toxicol. 2015;27:207–223. doi:10.3109/08958378.2015.1026620
175. Meng J, Cheng X, Liu J, et al. Effects of long and short carboxylated or aminated multiwalled carbon nanotubes on blood coagulation. PLoS One. 2012;7:e38995. doi:10.1371/journal.pone.0038995
176. Louro H, Pinhao M, Santos J, et al. Evaluation of the cytotoxic and genotoxic effects of benchmark multi-walled carbon nanotubes in relation to their physicochemical properties. Toxicol Lett. 2016;262:123–134. doi:10.1016/j.toxlet.2016.09.016
177. Kavosi A, Hosseini Ghale Noei S, Madani S, et al. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci Rep. 2018;8:8375. doi:10.1038/s41598-018-26790-x
178. Sohaebuddin SK, Thevenot PT, Baker D, et al. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol. 2010;7:22. doi:10.1186/1743-8977-7-22
179. Martinez CS, Igartua DE, Czarnowski I, et al. Biological response and developmental toxicity of zebrafish embryo and larvae exposed to multi-walled carbon nanotubes with different dimension. Heliyon. 2019;5:e02308. doi:10.1016/j.heliyon.2019.e02308
180. Shen Z, Wu J, Yu Y, et al. Comparison of cytotoxicity and membrane efflux pump inhibition in HepG2 cells induced by single-walled carbon nanotubes with different length and functional groups. Sci Rep. 2019;9:7557. doi:10.1038/s41598-019-43900-5
181. Harik VM. Geometry of carbon nanotubes and mechanisms of phagocytosis and toxic effects. Toxicol Lett. 2017;273:69–85. doi:10.1016/j.toxlet.2017.03.016
182. Sasaki T, Asakura M, Ishioka C, et al. In vitro chromosomal aberrations induced by various shapes of multi-walled carbon nanotubes (MWCNTs). J Occup Health. 2016;58:16–99.
183. Allegri M, Perivoliotis DK, Bianchi MG, et al. Toxicity determinants of multi-walled carbon nanotubes: the relationship between functionalization and agglomeration. Toxicol Rep. 2016;3:230–243. doi:10.1016/j.toxrep.2016.01.011
184. Haniu H, Saito N, Matsuda Y, et al. Biological responses according to the shape and size of carbon nanotubes in BEAS-2B and MESO-1 cells. Int J Nanomedicine. 2014;9:1979–1990. doi:10.2147/IJN
185. Hu X, Cook S, Wang P, et al. In vitro evaluation of cytotoxicity of engineered carbon nanotubes in selected human cell lines. Sci Total Environ. 2010;408:1812–1817. doi:10.1016/j.scitotenv.2010.01.035
186. El-Gazzar AM, Abdelgied M, Alexander DB, et al. Comparative pulmonary toxicity of a DWCNT and MWCNT-7 in rats. Arch Toxicol. 2019;93:49–59. doi:10.1007/s00204-018-2336-3
187. Fenoglio I, Aldieri E, Gazzano E, et al. Thickness of multiwalled carbon nanotubes affects their lung toxicity. Chem Res Toxicol. 2012;25:74–82. doi:10.1021/tx200255h
188. Rittinghausen S, Hackbarth A, Creutzenberg O, et al. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Particle & Fibre Toxicology. 2014;11:59. doi:10.1186/s12989-014-0059-z
189. Di Cristo L, Bianchi MG, Chiu M, et al. Comparative in vitro cytotoxicity of realistic doses of benchmark multi-walled carbon nanotubes towards macrophages and airway epithelial cells. Nanomaterials (Basel). 2019;9:982.
190. Sakamoto Y, Hojo M, Kosugi Y, et al. Comparative study for carcinogenicity of 7 different multi-wall carbon nanotubes with different physicochemical characteristics by a single intraperitoneal injection in male Fischer 344 rats. J Toxicol Sci. 2018;43:587–600. doi:10.2131/jts.43.587
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.