Back to Journals » Drug Design, Development and Therapy » Volume 11
Therapy with pamidronate in children with osteogenesis imperfecta
Authors Marginean O, Tamasanu RC, Mang N, Mozos I , Brad GF
Received 4 May 2017
Accepted for publication 27 June 2017
Published 28 August 2017 Volume 2017:11 Pages 2507—2515
DOI https://doi.org/10.2147/DDDT.S141075
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr James Janetka
Otilia Marginean,1 Raluca Corina Tamasanu,1 Niculina Mang,1 Ioana Mozos,2,3 Giorgiana Flavia Brad1
1First Department of Pediatrics, 2Department of Functional Sciences, 3Center for Translational Research and Systems Medicine, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania
Abstract: Osteogenesis imperfecta (OI) is a genetic disease characterized by excessive bone fragility with fractures consecutive to minor trauma. Considering lack of standardization of therapy with pamidronate in children, it was our aim to present our experience over a period of 10 years regarding evolution and treatment in patients diagnosed with osteoporosis and OI. Nine patients diagnosed with OI were admitted to the First Pediatric Clinic, Timisoara. They were investigated (clinical, biomarkers of bone metabolism and imaging studies), and a quality-of-life questionnaire was used to evaluate the impact of OI. Treatment was performed with pamidronate 1 mg/kg/cycle, every 3 months. The patients were evaluated every 3 months. The most frequent was type III (three patients), and two patients were diagnosed with type II, while the other patients were diagnosed with other forms such as types IV, V, VI and VIII. The clinical expression was polymorphic, and the number of fractures was variable. Bone pain ameliorated just after the first cycle of pamidronate, while the activity and mobility increased quickly. Osteodensitometry in children over 12 years showed a decreased bone mineral density (BMD) with a significant improvement after treatment. The values of the bone alkaline phosphatase and osteocalcin changed after the antiresorptive treatment, and the quality of life of the children and their family improved. Treatment with pamidronate is beneficial for the patient, family and society, increases mobility and bone density, improves quality of life and reduces family dependence in children with OI.
Keywords: osteoporosis, child, osteogenesis imperfecta, pamidronate
Introduction
Osteogenesis imperfecta (OI), known as brittle bone disease, is a heterogeneous, phenotypic and molecular group of inherited connective tissue diseases characterized by increased bone fragility.1 It is one of the most common skeletal dysplasias secondary to quantitative or qualitative abnormalities of collagen metabolism.2 The incidence is between 10,000 and 20,000 live births according to the statistics and national registers3 type I and IV disease, the most commonly represented, with a prevalence of 4–5 cases per 100,000.4 In Romania, there are no available data on the incidence and prevalence of this disease.
Traditionally, OI was considered a dominant disease caused by the mutations in COL1A1 or COL2A2 genes, which encodes α1 and α2 chains of collagen 4, responsible for the appearance of qualitative and quantitative deficiencies in the synthesis of type I collagen. Till now, more than 1,500 dominant mutations in the COL1A1 and COL2A2 genes have been identified and published, causing several skeletal phenotypes ranging from subclinical to lethal forms.5 More recently, recessive forms of OI have also been defined, caused by mutations in a variety of genes whose products interfere mainly with type I collagen quantity, structure, synthesis or function.6
In 1979, David Sillence classified OI in four classical forms based on clinical and radiological features.29 Later, the classification was supplemented by Rauch and Glorieux,1 and so far, till today, 16 types of OI and OI-like syndromes are known, divided in five groups based on the metabolic pathway affected regarding the collagen synthesis, structure, processing, posttranslational modification, folding and cross-linking, bone mineralization and osteoblast differentiation.7
Type I of OI is the most common and the mildest form as well. The quality of the collagen is normal, but its quantity is insufficient. Mild fractures, slight curvature of the spine, joint laxity and muscle hypotonia can occur. Type II is severe, usually lethal in the perinatal period secondary to respiratory failure or cerebral hemorrhage. In this form, collagen is quantitatively or qualitatively defective, which explains the intrauterine fractures and the blue sclera. Type III is progressive and deforming. Sometimes fractures may occur prior to birth. Bone deformities, often severe, cause a small waist, spinal curvature and barrel-shaped ribs, enabling a severe physical disability. In type IV, the bone deformities ranging from mild to moderate are associated with early hearing loss. Type IV A and IV B can be described depending on the presence or absence of dentinogenesis imperfecta. Types V and VI are characterized by the same clinical features as type IV, but have specific histological images (eye of the seine and the fish scale). Type VII, frequently encountered among a group of Indians in Quebec, has multiple fractures at birth. Fractures diminish in frequency in adults. Cabral et al8 emphasized type VIII of OI, a recessive form of great severity like type II, characterized by severe shortening of the long bones, vertebral compression fractures, arms longer than forearms, large anterior fontanel and round face. Patients with type IX have white sclerae and normal dentition, with their hand length that is proportionate for their age and without the metacarpal shortening. Although the osteoporosis in this type of OI is less severe than that in types VII and VIII, low bone mass and multiple long-bone fractures are described.9
The treatment of these patients should be performed as soon as possible with drugs that are able to maintain the quality of bone to access the autonomy necessary for long-term functionality. The recommended therapy consisted of treatment with bone antiresorptive drugs, but the doses and the period of administration in children are controversial.
Objectives
The authors aimed to present their experience over a period of 10 years regarding evolution and therapy in patients diagnosed with osteoporosis induced by OI admitted to the Endocrinology Department of the First Pediatric Clinic of the “Victor Babes” University of Medicine and Pharmacy, Timisoara, considering lack of standardization of therapy with pamidronate in children.
Patients and methods
Study population
A total of nine patients with OI admitted to the Endocrinology Department were analyzed. The group’s age ranged between 2 weeks and 17 years at admission, and five girls and four boys were included. This study complied with the Declaration of Helsinki and has been approved by the institutional ethics committee of the “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania. We obtained written informed consent from the children’s parents for using both the case details and their images in this publication.
The anamnesis pointed out the gestational age, the birth weight and height, the delivery history, the adopted kind of feeding (breast milk or formula milk), the history of fractures, the movement ability, the diet, the consumption of dairy products, the vitamin D intake and sun exposure. The patients were clinically examined (using anthropometric criteria, height and weight and then compared with weight-for-height and height-for-age considering age and sex) and a morphogram performed for each child enabled checking of harmonic growth. The pubertal development was evaluated using the Tanner stage. The examination on the systems was performed, and it described the characteristics of this disease with specific phenotype.
Markers of bone metabolism
The phosphate and calcium metabolism was investigated. 25-OH vitamin D was tested using immunochemistry method. It was defined as insufficient level of 25-OH vitamin D, a value between 10 and 30 ng/mL, while a serum level <10 ng/mL was suggestive of vitamin D deficiency.
Markers used to describe the bone metabolism were as follows: serum and bone alkaline phosphatase (Tandem®-MP Ostase® assay), osteocalcin (Elecsys N-MID osteocalcin reagent kit) and β-crosslaps (BM/Hitachi Elecsys 2010 method), and they were analyzed considering the age and sex of the patients. All the bone markers were tested during the first hospital admission, at 3 and 6 months after the start of the therapy and then annually.
Diagnosis of osteoporosis
Osteoporosis was defined according to the American Academy of Pediatrics (AAPs) and International Society for Clinical Densitometry (ISCD): the presence of both clinically significant fracture history and low bone mineral content or bone mineral density (BMD) showed by dual-energy X-ray absorptiometry (DXA) with the Z-score less than or equal to −2.0, adjusted for age, sex and body size.10–12 For young adults, the use of the T score is recommended, for the same purpose.12
Radiographies of the skeletal segments affected were performed to identify the fractures. The presence of osteoporosis in patients aged over 12 years was estimated using DXA method. This was not performed in children under 12 years old, because the software used for the interpretation of DXA results was not adequate. To perform this investigation in these younger patients, we had to adapt the software to the requirements.
Quality of life
Regarding the impact of the OI on the patients and their families’ life, a quality-of-life questionnaire was used. The Short Form-36 is a self-reported health assessment test which contains 36 items that evaluate many aspects of physical and mental health and is widely used to measure health outcomes. Each domain is scored from 0 to 100, a bigger score being correlated with better mental and physical health. This questionnaire was completed by the patients attending school and their parents, before and after pamidronate treatment.
Therapy
The treatment of the patients with OI was performed with intravenous pamidronate in glucose 5% solution (0.5 mg/kg – first dose followed by 1 mg/kg/cycle – next doses) over a period of 3–4 hours, every 3 months. A cycle represented a period of 1–3 days of administration. At the beginning of the treatment, intravenous calcium was administered to prevent the possible hypocalcemia associated with the first dose of pamidronate.
Clinical and biological changes were evaluated regularly every 3 months during antiresorptive treatment, and radiological and quality of life parameters were assessed yearly. The period of follow-up was between 3 and 6 years. The patients older than 20 years were transferred to adult medical service.
Statistical methods
Statistical data processing was performed using SPSS version 19 (IBM Corporation, Armonk, NY, USA). The Mann–Whitney nonparametric statistical test was used given the small number of patients sampled before and after treatment. The r correlation coefficient (Bravais–Pearson) was also used to correlate serum alkaline phosphatase and the number of fractures before and after treatment.
Results
In the past 10 years, we analyzed a total number of nine patients (four boys and five girls) diagnosed with osteoporosis induced by various forms of OI. The anthropometric measurements of the patients (aged between 2 weeks and 17 years) at their first hospital admission described them as being short statured with a mean score of standard deviation (SDS) of −5.78 (Table 1).
Their anamnesis revealed many fractures per patient and normal intake of calcium and vitamin D substituents.
According to the Sillence classification, the distribution of patients from our group was the following: type III of OI was encountered in most of the patients (three cases), two patients were diagnosed with type II, while the other forms such as types IV, V, VI and VIII were present in only one patient for each form.
The clinical picture was polymorphic and varied from fractures in a single segment of the limb to multiple ones (up to 39 fractures) localized in different segments. All the fractures were caused by minor trauma (Table 2).
  | Table 2 Clinical aspects of the study group |
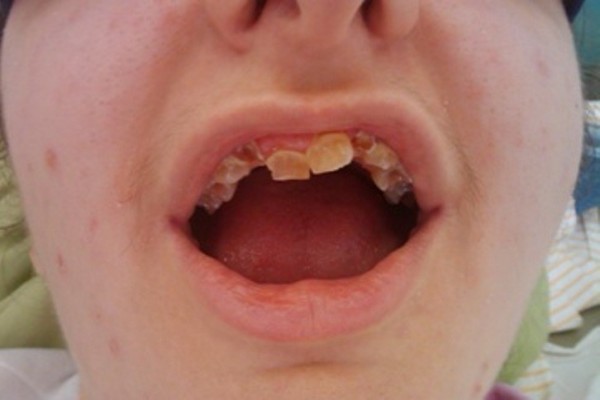
The phenotypes of these patients were very suggestive of the OI according to the age. The newborns were presented with intrauterine fracture, infants with triangle face with large head and barrel-shaped rib cage (Figure 1) and older children with short stature secondary to the limb fractures, sometimes with vicious callus (Figure 2). All the children had severe walking difficulties and were afraid to do it. Three patients had dentinogenesis imperfecta associated with brown–yellow spots, malposition and friability (Figure 3). Audiometry was performed in all the patients and was within the normal limits.
  | Figure 1 Newborn with triangle face. |
  | Figure 2 Short stature due to limb fractures with large head and barrel-shaped rib cage. |
  | Figure 3 Dentinogenesis imperfecta with brown–yellow spots, malposition and friability. |
All the patients had normal serum calcium levels before starting treatment with pamidronate. Regarding the 25-OH vitamin serum level, 25% of these patients had vitamin D deficiency and 55.5% were insufficient for vitamin D, for which vitamin D solution (800 UI/day) was prescribed.
In four children, the bone pain was very severe, and it ameliorated just after the first cycle of pamidronate and disappeared after the second. Their activity and mobility increased quickly. No side effects such as flu-like symptoms, headache, dizziness, gastrointestinal symptoms or hypocalcemia were described in our patients.
The X-rays of our patients consisted of very suggestive images for this pathology such as complete or incomplete fracture of the mid-diaphysis of long bones, less or more displaced, etc. One patient had an apophyseal avulsion fracture of tibia, which is less common. Usually, an aberrant vicious consolidation can appear associated with fracture and sometimes as a response to these fractures. Other X-ray-specific skeletal manifestations consisted of deformities of the spine (kyphosis, scoliosis), the rib cage (secondary to its thinning) or extremities (discrepancy in length, incurvation of the radius and ulna). The DXA showed the presence of osteoporosis in these children.
For 3-year treatment with pamidronate, the bone texture and mineral density evaluated by DXA improved, as indicated by the mean values of the Z-score in our cases that diminished from −2.85 to −2.25 (Figure 4).
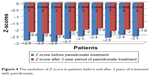  | Figure 4 The evolution of Z-score in patients before and after 3 years of treatment with pamidronate. |
The fractures were treated according to the fracture type and site using Dassault immobilization or surgical treatment in all patients, and in three cases the hip joint or long bones were consolidated.
The effectiveness after 3 years of treatment with pamidronate was confirmed by the reduction in the number of fractures to zero, with a significant P-value (Z=−2.375, P=0.018).
The markers of bone metabolism in our study group showed normal serum calcium and phosphorus level at the baseline and a stable serum normal level under treatment.
Concerning the variation of the serum alkaline phosphatase and the number of fractures before and after treatment with pamidronate, we calculated the r correlation coefficient (Bravais–Pearson) between these two variables. The scatter diagrams show that there is no significant correlation between the serum alkaline phosphatase values and the number of fractures in patients before treatment (r=1, P=0.613; Figure 5), neither between the serum alkaline phosphatase values and number of fractures in patients after 3 years of treatment (r=1, P=0.384; Figure 6).
  | Figure 5 Scatter diagram of serum alkaline phosphatase values and the number of fractures in patients before treatment. |
  | Figure 6 Scatter diagram between the serum alkaline phosphatase and number of fractures in patients after 3 years of treatment. |
Concerning the bone alkaline phosphatase before and after antiresorptive treatment, we found decreased values after the treatment (Figure 7).
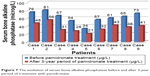  | Figure 7 The evolution of serum bone alkaline phosphatase before and after 3-year period of treatment with pamidronate. |
We analyzed the serum levels of osteocalcin and β-crosslaps in patients before and after pamidronate treatment (Table 3). In our study, we have a decreased level of osteocalcin and β-crosslaps (β–CTx) after treatment.
  | Table 3 The serum levels of osteocalcin and β-crosslaps (β–CTx) in these patients before and after pamidronate treatment |
Discussion
The current paper presents our experience over a period of 10 years related to evolution and treatment in children diagnosed with osteoporosis and OI, demonstrating the beneficial effect of pamidronate for bone density, mobility and quality of life.
Bisphosphonates are a class of medication frequently prescribed for the treatment of many bone disorders in adults such as primary and secondary osteoporosis, hypercalcemia secondary to different cancers or Paget’s disease.
The effect of bisphosphonates is based on the analogy with pyrophosphate and enabled joining the bone surfaces in contact with osteoclasts. This is the place where they exert their therapeutic activity. The mechanisms by which bisphosphonates act on osteoclast level can be divided into two categories: the physicochemical mechanism and the cellular one. It involves the increasing of osteoclast apoptosis together with the cellular functional damage. The apoptosis can be induced by both cytotoxic mechanisms and activation of mevalonate system.13 At the same time, the structure of the bisphosphonates induces the increased calcium affinity of the bone. All bisphosphonates have in common two phosphonates and one carbon atom (P–C–P), responsible for the affinity of bisphosphonates for hydroxyapatite.
The pharmacokinetics and the bioavailability vary between bisphosphonates, being responsible for good clinical outcomes obtained with minimal adverse effects. They are poorly absorbed through the entire gastrointestinal tract because of their poor lipophilicity. The antiresorptive medication is rapidly incorporated into the bones.14 After intravenous administration of pamidronate, the half-life in plasma is 1–2 hours being further renally excreted. A terminal half-life of 10 years has been detectable for alendronate, while pamidronate urinary excretion was up to 8 years after the ending of this long treatment.15,16
In pediatric age, bisphosphonates can be prescribed in both primary and secondary osteoporosis, increasing the bone mass and reducing the fracture rate and the bone pain.
Although there are oral bisphosphonates (alendronate and risedronate) and parenterally administered forms (pamidronate, neridronate or zoledronate), there is no consensus regarding the optimal form, dosage or duration of therapy. There are some studies analyzing the effects of oral bisphosphonates in primary osteoporosis. The oral treatment with alendronate for a period of 2 years was responsible for the decreased bone turnover and increased lumbar BMD,17 while the oral administration of risedronate increased the lumbar BMD, but weaker compared with intravenous pamidronate.18 In terms of the way of administration, the intravenous bisphosphonates are more efficient comparing with the oral forms in treating osteoporosis in children.19,20
The treatment of moderate and severe primary osteoporosis from OI with intravenous bone antiresorptive is approved in children by the European Medicines Evaluation Agency (EMEA) and Food and Drug Administration (FDA).21
Therapeutic efficacy in children with OI can be judged by the clinical and biological criteria, the decreased bone pain and the walking improvement with increased overall mobility, the reduced fracture rate, the elevated total BMD by increased trabeculae number and/or their mineralization, increased vertebral height preventing skeletal deformities and a global improvement in the quality of life. There is no adverse effect on growth.22
In the presented cases, the intravenous infusions with pamidronate showed a good tolerance without adverse effects or events. It was administered during 5–6 hours with a small infusion rate and a large fluid volume because high dilution makes a good tolerability.
The real benefit of this case series can be seen by the long-term follow-up of all studied patients. The height increase was observed in five patients who still had open growth cartilages at the beginning of pamidronate treatment, with a normal height according to their sex and age later. Other bisphosphonate effects described in our patients were: increased mobility and BMD showed by the Z-score at DXA evaluation, decreased pain and fracture rate and improved quality of life.
We presented our experience with patients with OI, treated with pamidronate for a minimum period of 3 years, a longer time compared to other studies where the treatment period was between 1 and 2 years.19–21 This means that our patients benefited by this medication for a longer period, explaining the good outcomes.
No long-term side effects were described. In a boy with severe OI, lost to follow-up for 1 year and 7 months, the treatment was interrupted; during this period, he had eight fractures caused by minor trauma. The literature mentions the effect of pamidronate for 8–10 years,23 but this statement was not confirmed in this case. It is recommended in such cases the continuation of bisphosphonate “rescue therapy” until the linear growth is possible.24
In patients treated for more than 2–3 years, we have identified bone bands on X-ray, as per the reported medical literature.25 They may occur after a longer period. It is quite sure that the post-therapeutic evolution of patients is greatly improved for both patient and family, who “escape” from a totally dependent patient, collaborating with a patient with cvasinormal mobility.
One patient from the orphanage had to stop this treatment for a period of 2 years in which he suffered other eight fractures. When he was brought again in our hospital, he restarted the treatment with pamidronate. He recovered his height with 3 cm but not adequate to his age and sex. This was the reason why we associated the growth hormone (GH) with the antiresorptive drug and the height improved with 8 cm in a year.
The effects of bisphosphonates in combination with recombinant human GH in pediatric OI patients were presented in the medical literature. This combined treatment gives an improvement in BMD and ensures a lumbar spine projected area, particularly in patients with quantitative defects. It is also responsible for the growth velocity and reduces the fracture risk.26
Puberty was not influenced under the bisphosphonate treatment. Two adolescent girls were stage V Tanner at the beginning of this therapy.
Biological investigations such as serum and bone alkaline phosphatase, osteocalcin and β-crosslaps bring us suggestive data for the diagnosis. Serum alkaline phosphatase is not a good marker for osteoporosis in children, while bone alkaline phosphatase shows a good correlation between clinical aspects and evolution under treatment.
Osteocalcin is produced by osteoblasts, and it is an important marker of bone formation and osteoblastic activity. Physiologically, serum osteocalcin is increased in children, particularly during the first year of life and during puberty period characterized by rapidity of physical growth.27
β-crosslaps are secreted and released into the bloodstream by osteoclasts during bone resorption together with a mixture of protease which degrade the collagen fibrils into molecular fragments. It is an important marker for the degradation of mature type I collagen and bone resorption. Its value is highest in neonates, reaches a nadir between 1 and 9 years of age and increases again during early adolescence in both sexes, attaining its peak earlier in girls than in boys.28 Pathological elevated levels of β-crosslaps are associated with osteoporosis and osteopenia.
When analyzing the serum levels of osteocalcin and β-crosslaps before and after pamidronate treatment, we observed a decrease in these bone markers with more than 20% for osteocalcin and more than 25% of the β-crosslaps after 6 months of pamidronate treatment.
The decrease in the serum osteocalcin after pamidronate treatment is a marker of an efficient response to it. The β-crosslaps decreased after antiresorptive medication, which means the improvement in bone metabolism with a stabile bone formation matrix under treatment, despite no statistical significance. All these factors demonstrate the complex mechanisms of this class of medication involved in bone matrix in OI patients.
According to our study, we can conclude that the serum alkaline phosphatase is not the best marker for the disturbance of the bone metabolism in OI but bone alkaline phosphatase together with serum osteocalcin is important bone turnover markers useful in the diagnosis and the monitoring of therapy in bone diseases.
The limitations of our study include the impossibility to perform genetic tests. In addition, it was very difficult to know in the cases presented, if OI was a familial disease or was a novo mutation because two patients came from orphanage.
In all our cases, the quality of life was improved for the patient and the family. Significant differences were found in the physical domain scores before and after antiresorptive treatment especially regarding body pain (43 versus 78, P<0.05) and physical functions (35 versus 85, P<0.05).
Conclusion
Treatment with pamidronate is beneficial for the patient with OI, family and society and increased the quality of life. It improved bone density and the clinical symptoms, improved mobility, reduced fracture frequency and prevented severe deformities. A significant improvement in the ability of walking and in the psycho-affective tonus of all patients with OI admitted in our hospital and their families was obtained after treatment with pamidronate. The independence and the autonomy of these patients increased, being able to move and/or to carry out normal light activities without fracture risk. Serum alkaline phosphatase is not a good marker for osteoporosis in children, while bone alkaline phosphatase significantly decreases after therapy with pamidronate and shows a good correlation between clinical aspects and evolution under treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–1385. | ||
OI Foundation [homepage on the Internet]. Available from: http://www.oif.org/site/PageServer?pagename=oif_mc_home_page. Accessed February 2017. | ||
Orphanet [webpage on the Internet]. Osteogenesis Imperfecta. Available from: http://www.orpha.net/consor/cgi-bin/OC_Exp.php?Expert=666. Accessed February 2017. | ||
Ramachandran M, Gellman H, Achan P, et al [webpage on the Internet]. Osteogenesis Imperfecta. Available from: http://emedicine.medscape.com/article/1256726-overview. Accessed February 2017. | ||
Leiden University Medical Center [database on the Internet]. Osteogenesis Imperfecta Variant Database. Available from: https://oi.gene.le.ac.uk. Accessed February 2017. | ||
Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7(9):540–557. | ||
Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387(10028):1657–1671. | ||
Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39(3):359–365. Erratum in. Nat Genet. 2008;40(7):927. | ||
Barnes AM, Carter EM, Cabral WA, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362(6):521–528. | ||
GABachrach LK, Sills IN. The Section on Endocrinology. Clinical report from the American Academy of Pediatrics – Bone Densitometry in Children and Adolescents. Pediatrics. 2011;127(1):189–194. | ||
International Society for Clinical Densitometry. 2007 Pediatric Official Position. Available from: http://www.iscd.org/wp-content/themes/iscd/pdfs/official-positions/ISCD2007OfficialPositions-Pediatric.pdf. Accessed February 2017. | ||
International Society for Clinical Densitometry. 2013 Adult& Pediatric Official Position. Available from: http://www.iscd.org/documents/2014/02/2013-iscd-official-position-brochure.pdf. Accessed February 2017. | ||
Szczepaniak-Kubat A, Kurnatowska O, Jakubowska-Pietkiewicz E, Chlebna-Sokół D. Assessment of quality of life of parents of children with osteogenesis imperfecta. Adv Clin Exp Med. 2012;21(1):99–104. | ||
Cremers S, Papapoulos S. Pharmacology of bisphosphonates. Bone. 2011;49:42–49. | ||
Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Porras AG. Clinical pharmacology of alendronate sodium. Osteoporos Int. 1993;3(suppl 3):S13–S16. | ||
Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. N Engl J Med. 2007;356:1075–1076. | ||
Ward LM, Rauch F, Whyte MP, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96:355–364. | ||
Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Bone Miner Res. 2009;24:1282–1289. | ||
Letocha AD, Cintas HL, Troendle JF, et al. Controlled trial of Pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005;20(6):977–986. | ||
Kok DH, Sakkers RJ, Janse AJ, et al. Quality of life in children with osteogenesis imperfecta treated with oral bisphosphonates (Olpadronate): a 2-year randomized placebo-controlled trial. Eur J Pediatr. 2007;166(11):1155–1161. | ||
Adler RA, Fuleihan GEH, Bauer CD, et al. Managing osteoporosis patients after long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16–35. | ||
Baroncelli GI, Bertelloni S. Use of bisphosphonates in the treatment of pediatric osteoporosis. Horm Res Paediatr. 2014;82(5):290–302. | ||
Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(suppl 2):S150–S162. | ||
Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of Pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339(14):947–952. | ||
Somalo L, Santos F. Clinical use of bisphosphonates in children. World J Pediatr. 2007;3(4):245–253. | ||
Antoniazzi F, Monti E, Venturi G, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfect. Eur J Endocrinol. 2010;163(3):479–487. | ||
Cioffi M, Molinari AM, Gazzerro P, et al. Serum osteocalcin in 1634 healthy children. Clin Chem. 1997;43(3):543–545. | ||
Crofton PM, Evans N, Taylor MR, Holland CV. Serum CrossLaps: pediatric reference intervals from birth to 19 years of age. Clin Chem. 2002;48(4):671–673. | ||
Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16(2):101–116. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

