Back to Journals » Drug Design, Development and Therapy » Volume 12
Therapeutic effect of protease-activated receptor 2 agonist SLIGRL-NH2 on loperamide-induced Sprague-Dawley rat constipation model and the related mechanism
Authors Zhang Y, Ge T, Xiang P, Mao H, Tang S, Li A, Lin L, Wei Y
Received 22 December 2017
Accepted for publication 24 April 2018
Published 1 August 2018 Volume 2018:12 Pages 2403—2411
DOI https://doi.org/10.2147/DDDT.S160628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Yonggang Zhang,1 Tingrui Ge,1 Ping Xiang,1 Haibing Mao,1 Shumin Tang,1 Aimin Li,2 Lin Lin,3 Yinting Wei4
1Department of Colorectal Surgery, The First People’s Hospital of Lianyungang, Lianyungang 222002, China; 2Department of Neurosurgery, The First People’s Hospital of Lianyungang, Lianyungang 222002, China; 3Department of Gastroenterology, Jiangsu Province Hospital, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China; 4Department of Gastroenterology, Lianyungang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Lianyungang 222000, China
Purpose: To investigate the therapeutic effects of protease-activated receptor 2 (PAR-2) agonist SLIGRL-NH2 on loperamide-induced Sprague-Dawley (SD) rat constipation animal models.
Materials and methods: Loperamide was injected subcutaneously to induce constipation twice a day for 3 days. SD rats (n = 30) were randomly divided into five groups: non-constipation group (control, n = 6), constipation group (constipation, n = 6), constipation + SLIGRL-NH2 low-dosage group (SLIGRL-NH2 low, n=6), constipation + SLIGRL-NH2 high-dosage group (SLIGRL-NH2 high, n = 6), and constipation + prucalopride (positive control, n = 6). The SLIGRL-NH2 low group and SLIGRL-NH2 high group were administered with 2.5 μmol/kg and 5 μmol/kg SLIGRL-NH2, respectively, and the prucalopride group received 2 mg/kg prucalopride. The control and constipation group received 1× PBS under the same pattern. SLIGRL-NH2 and prucalopride were orally administrated once daily for 7 days. On the final day of oral administration, food intake, water intake, the number of stool pellets, weight, and fecal water content was calculated; moreover, the colons of rats in different groups were collected and histological features were examined by hematoxylin and eosin staining; furthermore, the expression of anoctamin-1 was determined by Immunohistochemical methods, and the expressions of c-kit and PAR-2 were examined using real-time quantitative polymerase chain reaction and Western blot methods; finally, the expressions of neurotransmitter vasoactive intestinal peptide (VIP) and substance P (SP) were examined using enzyme-linked immunosorbent assay methods.
Results: The feeding and excretion behaviors, intestinal transit ratio, and the histological feature of the colon in the constipated rats were all improved by SLIGRL-NH2 treatment; moreover, SLIGRL-NH2 treatment induced significant increase in the expression of PAR-2 and also increased number of interstitial Cajal cells. Furthermore, SLIGRL-NH2 also decreased the contents of the inhibitory neurotransmitter VIP and increased the expression of the excitatory neurotransmitter SP. High dose of SLIGRL-NH2 has shown similar anti-constipation effects as prucalopride.
Conclusion: These results suggested that SLIGRL-NH2 can enhance gastrointestinal transit and alleviate in rats with loperamide-induced constipation.
Keywords: constipation, PAR-2, SLIGRL-NH2, loperamide, interstitial Cajal cells
Introduction
Constipation is a common gastrointestinal disorder characterized by difficulties during defecation, infrequent bowel movements, hard and dry feces, and incomplete bowel evacuation.1 A variety of therapies have been developed to treat constipation; however at current stage, there is no golden standard for treating constipation, and the best method for treatment of constipation is to include more fiber into the diet of the patients, recommend the patients to drink plenty of fluids, and do more exercise.2,3 In clinical applications, chemical laxatives, for example, gaviscon, correctol, senokot, and senna, have often been used to help patients pass the stool.4 However, most of these chemicals have undesirable side effects, which can cause damage to the cardiovascular system, for example, contraction of the artery and even myocardial infarction.5 Therefore, to identify novel medications with minimal side effects is a great need for treatment of constipation.
Protease-activated receptor 2 (PAR-2) belongs to the family of the PARs, and it is a transmembrane protein that couples to the guanosine nucleotide binding proteins.6 In human body, PAR-2 is widely expressed in different tissues.7 PAR-2 can be activated by the stimulation of different factors, for example, inflammation, bacterial infection, and endogenous trypsin.8,9 PAR-2 has also been reported to participate in the pathogenesis of many gastrointestinal diseases;10,11 however, the relationship between PAR-2 and constipation still requires further investigation.
Interstitial Cajal cells (ICCs) are a population of cells that can regulate gastrointestinal motility. It has been observed in previous studies that submucosal ICCs can generate smooth muscle electrical slow waves that determine the contractile activity of the smooth muscle.12 Decreased number of ICCs have been observed in the colon tissue samples of patients with constipation, which has been recognized as an important reason for disrupted motility of colon in constipation.13,14 However, to our knowledge, studies on relationship between PAR-2 and ICC were limited.
Loperamide is a peripheral m-opioid receptor agonist, and it has been widely applied for establishing constipation animal models.1,15,16 In the present study, we will focus on the effect of PAR-2 agonist SLIGRL-NH2 on loperamide-induced Sprague-Dawley (SD) rat constipation models. We hypothesized that SLIGRL-NH2 can alleviate the symptoms of constipation by increasing the number of ICCs, and results of our study may provide novel methods for treatment of constipation with improved therapeutic efficacy.
Materials and methods
Animals and treatment
The animal studies have been approved by the Animal Ethics Committee of The First People’s Hospital of Lianyungang. Thirty adult SD rats (all males, 3–4 weeks, weight 50–80 g) were maintained in a specific pathogen-free state at 23°C±2°C and 50%±10% humidity under 12/12 hours of light–dark cycle with ad libitum and standard diet and water. Rats (n=30) were randomly divided into five groups: non-constipation group (control, n = 6), constipation group (constipation, n = 6), constipation + 2.5 μmol/kg SLIGRL-NH2 group (SLIGRL-NH2 low, n = 6), constipation + 5 μmol/kg SLIGRL-NH2 (SLIGRL-NH2 high, n = 6), and constipation + prucalopride (positive control, n = 6). The dose of the SLIGRL-NH2 was determined as described by Kim et al.17 Constipation was induced by subcutaneous injection of loperamide (4 mg/kg) twice a day for 3 days, and the rats in the control group was injected with saline. After establishing the constipation models, the SLIGRL-NH2 low group and SLIGRL-NH2 high group were orally administered with 2.5 μmol/kg and 5 μmol/kg SLIGRL-NH2, respectively, and the prucalopride group received 2 mg/kg prucalopride. The control and constipation group received 1× PBS. Seven days after different treatment, all rats were sacrificed, and the colons were collected and divided into two parts, one part was embedded with paraffin and the other part was stored in liquid nitrogen until future analysis. This study has been performed in strict accordance with the guideline for the care and use of laboratory animals of the National Institutes of Health.
Evaluation of the feeding and excretion behaviors of the rats
On the final day of oral administration, the food intake and water intake of all the rats were recorded. The stool pellets of each rat were collected and numbers as well as weights of the pellets were recorded. The fecal moisture content was calculated according to the following equation: Fecal moisture content (%) = [(wet weight − dry weight) ÷ wet weight] × 100.
Intestinal transit ratio
Intestinal transit ratio was determined as described previously. Briefly, on the final day of the experiment, 10% charcoal aqueous suspension was prepared and administered orally at a volume of 2 mL. Forty minutes later, all rats were sacrificed and small intestines were collected, and total length as well as the distance covered by the charcoal were measured. The intestinal transit ratio was calculated as follow: Intestinal transit ratio (%) = (distance covered by the charcoal ÷ the length of the small intestine) × 100.
Hematoxylin and eosin (H&E) staining
The colons were collected from the rats and fixed with 10% formalin for 48 hours and then embedded with paraffin and sectioned into 5 μm slices. The sections were then stained with H&E (Sigma-Aldrich Co., St. Louis, MO, USA) using previously described methods. Then the morphological features of the colon samples were observed by light microscopy (Leica Microsystems, Wetzlar, Germany).
Real-time quantitative polymerase chain reaction (RT-qPCR)
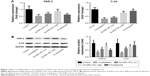
Total RNAs were extracted from the colon tissue samples of the rats using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA), and RT-qPCR has been performed to examine the expressions of c-kit and PAR-2 in different tissue samples using the SYBR ExScript RT-PCR kit (TaKaRa Bio Inc., Kusatsu, Japan). ABI 7300 RT PCR System (Thermo Fisher Scientific, Waltham, MA, USA) has been used for the amplification process. The thermo-cycling profiles were as follow: 95°C for 30 seconds; followed by 40 cycles of 95°C for 5 seconds and 60°C for 30 seconds. Primers were all synthesized by Sangon Biotech Engineering (Shanghai) Co. Ltd. (Shanghai, China). The relative expression of c-kit and PAR-2 in each sample was normalized to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCt method. In Figure 1, the relative expressions of c-kit and PAR-2 in other groups were presented as fold changes of the 2−ΔΔCt value compared to the average 2−ΔΔCt value of the control group, and the average 2−ΔΔCt value of the control group was set as onefold. The sequences of the primers were as follow: PAR-2, forward: 5′-GACTTTCTCTCGGTGCGTCC-3′; reverse: 5′-CCCCATAAATCCAGTTGTTGCC-3′. C-kit, forward: 5′-CCGACGCAACTTCCTTATGAT-3′; reverse: 5′-TCAGGACCTTCAGTTCCGACA-3′, GAPDH, forward: 5′-AGAAGGCTGGGGCTCATTTG-3′; reverse: 5′-AGGGGCCATCCACAGTCTTC-3′.
Western blotting
The colon tissues of the rats were lysed by radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China), and the concentration of the protein in each sample was determined by bicinchoninic acid kit (Beyotime Institute of Biotechnology). Then electrophoresis was performed, and the proteins on the gel were transferred onto polyvinylidene fluoride membranes (MilliporeSigma, Burlington, MA, USA). Next, the membranes were blocked by 5% nonfat milk and incubated with the primary antibodies (anti-c-kit cat.ab32363, 1:1,000, anti-PAR-2 cat.ab138479, 1:1,000 and anti-GAPDH cat.ab9485, 1:2,000, all purchased from Abcam plc, Cambridge, UK) overnight at 4°C. On day 2, the membranes were washed and incubated with horseradish peroxidase conjugated secondary antibodies (cat.ab6721, 1:5,000, purchased from Abcam plc) at room temperature for 45 minutes. Finally, the membranes were washed again and incubated with the enhanced chemiluminescent reagent (Beyotime Institute of Biotechnology). The signals were detected using Tanon 6100 Chemiluminescent Imaging System (Tanon Science & Technology Co., Ltd., Shanghai, China).
Immunohistochemical (IHC) analysis
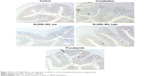
Tissue samples were embedded with paraffin and sectioned into 4 mm slides for the immunohistochemistry analysis. IHC was performed with ready-to-use immunohistochemistry hypersensitivity UltraSensitiveTM S-P kit (Maxim Integrated, San Jose, CA, USA) following the manufacturer’s protocols. Briefly, the tissue sections were deparaffinized and rehydrated, and then heat-fixed with the protein-blocking solution. Sections were subsequently incubated with primary antibodies (anti-anoctamin-1 [ANO1], cat.ab64085, 1:100, Abcam plc), followed by horseradish peroxidase-labeled secondary antibody. Diaminobenzidine was used for colorization.
Enzyme-linked immunosorbent assay (ELISA)
To examine the expressions of substance P (SP) and vasoactive intestinal peptide (VIP) in the colons of the rats, 100 mg colon of each rat was homogenated in PBS and centrifuged. The supernatants were collected and detected by SP or VIP ELISA kits (all purchased from Biocalvin, Suzhou, China) according to the manufacturer’s protocol.
Statistical analysis
All statistical analysis was performed using SPSS 19.0. software (IBM Corporation, Armonk, NY, USA). Data were presented as mean ± standard deviation, and one-way analysis of variance was performed for the comparison between multiple groups. p<0.05 has been considered as significant.
Results
Effect of SLIGRL-NH2 on the feeding and excretion behavior of loperamide-induced constipated rats
First of all, we investigated the effect of SLIGRL-NH2 on the feeding and excretion behaviors of the constipated rats. It was observed that compared with the control group, loperamide induced significant decrease in the food and water intake of the rats, while high dose of SLIGRL-NH2 and prucalopride treatment increased the food and water intake of the constipated rats (Table 1). The effect of low dose of SLIGRL-NH2 on the feeding behavior of the rats is not significant (p>0.05); moreover, loperamide also induced marked decrease in the fecal parameters of the rats, including the number, weight, and water contents of the fecal pellets. On the other hand, high dose of SLIGRL-NH2 and prucalopride treatment improved the fecal parameters of the constipated rats (Table 2, p<0.05).
  | Table 1 Effect of SLIGRL-NH2 on the feeding behavior of rats in different groups (mean ± standard deviation) |
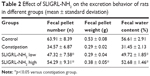  | Table 2 Effect of SLIGRL-NH2 on the excretion behavior of rats in different groups (mean ± standard deviation) |
Effect of SLIGRL-NH2 on the intestinal transit ratio of loperamide-induced constipated rats
Next, we further explored the effect of SLIGRL-NH2 on the intestinal transit ratio of the constipated rats. As shown in Table 3, loperamide treated rats have shown significant decrease in the intestinal transit ratio compared with the control group (p<0.05), and SLIGRL-NH2 treatment significantly increased the intestinal transit ratio of the constipated rats in a dose-dependent manner. Prucalopride treatment has shown similar effect as the high dose of SLIGRL-NH2.
  | Table 3 Effect of SLIGRL-NH2 on the intestinal transit ratio of loperamide-induced constipated rats (mean ± standard deviation) |
Effect of SLIGRL-NH2 on histological features of colon in loperamide-induced constipated rats
Furthermore, colons of the rats were collected and embedded with paraffin, and H&E staining has been performed to investigate the effect of SLIGRL-NH2 on the histological features of the constipated rats. We have observed a significant decrease in the thickness of the muscle layer, the number of goblet cells, and enterocytes in constipation group compared with the control group. On the other hand, the above histological features were significantly improved by the treatment of SLIGRL-NH2 or prucalopride (Figure 2 and Table 4, p<0.05) compared with the constipation group.
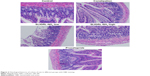  | Figure 2 Histological features of colons of rats in different groups with H&E staining. |
Effect of SLIGRL-NH2 on the expression of ANO1, PAR2, c-kit, and neurotransmitter VIP and SP in colons of loperamide-induced constipated rats
Finally, we investigated the underlying mechanism of the therapeutic effect of SLIGRL-NH2 for the treatment of constipation. The expressions of ANO1, PAR-2, c-kit, VIP, and SP in the colons of rats in different groups were examined. Using IHC methods, we observed that the expression of ANO1 was significantly increased by SLIGRL-NH2 or prucalopride treatment (Figure 3); moreover, as a PAR-2 agonist, SLIGRL-NH2 treatment induced significant increase in the expression of PAR-2 in constipated rats, while on the other hand, prucalopride had no significant effect on the expression of PAR-2 in constipated rats (Figure 1); furthermore, SLIGRL-NH2 or prucalopride also induced significant increase in the expression of c-kit, which a biomarker of ICCs, on both mRNA and protein level, and also lead to significant decrease in the contents of inhibitory neurotransmitter VIP and increased the expression of excitatory neurotransmitter SP (Figure 4, p<0.05).
Discussion
In the present study, we have explored the therapeutic effect of PAR-2 agonist SLIGRL-NH2 on loperamide-induced rat constipation models. We observed that SLIGRL-NH2 can alleviate the symptoms of constipation through increasing the number of ICCs in the colons of constipated rats. Our results have provided novel evidences that SLIGRL-NH2 can improve the feeding and excretion behaviors, and also improve the neuronal functions of the constipated rats.
Nowadays, the options for the management of chronic constipation were limited. Current methods for treatment of constipation include laxatives, saline, stimulants, and osmotics; however, in more than 50% of the cases, the therapeutic effects of current methods were unsatisfactory,18 which highlights the importance of searching for a more effective medication with improved therapeutic efficacy. The effects of PAR-2 on gastrointestinal transit function have been discussed in many previous studies. However, it is still controversial whether PARs can exert inhibitory or excitatory effect on gastrointestinal motility. PAR-2-derived receptor-activating peptide SLIGRL-NH2 can facilitate the gastrointestinal transit in a dose-dependent manner in mice,19 and the other study demonstrated that the activation of PAR-2-induced biphasic motor responses in the motility of gastrointestinal by enhancing spontaneous contractility and relaxation at the early phase (14 days) and excitation at the late phase (30 days).20 In current studies, the laxative effects of drugs were commonly evaluated by measuring the feeding and excretion parameters, including the food and water intake, the number, weight, and water content of the fecal pellets;21 moreover, the changes in the histopathological features of the colon, such as thickness of the muscle layer, the number of goblet cells, and enterocytes may also reflect the therapeutic efficacy of the drug. Loperamide has been known to increase the evacuation time of the stools, and on the other hand, also delayed the movement of the intestinal wall.22 In the present study, we used loperamide to establish the constipation rat models. Prucalopride is a selective 5-HT4 receptor agonist, and it has been widely used in many countries as one of the first-line medication for the management of chronic constipation.23 In the present study, prucalopride has been used as the positive control. We observed that administration of either prucalopride or 5 μmol/kg SLIGRL-NH2 significantly improved loperamide-induced decrease in food intake, water intake, the number, weight, and water contents of the fecal pellets of the constipated rats; moreover, prucalopride, 2.5 μmol/kg and 5 μmol/kg SLIGRL-NH2 also increased in the thickness of the muscle layer, the number of goblet cells, and enterocytes in the colons of the constipated rats. These results suggested that SLIGRL-NH2 can improve the feeding and excretion behaviors as well as the histopathological structure of colon in constipated rats.
To further explore the effects of SLIGRL-NH2 on the gastrointestinal function of the constipated rats, the gastrointestinal motility in different groups were also examined by charcoal aqueous suspension methods as described previously.22 We observed that loperamide induced significant delayed intestinal luminal transit, which was consistent with previous findings, and SLIGRL-NH2 treatment can increase the intestinal transit ratio of the constipated rats in a dose dependent manner. These results indicated that SLIGRL-NH2 has, at least partly, laxative effects that can improves the gastrointestinal motility.
c-kit has been originally identified as a proto-oncogene, and it belongs to the receptor tyrosine kinase superfamily. In some recent findings, the regulatory roles of c-kit in maintaining the ICC network have been discussed. c-kit has restricted expression pattern, it was highly expressed on the surface of mast cells, hematopoietic cells, as well as ICC. In the field of gastrointestinal motility study, it has now been widely recognized that the number of ICCs in the intestinal can be quantified by examining the expression of c-kit.24–27 ANO1 (also named transmembrane member 16A [TMEM16A]) is a calcium-activated chloride channel that expressed in epithelial cells and smooth muscle cells.28 It has been proved that ANO1 was highly expressed in ICCs, and now ANO1 has been considered as a selective marker of ICCs. Interestingly, PAR-2 can regulate the pacemaker activity of colonic ICCs,29 and the other study indicated that PAR-2 may modulate the excitability of colonic smooth muscles in rats via affecting the colonic ICCs.30 Thus, to determine whether ICC was involved in the mechanism of SLIGRL-NH2 induced anti-constipation effects, the number of ICCs in the colons of different treatment were quantified by examining the expression of c-kit and ANO1. It was observed that loperamide treatment induced significant decrease in the expression of c-kit and ANO1, while SLIGRL-NH2 or prucalopride treatment can partially increase the expression of c-kit and ANO1; furthermore, dysfunction of the neurotransmitter VIP and SP may contribute to the incidence of constipation,31 and in the present study, we observed that SLIGRL-NH2 or prucalopride also lead to significant decrease in the contents of inhibitory neurotransmitter VIP and increased the expression of excitatory neurotransmitter SP. Taken together, our results indicated that administration of SLIGRL-NH2 can increase the number of ICCs as well as the expression of neurotransmitters in the colons of constipated rats, improve the excitability of colonic smooth muscle cells, and alleviate the symptoms of constipation, and the high dose of SLIGRL-NH2 has shown similar effects as prucalopride.
Our studies have limitations. In the present study, we mainly focused on the short time (within 1 week) anti-constipation effects of SLIGRL-NH2; however, whether long-term administration of SLIGRL-NH2 can lead undesirable side effects is still unknown. It has been discussed that intracolonic PAR-2 activation (using trypsin, tryptase, or the peptide SLIGRL-NH2) results in mucosal damage and inflammation,32 bowel wall thickening, and release of myeloperoxidase activity, which may cause colitis or inflammatory bowel diseases.33 So in future study, we will investigate long-term side effects of SLIGRL-NH2 to determine the feasibility as well as the optimum dose of SLIGRL-NH2 for the treatment of constipation.
Conclusion
We reported for the first time that SLIGRL-NH2 can enhance gastrointestinal transit and alleviate in rats with loperamide-induced constipation. Although further investigations still need to be performed for exploring the underlying mechanism as well as the safety issue, our study has provided potential therapeutic methods for treatment of constipation.
Acknowledgment
This study was supported by the Post-doc Fund of Jiangsu Province, the Post-doc Fund of Lianyungang, and Fund from the “521 High-Level Talents Training Project” in Lianyungang.
Disclosure
The authors report no conflicts of interest in this work.
References
Kim JE, Lee YJ, Kwak MH, et al. Metabolomics approach to serum biomarker for loperamide-induced constipation in SD rats. Lab Anim Res. 2014;30(1):35–43. | ||
Kartal A, Yalcin M, Citgez B, Uzunkoy A. The effect of chronic constipation on the development of inguinal herniation. Hernia. 2017;21(4):531–535. | ||
Krogh K, Chiarioni G, Whitehead W. Management of chronic constipation in adults. United European Gastroenterol J. 2017;5(4):465–472. | ||
Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(Suppl 1):S5–S21. | ||
Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015;5:CD003448. | ||
Indrakusuma I, Romacho T, Eckel J. Protease-activated receptor 2 promotes pro-atherogenic effects through transactivation of the VEGF receptor 2 in human vascular smooth muscle cells. Front Pharmacol. 2016;7:497. | ||
Wang Y, He Y, Wang M, Lv P, Liu J, Wang J. Role of protease-activated receptor 2 in regulating focal segmental glomerulosclerosis. Cell Physiol Biochem. 2017;41(3):1147–1155. | ||
de Boer JD, Van’t Veer C, Stroo I, et al. Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun. 2014;20(6):618–625. | ||
Lee SE, Kim JM, Jeong SK, Choi EH, Zouboulis CC, Lee SH. Expression of protease-activated receptor-2 in SZ95 sebocytes and its role in sebaceous lipogenesis, inflammation, and innate immunity. J Invest Dermatol. 2015;135(9):2219–2227. | ||
Suckow SK, Anderson EM, Caudle RM. Lesioning of TRPV1 expressing primary afferent neurons prevents PAR-2 induced motility, but not mechanical hypersensitivity in the rat colon. Neurogastroenterol Motil. 2012;24(3):e125–e135. | ||
Suckow SK, Caudle RM. NMDA receptor subunit expression and PAR2 receptor activation in colospinal afferent neurons (CANs) during inflammation induced visceral hypersensitivity. Mol Pain. 2009;5:54. | ||
He CL, Burgart L, Wang L, et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118(1):14–21. | ||
Shafik A, Shafik AA, El-Sibai O, Shafik IA. Interstitial cells of cajal in patients with constipation due to total colonic inertia. J Invest Surg. 2006;19(3):147–153. | ||
Wang LM, McNally M, Hyland J, Sheahan K. Assessing interstitial cells of Cajal in slow transit constipation using CD117 is a useful diagnostic test. Am J Surg Pathol. 2008;32(7):980–985. | ||
Chen J, Liu C, Xie L, et al. [Effect of protease-activated receptor-2 agonist on gastrointestinal motility in mice]. J Chin Pract Diag Ther. 2016;30(1):39–41. Chinese [with English abstract]. | ||
Kim JE, Go J, Koh EK, et al. Gallotannin-enriched extract isolated from Galla Rhois may be a functional candidate with laxative effects for treatment of loperamide-induced constipation of SD Rats. PLoS One. 2016;11(9):e0161144. | ||
Kim JE, Go J, Sung JE, et al. Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats. Lab Anim Res. 2016;32(1):16–23. | ||
Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25(5):599–608. | ||
Sriwai W, Mahavadi S, Al-Shboul O, Grider JR, Murthy KS. Distinctive G Protein-dependent signaling by protease-activated receptor 2 (PAR2) in smooth muscle: feedback inhibition of RhoA by cAMP-independent PKA. PLoS One. 2013;8(6):e66743. | ||
Fernandez-Blanco JA, Hollenberg MD, Martinez V, Vergara P. PAR-2-mediated control of barrier function and motility differs between early and late phases of postinfectious gut dysfunction in the rat. Am J Physiol. 2013;304(4):G390–G400. | ||
Kim JE, Go J, Sung JE, et al. Uridine stimulate laxative effect in the loperamide-induced constipation of SD rats through regulation of the mAChRs signaling pathway and mucin secretion. BMC Gastroenterol. 2017;17(1):21. | ||
Li C, Nie SP, Zhu KX, et al. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr. 2015;66(5):533–538. | ||
Bassotti G, Gambaccini D, Bellini M. Prucalopride succinate for the treatment of constipation: an update. Expert Rev Gastroenterol Hepatol. 2016;10(3):291–300. | ||
Breuer C, Oh J, Molderings GJ, et al. Therapy-refractory gastrointestinal motility disorder in a child with c-kit mutations. World J Gastroenterol. 2010;16(34):4363–4366. | ||
Yin J, Liang Y, Wang D, et al. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. 2018;41(2):649–658. | ||
Chai Y, Huang Y, Tang H, et al. Role of stem cell growth factor/c-Kit in the pathogenesis of irritable bowel syndrome. Exp Ther Med. 2017;13(4):1187–1193. | ||
Yamada K, Onoda Y. Comparison of the effects of T-1815, yohimbine and naloxone on mouse colonic propulsion. J Smooth Muscle Res. 1993;29:47–53. | ||
Wang XY, Chen JH, Li K, Zhu YF, Wright GW, Huizinga JD. Discrepancies between c-Kit positive and Ano1 positive ICC-SMP in the W/Wv and wild-type mouse colon; relationships with motor patterns and calcium transients. Neurogastroenterol Motil. 2014;26(9):1298–1310. | ||
Shin DH, Kim MW, Choi S, et al. Regulation of the pacemaker activity of colonic interstitial cells of cajal by protease-activated receptors: involvement of hyperpolarization-activated cyclic nucleotide channels. Pharmacology. 2016;98(3–4):171–182. | ||
Sung TS, Kim HU, Kim JH, Lu H, Sanders KM, Koh SD. Protease-activated receptors modulate excitability of murine colonic smooth muscles by differential effects on interstitial cells. J Physiol. 2015;593(5):1169–1181. | ||
Moriya R, Fujikawa T, Ito J, et al. Pancreatic polypeptide enhances colonic muscle contraction and fecal output through neuropeptide Y Y4 receptor in mice. Eur J Pharmacol. 2010;627:258–264. | ||
Kawabata A, Matsunami M, Sekiguchi F. Gastrointestinal roles for proteinase-activated receptors in health and disease. Br J Pharmacol. 2008;153(Suppl 1):S230–S240. | ||
Lohman RJ, Cotterell AJ, Suen J, et al. Antagonism of protease-activated receptor 2 protects against experimental colitis. J Pharmacol Exp Ther. 2012;340(2):256–265. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.