Back to Archived Journals » ChronoPhysiology and Therapy » Volume 4
The use of melatonin for treating sleep disorders in patients with Parkinson's disease
Authors Srinivasan V, De Berardis D, Partonen T , Zakaria R, Othman Z
Received 22 March 2014
Accepted for publication 23 April 2014
Published 14 August 2014 Volume 2014:4 Pages 51—57
DOI https://doi.org/10.2147/CPT.S44802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Video abstract presented by Timo Partonen
Views: 4232
Venkataramanujam Srinivasan,1 Domenico De Berardis,2,3 Timo Partonen,4 Rahimah Zakaria,5 Zahiruddin Othman6
1Sri Sathya Sai Medical Educational and Research Foundation, Coimbatore, India; 2Psychiatric Service of Diagnosis and Treatment, Giuseppe Mazzini Hospital, Teramo, 3Department of Neuroscience and Imaging, Gabriele d'Annunzio University, Chieti, Italy; 4Department of Mental Health and Substance Abuse Services, National Institute for Health and Welfare, Helsinki, Finland; 5Department of Physiology, 6Department of Psychiatry, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
Abstract: Most patients with Parkinson's disease (PD) experience sleep-related problems, such as difficulty in initiating and maintaining sleep, excessive daytime sleepiness, sleep fragmentation, reductions in non-rapid eye movement (NREM) or rapid eye movement (REM) sleep, and REM sleep behavior disorder. Although motor symptoms of PD are treated with dopaminergic drugs, the nonmotor symptoms pose a big problem, and they often precede the onset of the disease. Treating the nonmotor symptoms, such as sleep and associated behavioral disorders, is beneficial, for it not only relieves the symptoms but also helps to slow the progression of the disease. Treating PD patients with melatonin has been shown to be beneficial in treating sleep and behavior problems. The finding of reduced expression of the MT1 and MT2 melatonin receptors in amygdalae and substantia nigra of PD patients supports the involvement of melatonergic system in the etiology of PD. Hence, the use of melatonin or its analogs may even be beneficial not only for improving sleep quality but also for enhancing neuroprotection in PD.
Keywords: REM sleep-behavior disorder, insomnia, melatonin receptors, circadian dysregulation
Introduction
Parkinson’s disease (PD) affects millions of people all over the world, and its key symptoms include tremor, rigidity, postural instability, and bradykinesia. Nonmotor symptoms of PD include rapid eye movement (REM) sleep behavior disorder (RBD), depression, and mania, which may often precede the onset of disease, and of these, RBD even serves as a preclinical marker of PD.1 The prevalence of sleep disturbances in PD is very high.2–6 Although the etiology of sleep disorders in PD is not clearly known, dysregulation of the circadian components of the sleep-regulating system is said to be involved in this disease.7 A number of studies involving PD patients have shown that both sleep induction and sleep maintenance are affected. The onset and timing of REM sleep is very much impaired in PD patients.8 Treatment of sleep disorders associated with PD with appropriate drugs will not only help in promoting sleep and reducing the severity of sleep problems but also will help in slowing the progression of PD itself.9 As melatonin, the major neurohormone secreted by the pineal gland, has significant circadian rhythm regulatory function through its MT1 and MT2 receptors in the central circadian clock, namely the suprachiasmatic nucleus (SCN), interest has been focused on the use of melatonin in treating sleep disorders associated with PD. This review discusses the sleep disturbances associated with PD and also the treatment options, mainly melatonin and its agonists in treating PD.
Circadian dysregulation in PD
There is much evidence for the possible involvement of the retinohypothalamic system in the etiology of PD, since circadian rhythm disturbances are common in this disease.10,11 The SCN seems to be intact in PD patients, and the possible cause for circadian rhythm sleep disorders in PD is that a decrease in melatonergic receptors occurs in the amygdala and the substantia nigra of PD patients,12 and that this reduction coexists with PD-associated sleep disturbances.
Parkinsonian symptoms themselves undergo circadian fluctuations.7 Patients with PD often experience worsening of symptoms in the afternoon and evening. They experience time-dependent responsiveness to dopaminergic stimulation.10 Fertl et al reported that levodopa-treated PD patients but not de novo PD patients showed a phase advance of the melatonin circadian rhythm.13,14 The phase advance in PD patients treated with l-dopa/decarboxylase inhibitor is possibly due to a central nervous dopaminergic effect elicited by L-dopa administration and not inherent to Parkinson’s disease per se.14 Bordet et al confirmed those results, and showed no changes in locomotor activity, cortisol, or temperature rhythms in de novo PD patients.15 In this study, a decrease in amplitude of circulating melatonin rhythm was also observed. In a recent study, PD patients with excessive daytime sleepiness had a significantly lower amplitude of the melatonin rhythm and 24-hour melatonin area under the curve (P<0.001) compared to patients without excessive daytime sleepiness (Epworth Sleepiness Scale score ≥10).11 In this study, circadian dysfunction may have underlain excessive sleepiness in PD. However, the nature of this association needs to be explored further in longitudinal studies.
Recently, many studies of molecular clock mechanisms regulating circadian physiology and behavior in mammals have been undertaken in the central circadian pacemaker, the SCN, and various peripheral tissues and cells.16 Several circadian genes, known as key “clock genes”, have been identified. Of these, PER1 and BMAL1 are regarded as the best markers of the molecular clock. Disruptions of BMAL1 and PER1 in mice have been shown to cause altered circadian behavior and dysregulation of circadian patterns in gene expression.17–19 The circadian clock genes PER1 and BMAL1 have been located in leukocytes of healthy humans and hence, study of these genes in patients with PD has been recently undertaken.20,21 PER1 and BMAL1 expression in leukocytes of patients with PD and normal controls were investigated between 9 pm and 9 am. It was noticed that the expression of BMAL1 but not PER1 was greatly reduced during this dark phase, suggesting that a peripheral molecular clock is altered in PD patients. Moreover, BMAL1 expression in PD patients correlated with the Unified PD Rating Scale score at 6 am and 9 am and with the Pittsburgh Sleep Quality Index Score at 6 am.20,21
Sleep disorders in PD
Sleep disorders constitute one of the major nonmotor features of PD, and even serve as a preclinical marker of the disease. Between 42% and 98% of patients with PD experience sleep-related symptoms.22,23 Sleep disturbances in PD encompass difficulty initiating sleep, frequent nighttime awakening and sleep fragmentation, nocturia, restless leg syndrome/periodic limb movements, sleep breathing disorders, drug-induced symptoms, RBD, sleep attacks, reduced sleep efficiency, and excessive daytime sleepiness.24
These sleep disorders are classified as primary sleep disorders that are intrinsic to PD and those that are secondary to medication, etc.25 Among the nonmotor symptoms of PD, sleep disturbances and particularly RBD are very important, and even predict the diagnosis of PD as ascertained by motor symptoms.26,27
RBD in PD
RBD in PD is poorly understood, and may occur as a prodromal feature predating motor symptoms by several years. Its prevalence is about 60% in PD patients, and it has been suggested to be predictive of dementia in longitudinal studies.26,27 However, mild cases of RBD may be missed by clinical interview but revealed by polysomnography. In the International Classification of Sleep Disorders, second edition, RBD is subcategorized under parasomnias usually associated with REM sleep, and polysomnography findings are required to establish the diagnosis.28 RBD is characterized by a loss of atonia, with prominent motor activity and dreaming.22 Numerous cases of RBD have been found in clinically diagnosed PD.29–31
The patient states of being, ie, wakefulness, non-REM sleep, and REM sleep, are not necessarily mutually exclusive, and components of these states may appear in various combinations, with intriguing clinical consequences. RBD is an intrusion of wakefulness into REM sleep, due to the absence of REM sleep atonia.24,32 The loss of REM sleep atonia and/or increased locomotor drive is suggested as the likely mechanism for the clinical expression of human RBD.31 The loss of REM sleep atonia manifests as a dream disorder similar to REM motor disorder, and there is a tendency for the dream content to involve an aggressive, attacking, or chasing theme. Nightmare behaviors like screaming, kicking, punching, and injuring the bed partner are quite common.29–31 Nocturnal disturbances and sleep arousals, as measured by actigraphy, are specific to the RBD seen in PD patients. In a study conducted on the hypothalamuses of eleven PD patients and five control subjects, a loss of hypothalamic hypocretin and melanin-concentrating hormone-producing neurons has been found in PD.33 However, a recent study undertaken with regard to these issues indicated that changes in orexin/hypocretin do not necessarily underpin RBD sleep disturbances.34 It is suggested that probing into the components of the circadian system that mediates the onset and timing of REM sleep, including the pattern and timing of melatonin secretion, combined with clinical pathological studies may prove to be vital for defining the neuroanatomical correlates of RBD in PD.7
Sleep fragmentation and difficulties in initiation and maintenance of sleep
Sleep fragmentation is the most common sleep-related manifestation of PD; its prevalence is nearly 40%,35 and it occurs concomitantly with PD motor disorders.36 Overall difficulties in falling asleep or maintaining restorative sleep are common in PD. Disorders of sleep initiation and maintenance are characterized either by reduction in stages 3 and 4 of non-REM sleep or by decrease of REM sleep.37 Restless leg syndrome is most prevalent in PD,38 and an obstructive and central type of apnea is seen in 20% of PD patients.4,5,39 In a study conducted on PD patients using a self-rated sleep log for assessing sleep quality, it was found that daily well-being was more severely affected in moderately/severely affected patients than less severely affected patients.40 The most commonly used dopaminergic drugs may affect sleep and degrade quality of life in PD patients.41 A primary approach for treating sleep disorders in PD is to use sleep hygiene, but the results have not been encouraging, and hence hypnotic drugs have been recommended. However, these drugs need to be used with caution because of their potential side effects.42 Using short-acting hypnotic drugs, such as zolpidem,43 which have less impact on muscle relaxation, is recommended to prevent falls associated with sleep aids, especially in elderly subjects. The relative lack of safe pharmacological tools for tackling PD sleep problems has necessitated turning to safer drugs like the naturally occurring melatonin, its slow-release preparations, or its agonists like ramelteon or agomelatine.44
Melatonin and its receptors
Melatonin is mainly synthesized in the pineal gland, and its synthesis is regulated by the SCN, which also acts as the central circadian pacemaker. The production and release of melatonin is very high during the dark of night – around 200 pg/mL – and is low in the daytime – 10 pg/mL.45 Synthesis of melatonin occurs in other regions of the body, such as the retina, gastrointestinal tract, skin, ovaries, lymphocytes, and thymus.46 Melatonin exerts its physiological actions by acting through G-protein-coupled receptors, namely MT1 and MT2 melatonin receptors, expressed either singularly as combined together in various cells and tissues of the body.47 Melatonin receptors have been identified in different regions of the brain, such as the SCN,48 hippocampus,49 and central dopaminergic pathways, including the substantia nigra, caudate, putamen, ventral tegmental area, and nucleus accumbens.50 The signal-transduction pathways for MT1 melatonin receptors are coupled to different G proteins that cause adenylyl cyclase inhibition and phospholipase C activation.51–53 MT2 receptors are coupled to a number of signal-transduction pathways that cause phosphoinositide production, inhibition of adenylyl cyclase, and guanylcyclase.54 A third melatonin-binding site known as MT3 has been identified as quinine reductase.55 Melatonin also exerts some of its actions by binding with calmodulin, reticulin, and also with orphan nuclear receptors of retinoic acid superfamily retinoid Z receptor-β, retinoic acid receptor (RAR)-related orphan receptor (ROR)-α and RORα-2.56–58 Studies on the expression of MT1 and MT2 receptors in human amygdalae and substantia nigra have shown that they are very much decreased when compared to normal subjects.12
Melatonin as a therapeutic agent in the treatment of PD
Experimental animal studies have shown that melatonin, an effective antioxidant, offers neuroprotection and has potential for treating cognitive disorders in PD.59,60 Also, melatonin has been used for treating sleep problems, insomnia, and daytime sleepiness. In a study involving 40 patients with PD (29 males and eleven females, mean age 61.7±8.4 years, range 43–76 years) for a treatment period of 2 weeks, melatonin was administered in doses ranging from 5 mg to 50 mg/day.53 Melatonin was administered (5 or 50 mg) 30 minutes before bedtime to avoid any possible circadian phase shift, as such shifts can occur if melatonin is administered at any other time. All subjects were taking stable doses of antiparkinsonian medications during the course of the study. Treatment with 50 mg of melatonin significantly increased nighttime sleep (P<0.05) compared to placebo, as revealed by actigraphy. Subjective reports of overall sleep disturbance improved significantly on 5 mg of melatonin when compared to 50 mg or placebo. It was found that high doses of melatonin were well tolerated in this study.61
In a double-blind placebo-controlled trial of RBD that utilized melatonin at a dosage of 3 mg/day, four patients had complete resolution of the disturbance, two patients had marked improvement, one patient showed a little improvement, and one patient remained unchanged. The data suggest that melatonin might be a second useful agent besides clonazepam in the treatment of RBD.62 In a later study, a higher dosage of melatonin, 15 mg at bedtime, was reported to be effective.63 In a recent study conducted on 38 patients with PD (15 males and 23 females, mean age 67.3±4.8 years) and complaints of sleep disorders, either melatonin 3 mg along with the usual dopaminergic drug or clonazepam 2 mg along with a dopaminergic agonist was administered at night for 6 weeks. It was noted in this study that both melatonin and clonazepam reduced sleep disorders in their respective groups. With clonazepam, patients showed daytime sleepiness, which was not seen with melatonin treatment. The PD Sleep Scale was employed in this study. Neuropsychological testing, as assessed by the Mini-Mental State Examination, showed that the melatonin group had better scores. From this study, it was concluded that melatonin has high treatment efficacy for the treatment of sleep disorders associated with PD.64
In another study involving 30 patients with early and late stages of PD, a decrease by 21% (P<0.05) in chronic fatigue syndrome on the Parkinson Fatigue Scale, improvement of sleep on the PD Sleep Scale (PDSS), a decrease in the anxiety state on Spielberger’s scale, and an improvement in quality of life on the 39-item Parkinson’s Disease Questionnaire (P<0.05) were found after melatonin treatment. From this study, it was concluded that melatonin together with optimized antiparkinsonian treatment can treat chronic fatigue syndrome and improve the sleep and quality of life of PD patients.65
The therapeutic use of melatonin in treating sleep disorders associated with PD, including RBD, was supported in a recent review paper.66 Using salivary dim-light melatonin onset and actigraphy, research has shown that PD patients treated with dopaminergic therapy (medicated PD) demonstrated less circadian disturbance than unmedicated PD group and controls.67 In another study, it was found that serum melatonin levels correlated with PD severity according to the Hoehn and Yahr scale.68
With the finding of reduced expression of the MT1 and MT2 melatonin receptors in patients with PD, there is a possibility that the melatonergic system is involved in the abnormal sleep mechanisms seen in PD and in the pathophysiology of PD. Therefore, therapeutic strategies should target the use of melatonin and its agonists, such as ramelteon, to treat subjective sleep disturbances, sleep quantity, and daytime sleepiness.69 The use of melatonin as an adjunct therapy to either halt disease progression or provide symptomatic relief in PD has been gaining more importance in recent times. The neuroprotective actions of melatonin in Parkinson’s disease are presented in Figure 1.
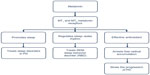  | Figure 1 Melatonin’s neuroprotective and sleep regulating role in PD. |
Bright-light treatment, melatonin, and PD
Exposure to light of 1,000–1,500 l× for 1–1.5 hours, 1 hour prior to bedtime at 10 pm for 2–5 weeks, in 12 patients has improved the bradykinesia and rigidity observed in PD.70 A reduction in agitation and psychiatric side effects were also reported in this study. Activation of the circadian system by antagonizing melatonin secretion with bright light has been suggested as the possible mechanism for treating the symptoms of PD.10 Bright light has been employed in treating depressive symptoms. However, suppression of melatonin secretion is not the likely mechanism by which artificial light exerts its therapeutic effect.71 Two possible mechanisms have been proposed for the possible therapeutic effects of bright light. First, bright light could reset the phase of abnormal circadian rhythms seen in depressed patients.71 Second, evening bright-light exposure, though it produces momentary melatonin suppression, actually causes a rebound increase of melatonin secretion in the late-night period.72 Bright-light exposure ultimately facilitates melatonin secretion rather than suppressing it, and this is said to be responsible for the therapeutic efficacy of bright light in affective disorders. Therefore, in case of PD, bright light might also improve the symptoms of PD, certainly not by antagonizing melatonin secretion but instead inducing the rebound effect of enhancing melatonin secretion. Light therapy has been shown to offer the advantages of neuroprotection, which is not provided by dopamine-replacement therapy, and hence it is advocated for treating the disease itself.73
Conclusion and implications for future therapy
Animal models of PD indicate that free radical generation in the nigrostriatal system is involved in the pathogenesis of PD. Since nonmotor symptoms of PD, such as sleep disorders, particularly REM sleep disorder, occur in the majority of PD patients and can present long before PD motor symptom manifestations, treating the sleep disorders of PD may be essential both for preventing and delaying the progression of this disease. Current evidence points to melatonin and melatonin receptors in PD pathophysiology. Therapeutic strategies for the control of PD should consider the therapeutic application of melatonin or its receptor agonists, such as ramelteon and the melatonergic antidepressant agomelatine. To date, melatonin alone has been employed for treating sleep disorders and RBD, and has been found to be effective in treating nonmotor symptoms of the disease. In future, large numbers of clinical trials are required, as melatonin not only sets right the sleep disorders associated with the disease but treats the disease itself, since it has sufficient antioxidant activity. By its dual therapeutic actions acting as a chronohypnotic (a drug that can promote sleep and set right a disturbed sleep–wake rhythm) and as an effective antioxidant, melatonin can arrest the progression of PD when administered in the early stages of disease.
Disclosure
The authors report no conflicts of interest in this work.
References
Postuma RB, Gagnon JF, Montplaisir J. Clinical prediction of Parkinson’s disease: planning for the age of neuroprotection. J Neurol Neurosurg Psychiatry. 2010;81:1008–1013. | |
Trendwalker C. Sleep dysfunction in Parkinson’s disease. Clin Neurosci. 1998;5:107–114. | |
Happe S, Lüdemann P, Berger K. The association between disease severity and sleep-related problems in patients with Parkinson’s disease. Neuropsychobiology. 2002;46:90–96. | |
Garcia-Borreguero D, Larrosa O, Bravo M. Parkinson’s disease and sleep. Sleep Med Rev. 2003;7:115–129. | |
Comella CL. Sleep disturbances and excessive daytime sleepiness in Parkinson’s disease: an overview. J Neurol Transm Suppl. 2006;70:349–355. | |
Comella CL. Sleep disorders in Parkinson’s disease: an overview. Mov Disord. 2007;22 Suppl 17:S367–S373. | |
Willis GL. Parkinson’s disease as a neuroendocrine disorder of the circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. 2008;19:245–316. | |
Naismith SL, Rogers NL, Mackenzie J, Hickie IB, Lewis SJ. The relationship between actigraphically defined sleep disturbance and REM sleep behaviour disorder in Parkinson’s disease. Clin Neurol Neurosurg. 2010;112:420–423. | |
Srinivasan V, Cardinali DP, Srinivasan US, et al. Therapeutic potential of melatonin and its analogs in Parkinson’s disease: focus on sleep and neuroprotection. Ther Adv Neurol Disord. 2011;4:297–317. | |
Bruguerolle B, Simon N. Biologic rhythms and Parkinson’s disease: a chrono-pharmacologic approach to considering fluctuations in function. Clin Neuropharmacol. 2002;25:194–201. | |
Videnovic A, Noble C, Reid KJ, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71:463–469. | |
Adi N, Mash DC, Ali Y, Singer C, Shehadeh L, Papapetropoulos S. Melatonin MT1 and MT2 receptor expression in Parkinson’s disease. Med Sci Monit. 2010;16:BR61–BR67. | |
Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1991;3:41–47. | |
Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase shifting properties of l-dopa. J Neural Transm Park Dis Dement Sect. 1993;5:227–234. | |
Bordet R, Devos D, Brique S, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol. 2003;26:65–72. | |
Liu S, Cai Y, Sothern RB, Guan Y, Chan P. Chronobiological analysis of circadian patterns in transcription of seven clock genes in six peripheral tissues in mice. Chronobiol Int. 2007;24:793–820. | |
Cermakian N, Monaco L, Pando MP, Dierich A, Sasone-Corsi P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period 1 gene. EMBO J. 2001;20:3967–3974. | |
Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early age related pathologies in mice deficient in BMAL1, the core components of the circadian clock. Genes Dev. 2006;20:1868–1873. | |
Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–4145. | |
Fukuya H, Emoto N, Nonaka H, Yagita K, Okamura H, Yokoyama M. Circadian expression of clock genes in human peripheral leukocytes. Biochem Biophys Res Commun. 2007;354:924–928. | |
Cai Y, Liu S, Sothern RB, Xu S, Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur J Neurol. 2010;17:550–554. | |
Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson’s disease. Mov Disord. 2002;17:775–781. | |
Brotini S, Gigli GL. Epidemiology and clinical features of sleep disorders in extrapyramidal disease. Sleep Med. 2004;5:169–179. | |
Raggi A, Bella R, Pennisi G, Neri W, Ferri R. Sleep disorders in Parkinson’s disease: a narrative review of the literature. Rev Neurosci. 2013;24:279–291. | |
Vendette M, Gagnon JF, Décary A, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson’s disease without dementia. Neurology. 2007;69:1843–1849. | |
Marion MH, Qurashi M, Marshall G, Foster O. Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson’s disease? J Neurol. 2008;255:192–196. | |
Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–339. | |
Miyamoto M, Miyamoto T, Suzuki K, Iwanami M, Hirata K. Screening methods for REM sleep behavior disorder. In: Idzikowski C, editor. Sleep Disorders. Rijeka, Croatia: InTech; 2012:181–190. | |
Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–393. | |
Boeve B, Silber M, Ferman T. REM sleep behavior disorder in Parkinson’s disease and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2004;17:146–157. | |
Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. | |
Mahowald MW, Schenck CH. Evolving concepts of human state dissociation. Arch Ital Biol. 2001;139:269–300. | |
Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. | |
Compta Y, Santamaria J, Ratti L, et al. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson’s disease dementia. Brain. 2009;132:3308–3317. | |
Tandberg E, Larsen JP, Karlsen NK. Excessive daytime sleepiness and sleep benefit in Parkinson’s disease. A community based study. Mov Disord. 1999;14:922–927. | |
Larsen JP. Sleep disorders in Parkinson’s disease. Adv Neurol. 2003;91:329–334. | |
Adler CH, Thorpy MJ. Sleep issues in Parkinson’s disease. Neurology. 2005;64:S12–S20. | |
Nomura T, Inoue Y, Miyake M, Yasui K, Nakashima K. Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson’s disease. Mov Disord. 2006;21:380–384. | |
Arnulf I, Konofol E, Merino-Andreu M, et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology. 2002;58:1019–1024. | |
Perez-Lloret S, Rossi M, Nouzeilles MI, Trenkwalder C, Cardinali DP, Merello M. Parkinson’s disease, sleep scale, sleep logs, and actigraphy in the evaluation of sleep in parkinsonian patients. J Neurol. 2009;256:1480–1484. | |
Bonnet AM, Jutras MF, Czernecki V, Corvol JC, Vidailhet M. Nonmotor symptoms in Parkinson’s disease in 2012: relevant clinical aspects. Parkinsons Dis. 2012;2012:198316. | |
Ceravolo R, Rossi C, Kiferle L, Bonuccelli U. Nonmotor symptoms in Parkinson’s disease: the dark side of the moon. Future Neurol. 2010;5:851–871. | |
Abe K, Hikita T, Sakoda S. A hypnotic drug for sleep disturbances in patients with Parkinson’s disease. Brain Nerve. 2005;57:301–305. | |
Srinivasan V, Srinivasan US, Kaur C, et al. Melatonin in Parkinson’s disease and its therapeutic potential. In: Srinivasan V, Brzezinski A, Oter S, Shillcutt SD, editors. Melatonin and Melatonergic Drugs in Clinical Practice. New Delhi: Springer; 2014:239–252. | |
Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25–39. | |
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin – a pleiotropic, orchestrating regulator molecule. Progr Neurobiol. 2011;93:350–384. | |
Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV, Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. | |
Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. | |
Savaskan E, Olivieri G, Meier F, et al. Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer’s disease patients. J Pineal Res. 2002;32:59–62. | |
Uz T, Arslan AD, Kurtuncu M, et al. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136:45–53. | |
Bryden L, Roka F, Petit L, et al. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3) and G(q/11) proteins. Mol Endocrinol. 1999;13:2025–2038. | |
Ho MK, Yung LY, Chan JS, Chan JH, Wong CS, Wong YH. Gα(14) links a variety of G(i)- and G(s)-coupled receptors to the stimulation of phospholipase C. Brit J Pharmacol. 2001;132:1431–1440. | |
Chan AS1, Lai FP, Lo RK, Voyno-Yasenetskaya TA, Stanbridge EJ, Wong YH. Melatonin MT1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and insensitive G proteins. Cell Signal. 2002;14:249–257. | |
Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–419. | |
Nosjean O, Ferro M, Coge F, et al. Identification of the melatonin-binding site MT3 as the quinine reductase 2. J Biol Chem. 2000;275:31311–31317. | |
Benítez-King G. Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res. 2006;49:1–9. | |
Macías M, Escames G, Leon J, et al. Calreticulin-melatonin. An unexpected relationship. Eur J Biochem. 2003;270:832–840. | |
Wiesenberg I, Missbach M, Kahlen JP, Schräder M, Carlberg C. Transcriptional activation of the nuclear RZRα by the pineal gland hormone melatonin and identification of CGP 52608 as a synthetic ligand. Nucleic Acids Res. 1995;23:327–333. | |
Arushanian EB. [A hormonal drug melatonin in the treatment of cognitive function disorders in parkinsonism]. Eksp Klin Farmacol. 2010;73:35–39. Russian. | |
Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 2005;6:459–466. | |
Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhães MC, de Lourdes Seabra M, de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J Neurol. 2007;254:459–464. | |
Kunz D, Mahlberg RA. Two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res. 2010;19:591–596. | |
Schenck CH, Mahowald MW. REM sleep parasomnias in adults: REM sleep behavior disorder, isolated sleep paralysis and nightmare disorder. In: Barkoukis TJ, Matheson JK, Ferber R, Doghramji K, editors. Therapy in Sleep Medicine. Philadelphia: Elsevier; 2011:549–558. | |
Litvinenko IV, Krasakov IV, Tikhomirova OV. [Sleep disorders in Parkinson’s disease without dementia: a comparative randomized controlled study of melatonin and clonazepam]. Zh Nevrol Psikhiatr Im S S Korsakova. 2012;112:26–30. Russian. | |
Datieva VK, Rosinskaia AV, Levin OS. The use of melatonin in the treatment of chronic fatigue syndrome and circadian rhythm disorders in Parkinson’s disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2013;113:77–81. | |
Trotti LM, Bliwise DL. Treatment of the sleep disorders associated with Parkinson’s disease. Neurotherapeutics. 2014;11:68–77. | |
Bolitho SJ, Naismith SL, Rajaratnam SM, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014;15:342–347. | |
Lin L, Du Y, Yuan S, Shen J, Lin X, Zheng Z. Serum melatonin is an alternative index of Parkinson’s disease severity. Brain Res. 2014;1547:43–48. | |
Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 2005;6:459–466. | |
Wills GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. 2007;24:521–537. | |
Beck-Friis J, Borg G, Wetterberg L. Rebound increase of nocturnal serum melatonin levels following evening suppression of bright light exposure in healthy men: relation to cortisol levels and morning exposure. Ann N Y Acad Sci. 1985;453:371–375. | |
Srinivasan V. Psychoactive drugs, pineal gland and affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:653–664. | |
Johnstone DM, Coleman K, Torres N, et al. The potential of light therapy in Parkinson’s disease. Chronophysiol Ther. 2014;4:1–14. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
