Back to Journals » Clinical Interventions in Aging » Volume 14
The therapeutic effect and complications of oro-esophageal tube training in stroke patients
Authors Kang S, Lee SJ , Park MK, Choi E, Lee S
Received 20 February 2019
Accepted for publication 30 April 2019
Published 11 July 2019 Volume 2019:14 Pages 1255—1264
DOI https://doi.org/10.2147/CIA.S204835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Saerom Kang,1 Sook Joung Lee,1 Min Kyu Park,2 Eunseok Choi,1 Sangjee Lee1
1Department of Physical Medicine and Rehabilitation, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 2Department of Clinical Pharmacology and Therapeutics, Chung Buk National University Hospital, College of Medicine, Cheongju-si, Chungcheongbuk-do, Republic of Korea
Background: Patients with severe dysphagia after stroke are usually fed using a nasogastric tube. However, this method is inconvenient and causes complications. The oro-esophageal (OE) tube has been used as an alternative parenteral feeding method for patients for whom safe oral feeding is impossible. This study aimed to evaluate the therapeutic effects and complications of OE tube feeding in stroke patients with dysphagia.
Methods: This study was designed as a retrospective medical chart review of dysphagic stroke patients who were recommended for OE tube feeding. Thirty-eight stroke patients were recommended for OE tube feeding according to videofluoroscopic swallowing study (VFSS) findings. Of those patients, 17 received OE tube feeding training and conventional dysphagia therapy. Follow-up VFSSs were performed sequentially based on the patients’ conditions. When a patient was able to swallow therapeutic foods with specific viscosities during the VFSS, oral feeding was considered to be initiated. Patients were divided into two groups according to final feeding methods.
Results: Seventeen patients attempted OE tube feeding. Among them, 64.7% of the patients could change to full oral feeding at their follow-up VFSS evaluation. Additionally, 70.6% of the patients showed gastroesophageal reflux disease regardless of whether they changed to oral feeding. On individual items of the Functional Dysphagia Scale, both groups showed significant improvements in the triggering of pharyngeal swallowing, the amount of residue, and the pharyngeal transit time. These functions were better improved in the patients who could change to oral feeding than in those who could not. Both groups showed significant aggravation of nasal penetration.
Conclusion: Our study quantitatively shows the therapeutic effects and complications of OE tube training. OE tube feeding can facilitate the swallowing process and assist patients in transitioning to oral feeding. This easy-to-apply technique may significantly impact future treatment strategies in stroke patients with severe dysphagia.
Keywords: stroke, swallowing disorder, oro-esophageal tube, tube feeding, videofluoroscopy
Introduction
Stroke is a leading cause of disability in elderly individuals worldwide. Progressively increasing life expectancy has led to growing numbers of elderly stroke patients.1 After stroke, many patients suffer from swallowing disorders during the acute stage, leading to a number of complications, such as aspiration pneumonia, malnutrition or dehydration, and even life-threatening conditions.2–4
Acute stroke patients with severe dysphagia usually use compensatory feeding methods such as a nasogastric (NG) tube or parenteral feeding.5,6 NG tube feeding can be a proper alternative enteral feeding method; however, it is inconvenient and sometimes causes complications such as aspiration pneumonia, gastroesophageal reflux, and diarrhea.7 Furthermore, NG tube feeding can interfere with hyoid bone movement in stroke patients during swallowing.8 In addition, long-term use of NG tubes can lead to gastric mucosal ulcers, reflux, or bleeding. Thus, short-term use of NG tube feeding is recommended.7 Furthermore, parenteral feeding cannot provide appropriate nutrition and may increase the complication rates of bacterial infection and diarrhea.5,6
The oral feeding catheter was first used by Funahashi et al in 1985 for nutritional support in children with dysphagia.7,9 In 1988, Campbell-Taylor designed a procedure called “oro-esophageal (OE) tube feeding”, which uses a Nelaton rubber catheter in dysphagic patients.7 OE tube feeding has been used for dysphagic stroke patients.10–12 Previous studies report that the OE tube can stimulate the pharyngeal wall during insertion and facilitate swallowing and relaxation of the upper esophageal sphincter.12 Thus, OE tube training itself can promote the swallowing reflex and assist patients in transitioning to oral feeding.10,11,13,14
However, stroke is a very heterogeneous disease. Different types of swallowing disorders after stroke may occur according to the stroke lesion and present with various clinical symptoms.2,15,16
Furthermore, as stroke patients show different recovery processes and multiple factors are related to the successful transition to oral feeding, early detection of dysphagia and appropriate management according to its symptoms are very important strategies.
We aimed to evaluate the therapeutic effect and complications of OE tube training in dysphagic stroke patients. We also attempted to demonstrate which factors could be related to achieving oral feeding.
Materials and methods
Subjects
This study was designed as a retrospective medical chart review. We reviewed the medical records of stroke patients with dysphagia who were recommended for OE tube training from May 2013 through June 2017. Patients who had a previous stroke history, comorbid cardiopulmonary disease, or head and neck cancer were excluded. Thirty-eight stroke patients were recommended for OE tube training according to the videofluoroscopic swallowing study (VFSS) findings. Of these patients, 17 agreed to and received OE tube feeding training and conventional dysphagia therapy; thus, they were enrolled in the study. The study protocol was approved by the institutional review board of the Catholic University of Korea, Daejeon St. Mary’s Hospital (IRB No.: DC19RESE0013). Because of the nature of retrospective design, the Ethics Committee of the Catholic University of Korea, Daejeon St. Mary’s Hospital approved the current study and also determined that the informed consent was not required. All patient-specific identifiers were removed from the data set before analysis.
OE tube training
Indications for OE tube training were severe dysphagic stroke patients with a decreased gag reflex but who had the potential cognitive ability and hand motor function to perform OE tube insertion according to the physiatrist’s instruction.
During the VFSS, the patient inserted an 8–10 Fr. barium-coated Nelaton rubber catheter into his or her mouth with the examiner’s guidance. The patient swallowed and pushed the catheter until the catheter’s tip was passed through the upper esophageal sphincter (UES) and placed in the middle esophagus (Figure 1). Appropriate placement of the catheter tip into the middle esophagus was verified via fluoroscopy. Then, a 10-cc bolus of dilute barium was administered using a syringe, and after confirming that the diluted-barium went into the esophagus without causing choking, vomiting, or reflux, the catheter was removed.
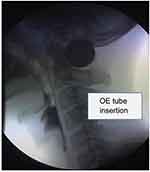 |
Figure 1 The oro-esophageal tube is inserted into the pharynx, passing the upper esophageal sphincter during the videofluoroscopic swallowing study.Abbreviation: OE, oro-esophageal. |
After confirming OE tube insertion during the VFSS, the patient tried OE tube feeding in the ward at every meal time. Because there was no way to check the tube placement with fluoroscopy in the ward, physiatrists initially supervised and instructed the patients several times. Before supplying feeding supplements, the patient and the physiatrist verified suitable insertion of the OE tube by a lack of clinical signs such as gag reflex, choking, or wet voice changes. Then, 10 cc was injected continuously into the OE tube using a syringe for safety confirmation. Next, the feeding supplement that was used for the previous enteral formula was administered in the sitting position. Medications were also applied through the OE tube in powder form mixed with water.
Evaluations
Swallowing function was assessed using the Functional Dysphagia Scale (FDS), Penetration-Aspiration Scale (PAS), and Dysphagia Outcome and Severity Scale (DOSS) based on the VFSS results. The VFSSs were performed with a modified Logerman’s protocol by physiatrists.17 Patients were assessed in the sitting position to allow a lateral view of the X-ray after the NG tube was removed. First, 2 mL of a thick-liquid containing barium was administered to the subject. Next, pureed foods, semisolid foods, solid foods, and thin-liquid were administered in this order. All kinds of the test foods contained barium and were given two or three times. All the VFSS processes were recorded, and the results were interpreted by 2–3 physiatrists.
The FDS is a system that was invented to measure the severity of dysphagia. It also correlates well with the American Speech Language-Hearing Association’s National Outcome Measurement System criteria.18 The scale is composed of 11 items with weighted values, including 4 oral functions and 7 pharyngeal functions (Table S1), which can be observed in the VFSS. To evaluate the differences in the parameters related to the OE tube training, the FDS score was divided into subscores and analyzed.
The PAS evaluates airway invasion,19 and the scores are decided based on the depth to which materials pass into the airway and whether material entering the airway can be expelled. The penetration categories correspond to level 5 on the scale, and levels 6–8 correspond to laryngotracheal aspiration. A PAS score of 8 indicates that materials enter the airway, pass below the vocal folds, and no effort is produced for the material expectoration.
The DOSS is a 7-point scale developed to systematically rate the functional severity of dysphagia.20 It is used for documenting the functional status of swallowing and diet based on the results of the VFSS. A DOSS level 1 indicates that patients showed severe dysphagia and thus, were unable to tolerate any per-oral nutrition. Level 7 indicates normal swallowing function in all situations. All patients included in this study showed DOSS level 1 which represents severe dysphagia.
Follow-up VFSSs were performed sequentially, based on patient condition, every 2 or more weeks. If a patient could swallow a therapeutic diet with a certain viscosity without serious aspirations or choking and showed a low amount of pharyngeal residue after swallowing during the VFSS, oral feeding was considered initiated. All subjects received individualized dietary prescriptions based on the VFSS results and were administered conventional swallowing therapy. Conventional swallowing therapy included neuromuscular electrical stimulation using VitalStim® (Chattanooga group, Hixson, TN, USA), Shaker exercise, oromotor facilitation, effortful swallowing, and vocal cord adduction strengthening exercise, etc.
Patients’ clinical information, including stroke characteristics, such as stroke lesion type, duration of OE tube training, and complications related to OE tube training, were evaluated. Subjects were divided into two groups; patients who could change or could not change to oral feeding.
Statistical analysis
SPSS 21.0 (IBM Corporaion, Armonk, NY, USA) for Windows was used for the statistical analysis. Changes in swallowing function in each group were analyzed using the Wilcoxon signed-rank test before and after OE tube training. The Mann–Whitney U test was used to compare swallowing function improvement between the two groups. The chi-square test was applied to evaluate the relationship between oral feeding and stroke characteristics. A p-value <0.05 was considered indicative of statistical significance.
Results
Seventeen patients attempted OE tube feeding. Table 1 shows the demographic characteristics of the subjects according to the feeding methods. Among them, 11 (64.7%) patients could change to full oral feeding without tube feeding on their last follow-up VFSS evaluation. The mean duration of OE tube feeding was 36.1±17.6 days in patients who could change to oral feeding and 54.8±38.5 days in patients who could not change to oral feeding. Their baseline demographic characteristics were compared, and the initial swallowing functions did not differ significantly between the two groups. Full oral feeding was successful in younger patients. The mean age was 56.3±21.7 years in patients who could change to oral feeding and 72.2±15.4 years in patients who could not change to oral feeding. Patients who could not change to oral feeding all had brainstem lesions. Among 9 patients with lateral medullary infarctions, 5 (55.6%) patients were able to eat orally. There were several complications; a total of 70.6% of the patients showed gastroesophageal reflux disease (GERD) and received GERD medications regardless of changing to oral feeding.
 |
Table 1 Demographic factors of patients according to the feeding methods (N=17) |
Table 2 shows changes in the swallowing function in each group. Both groups showed improvement in swallowing. Patients who could change to oral feeding showed significant improvements in the total scores on the FDS and in many subscores of the oral and pharyngeal phases of the FDS. Patients who could not change to the oral feeding group also showed significant improvements in the total scores on the FDS, but fewer FDS subscores were improved. Among individual items of the FDS, both groups showed significant improvements in the triggering of pharyngeal swallowing, the amount of pharyngeal residue, and the pharyngeal transit time. Both groups showed significant aggravation of nasal penetration. PAS scores were improved only in the group of patients who could change to oral feeding.
 |
Table 2 Changes in swallowing function in each group |
When comparing the changes in swallowing function between the two groups (Table 3), patients who could change to oral feeding showed significantly greater improvement in the scores on the total FDS, on the oral phase of the FDS, and on the PAS. Nasal penetration was aggravated in both groups; however, there were no intergroup differences.
 |
Table 3 Comparison of changes in swallowing function between the two groups |
Discussion
In the present study, we aimed to investigate the therapeutic effect and complications of OE tube training in severe dysphagic patients after stroke. We also attempted to demonstrate which factors may be related to achieving successful oral feeding.
OE tube feeding may be an attractive and safe compensatory feeding method for patients with severe dysphagia. The OE tube is not an indwelling catheter, such as an NG tube or a parenteral feeding tube. Rather, the OE tube is convenient, needs no visual avoidance, and makes it comfortable to breathe by not occupying space in the patient’s nose. The OE tube is an easy-to-use procedure in swallowing therapy, enabling interventions such as oro-motor facilitation and therapeutic diets because there is no space-occupying material in the oral cavity, as with the NG tube. The NG tube itself could interfere with hyoid bone movement in stroke patients during swallowing.8 The OE tube also induces a lower risk of complications, such as aspiration pneumonia, gastric mucosal ulceration or bleeding, and diarrhea, compared with the NG tube.7 For these reasons, OE tube training may improve the patient’s quality of life. In this study, we quantitatively demonstrated that OE tube training has therapeutic effects in stroke patients with severe dysphagia.
Therapeutic effects of the OE tube
The mechanisms of dysphagia in stroke include damage to the corticobulbar and/or pseudobulbar tract or disorders of the central pattern generator due to lesions of the brainstem swallowing center.21,22 In these patients, there is marked weakness of pharyngeal contraction with a delayed swallowing reflex, and relaxation of the UES is incomplete and may lead to aspiration.23
The OE tube may stimulate the pharyngeal wall during insertion by forming an angle when it bends, thereby affecting pharyngeal efferent nerves and triggering pharyngeal and swallowing reflexes.11 Compared to the NG tube, the OE tube not only improves the pharyngeal swallowing reflex by stimulating the pharyngeal wall during insertion but also reduces the amount of time spent during a meal, decreases acid reflux, and prevents pharyngeal bacterial growth and ulcer development in the skin and mucous membranes.10,14 A previous study showed that such positive effects improve the quality of life of patient with lateral medullary infarction who have dysphagia.24
Our results quantitatively reveal that after OE tube training, all patients significantly improved on the pharyngeal phase of FDS, improved the triggering of pharyngeal swallowing, decreased the amount of pharyngeal residue, and shortened the pharyngeal transit time regardless of the transition to oral feeding. The relaxation of the UES was also improved (Table 1). These results represent improvements in the pharyngeal phase of swallowing, which may be due to the OE tube training effects. The results of this study were consistent with those from previous investigations,14 which included only brainstem stroke patients.
Furthermore, both groups showed significant improvements in the amount of pharyngeal residue regardless of oral feeding after OE tube training (Table 2). Previous studies enrolled patients who had a weak gag reflex and severe dysphagia.10,13,14 From these results, we proposed that patient who shows a large amount of pharyngeal residue on the VFSS may be appropriate for OE tube training.
Factors associated with successive oral feeding
We attempted to demonstrate which factors may be related to successive oral feeding and showed that full oral feeding was achieved in younger patients. This result was concordant with previous studies showing that younger patients have a better prognosis than do older patients among stroke survivors. Previous studies have reported that younger age, good functional status, and higher cognition scores could predict oral feeding outcomes.25–27 Ickenstein et al28 showed that cognitive function might play an important role in oral feeding. However, this study included only patients with good cognitive function; thus, cognitive function could not be analyzed.
In terms of stroke lesions, considering the role of brainstem structures (including the nucleus ambiguus and nucleus solitarius), strokes involving brainstem lesions would cause more severe dysphagia.21,29 However, statistical significance according to the stroke lesion location was not found in the present study due to the small number of subjects. Among the 9 patients with lateral medullary infarction, 5 (55.6%) were able to eat orally. Our previous study revealed that swallowing functions differ according to the stroke lesion16 and that multiple factors are related to successful oral feeding. Thus, early detection of dysphagia and appropriate management according to its symptoms are very important strategies.2,15
Complications related to OE tube feeding
After OE tube training, both groups showed significant aggravation in terms of nasal penetration. Nasal penetration, which is represented as velopharyngeal incompetence (VPI), is caused by inappropriate velopharyngeal closure and is associated with nasopharyngeal muscle weakness.30,31 OE tube insertion apparently stimulates the pharyngeal and nasopharyngeal walls, inducing nasopharyngeal reflux. If a patient has severe cricopharyngeal dysfunction that makes it hard to relax the UES, then the pressure of the UES is higher than the pressure of the nasopharynx and could induce nasopharyngeal reflux. Our previous study reported that mechanical inspiration and expiration (MIE) exercises affect nasal penetration in post-stroke patients;32 thus, patients who show nasal penetration after OE tube training may benefit from MIE exercises.
A total of 70.6% of the patients showed GERD and received GERD medications regardless of changing to oral feeding. Nakajima et al10 also reported that a disadvantage of the OE tube is esophageal hiatal hernia or reflux into the oral cavity. Our subjects did not exhibit the GERD symptoms; however, following the VFSS, they showed esophageal reflux. Whenever the OE tube was inserted, it passed the UES; thus, this training could make the UES weak.
Mis-insertion could also be a problem. As described in a previous study, OE tube training should not be applied to patient who cannot recognize the OE tube insertion process.10,13,14 Blind insertion is related to the risk of aspiration pneumonia in unconscious patients, as the tube may be placed into the larynx or its insertion may cause torsion at the oropharyngeal level.10,33 Shin et al suggested that using the oropharyngeal airway could be helpful during OE tube feeding in patients with dysphagia.5
Strengths and limitations
Our study has several strengths. First, this study quantitatively showed the therapeutic effects and complications of OE tube training using the FDS, PAS, and DOSS. Second, the present study revealed “OE tube training” as an intermediate phase from non-oral feeding to oral feeding. Most previous studies showed OE tube feeding as a compensative feeding method for non-oral feeding, such as NG tube and percutaneous endoscopic gastrostomy tube. Finally, we showed the factors associated with successive oral feeding change when using OE tube training.
However, because our study was a retrospective medical chart review and not a randomized controlled trial, the findings are not sufficient for evaluating the therapeutic effects of OE tube training as generalized to all stroke patients. In addition, this study enrolled a small number of participants, and we could not divide patients according to the stroke lesion characteristics. Therefore, further studies involving more patients and a longer follow-up period are needed.
Conclusion
Our study quantitatively shows the therapeutic effects and complications of OE tube training, and these results indicate the rationale for proper treatment strategies for severe dysphagia after stroke. OE tube insertion itself could facilitate the swallowing process and assist patients in transitioning to oral feeding. This easy-to-use technique may significantly impact future treatment strategies for stroke patients with severe dysphagia.
Abbreviations list
FDS, Functional Dysphagia Scale; GERD, gastroesophageal reflux disease; NG, nasogastric; OE, oro-esophageal; PAS, Penetration Aspiration Scale; UES, upper esophageal sphincter; VFSS, videofluoroscopic swallowing study.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Azkune Calle I, Bocos Portillo J, Anton-Ladislao A, et al. Clinical outcome of mechanical thrombectomy for stroke in the elderly. J Stroke Cerebrovascular Dis. 2017;26(3):582–588. doi:10.1016/j.jstrokecerebrovasdis.2016.11.117
2. Smithard DG, O’Neill PA, Parks C, Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996;27(7):1200–1204.
3. Huang JF. Prevention and management of poststroke complications. Continuum. 2017;23(1,Cerebrovascular Disease):93–110. doi:10.1212/CON.0000000000000421
4. McHorney CA, Robbins J, Lomax K, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17(2):97–114. doi:10.1007/s00455-001-0109-1
5. Shin HK, Koo KI, Hwang CH. Intermittent oroesophageal tube feeding via the airway in patients with dysphagia. Ann Rehabil Med. 2016;40(5):794–805. doi:10.5535/arm.2016.40.5.794
6. Gomes GF, Pisani JC, Macedo ED, Campos AC. The nasogastric feeding tube as a risk factor for aspiration and aspiration pneumonia. Curr Opin Clin Nutr Metab Care. 2003;6(3):327–333. doi:10.1097/01.mco.0000068970.34812.8b
7. Campbell-Taylor I, Nadon GW, Sclater AL, Fisher R, Harris-Kwan J, Rosen I. Oro-esophageal tube feeding: an alternative to nasogastric or gastrostomy tubes. Dysphagia. 1988;2(4):220–221.
8. Kwak HJ, Kim L, Ryu BJ, et al. Influence of nasogastric tubes on swallowing in stroke patients: measuring hyoid bone movement with ultrasonography. Ann Rehabil Med. 2018;42(4):551–559. doi:10.5535/arm.2018.42.4.551
9. Funahashi M, Nakajima S, Ishihara K, Nishimura F. [Intermittent use of an oral catheter for feeding dysphagic children]. No to Hattatsu. 1985;17(1):3–9.
10. Nakajima M, Kimura K, Inatomi Y, et al. Intermittent oro-esophageal tube feeding in acute stroke patients – a pilot study. Acta Neurol Scand. 2006;113(1):36–39. doi:10.1111/j.1600-0404.2005.00534.x
11. Kisa T, Igo M, Inagawa T, Fukada M, Saito J, Setoyama M. Intermittent oral catheterization (IOC) for dysphagic stroke patients. Jpn J Rehabil Med. 1997;34:113–120. doi:10.2490/jjrm1963.34.113
12. Kim J, Seo HG, Lee GJ, Han TR, Oh BM. The feasibility and outcome of oro-esophageal tube feeding in patients with various etiologies. Dysphagia. 2015;30(6):680–685. doi:10.1007/s00455-015-9644-z
13. Kisa T, Sakai Y, Okano K, Iwanari M. Proper use of intermittent oral catheter feeding (IOC) and gastrostomy for dysphagic stroke patients. Jpn J Stroke. 2010;32:41–47. doi:10.3995/jstroke.32.41
14. You DS, Chun MH, Kim HJ, et al. The effectiveness of oro-phageal tube feeding with dysphagia after brainstem stroke. J Korean Acad Rehabil Med. 2011;35:27–33.
15. Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620–625.
16. Lee SJ, Lee KW, Kim SB, Lee JH, Park MK. Voluntary cough and swallowing function characteristics of acute stroke patients based on lesion type. Arch Phys Med Rehabil. 2015;96(10):1866–1872. doi:10.1016/j.apmr.2015.06.015
17. Palmer JB, Kuhlemeier KV, Tippett DC, Lynch C. A protocol for the videofluorographic swallowing study. Dysphagia. 1993;8(3):209–214.
18. Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001;82(5):677–682. doi:10.1053/apmr.2001.21939
19. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98.
20. O’Neil KH, Purdy M, Falk J, Gallo L. The dysphagia outcome and severity scale. Dysphagia. 1999;14(3):139–145. doi:10.1007/PL00009595
21. Crary MA. A direct intervention program for chronic neurogenic dysphagia secondary to brainstem stroke. Dysphagia. 1995;10(1):6–18.
22. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–2763. doi:10.1161/01.STR.0000190056.76543.eb
23. Lee JH, Choi KH, Ha SB. The comparison of videofluoroscopic findings between the patients with lateral medullary infarct and middle cerebral artery territorial infarct. J Korean Acad Rehabil Med. 2001;25:396–403.
24. Toshiki MTMSN, Yugo N, Shigeki K. Successful treatment of dysphagia with intermittent oro-esophageal tube feeding in a case of wallenberg syndrome. Neurol Ther. 2005;22:529–533.
25. Lee JH, Kim SB, Lee KW, Lee SJ, Park JG, Ri JW. Associating factors regarding nasogastric tube removal in patients with dysphagia after stroke. Ann Rehabil Med. 2014;38(1):6–12. doi:10.5535/arm.2014.38.1.6
26. Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30(4):744–748.
27. Oto T, Kandori Y, Ohta T, Domen K, Koyama T. Predicting the chance of weaning dysphagic stroke patients from enteral nutrition: a multivariate logistic modelling study. Eur J Phys Rehabil Med. 2009;45(3):355–362.
28. Ickenstein GW, Stein J, Ambrosi D, Goldstein R, Horn M, Bogdahn U. Predictors of survival after severe dysphagic stroke. J Neurol. 2005;252(12):1510–1516. doi:10.1007/s00415-005-0906-9
29. Kwon M, Lee JH, Kim JS. Dysphagia in unilateral medullary infarction: lateral vs medial lesions. Neurology. 2005;65(5):714–718. doi:10.1212/01.wnl.0000174441.39903.d8
30. Glade RS, Deal R. Diagnosis and management of velopharyngeal dysfunction. Oral Maxillofac Surg Clin North Am. 2016;28(2):181–188. doi:10.1016/j.coms.2015.12.004
31. Folkins JW. Velopharyngeal nomenclature: incompetence, inadequacy, insufficiency, and dysfunction. Cleft Palate J. 1988;25(4):413–416.
32. Jang KW, Lee SJ, Kim SB, Lee KW, Lee JH, Park JG. Effects of mechanical inspiration and expiration exercise on velopharyngeal incompetence in subacute stroke patients. J Rehabil Med. 2019;51(2):97–102. doi:10.2340/16501977-2506
33. Han TR, Paik NJ, Park JW, Kwon BS. The prediction of persistent dysphagia beyond six months after stroke. Dysphagia. 2008;23(1):59–64. doi:10.1007/s00455-007-9097-0
Supplementary materials
 |
Table S1 The functional dysphagia scale |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
