Back to Journals » Drug Design, Development and Therapy » Volume 9
The rapid effects of budesonide plus formoterol in patients with obstructive airway diseases
Authors Bayiz H, Ozkaya , Dirican A, Ece F
Received 15 June 2015
Accepted for publication 4 August 2015
Published 21 September 2015 Volume 2015:9 Pages 5287—5290
DOI https://doi.org/10.2147/DDDT.S90504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Hulya Bayiz,1 Sevket Ozkaya,1 Adem Dirican,2 Ferah Ece1
1Department of Pulmonary Medicine, Faculty of Medicine, Bahcesehir University, Istanbul, 2Department of Pulmonary Medicine, Samsun Medical Park Hospital, Samsun, Turkey
Introduction: The use of a combination inhaler containing budesonide and formoterol (BUD/FOR) to both maintenance and quick relief therapy has been recommended as an improved method of using inhaled corticosteroid/long-acting β agonist therapy. The aim of this study was to investigate the acute effects of BUD/FOR and testing the availability of BUD/FOR for early reversibility test in patients with airway obstruction.
Patients and methods: The study was conducted on patients who were admitted to the Department of Pulmonary Medicine, Samsun Medical Park Hospital, Samsun, Turkey.
Results: A total of 44 patients were included in the study. The mean age of patients was 48.5±17.3 (range 10–75) years and the male-to-female ratio was 36:8. The pre-bronchodilator pulmonary function test results are as follows: the mean forced vital capacity, 3,025±1,162 mL (76.3%±23.2%); mean forced expiratory volume in 1 second (FEV1), 1,898±725 mL (59.2%±19.1%); mean FEV1/forced vital capacity, 62.8±6.3% (range 42%–70%); mean peak expiratory flow, 3,859±1,779 mL (48.0%±19.7%); and forced expiratory flow 25%–75%, 1,295±486 mL (35.8%±12.3%). The reversibility was positive in 26 (59.1%) patients. The absolute change and percentage of change in FEV1 were 318±228 mL and 17.7%±11.9%, respectively. The patients were divided into two groups according to reversibility (reversible and irreversible) and both groups were compared with changes according to spirometric results. FEV1 values were statistically different between the two groups.
Conclusion: The fixed combination of BUD/FOR has rapid bronchodilator effect, and they can be used for early reversibility test.
Keywords: formoterol/budesonide, reversibility test, asthma, chronic obstructive pulmonary disease
Introduction
Obstructive lung diseases are one of the most common chronic diseases worldwide, and they are effectively controlled in most patients with a combination inhaler containing a corticosteroid and a long-acting β2 agonist (LABA). In patients with airway obstruction, evaluation of acute response to bronchodilators (the test of reversibility of airway obstruction) is a commonly used procedure in clinical and research studies. We know that the use of a combination inhaler containing budesonide and formoterol (BUD/FOR) to both maintenance and quick relief therapy has been recommended as an improved method of using inhaled corticosteroid/LABA.1 There is no clear consensus on what constitutes reversibility or a significant acute response to a bronchodilator. The aim of this study was to examine the acute effects of using BUD/FOR and testing the availability of BUD/FOR for early reversibility test in patients with airway obstruction.
Patients and methods
The study was conducted on patients who were admitted to the Department of Pulmonary Medicine, Samsun Medical Park Hospital, Samsun, Turkey. The following inclusion criteria were evaluated:
Symptomatic patients (cough, dyspnea, and/or wheezing);
Obstructive breath sounds on chest auscultation;
Presence of airway obstruction in spirometry (forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] ≤70% of expected);
Patients who had never used bronchodilators before, or;
Patients who had not received short- or long-acting inhaled bronchodilator therapy within the recent 12 hours.
The study was performed in accordance with the ethical principles in the Good Clinical Practice guidelines, in addition to applicable local regulatory requirements, and the protocol was approved by local Bahcesehir University Clinical Research Ethics Committee. The patients were informed of the study procedure, and written informed consents were obtained.
Pulmonary function test and reversibility assessment
The values of basal FVC, FEV1, FEV1/FVC, peak expiratory flow (PEF), and forced expiratory flow 25%–75% (FEF 25%–75%) were measured using the MIR (MIR Medical International Research Srl, Rome, Italy) MiniSpir PC-Based USB Spirometer by the same physician (Sevket Ozkaya) following a 30-minute resting period in an outpatient clinic setting. The test was performed in the seated position, when the nose was clamped and nasal respiration was hindered. The patients performed the forced expiratory maneuver at least three times, and the maximum FEV1 value was recorded as the basal value.
Reversibility test
Following baseline spirometry, patients inhaled a fixed combination of BUD/FOR (Rolastym Combi®, Deva®, Turkey; 12/400 μg [Halkali Merkez Mah. Basin Ekspres Caddesi, Istanbul, Turkey]) and dry powder inhaler capsule, and then spirometry was repeated after 15 minutes. Reversibility levels were evaluated as the absolute change in FEV1 and the percentage of change from the initial FEV1, calculated as FEV1%Δinit: post-FEV1 - pre-FEV1/pre-FEV1×100 (according to American Thoracic Society guidelines), and bronchial reversibility is defined as a drug-induced increase in FEV1 of ≥200 mL and ≥12% baseline.
Statistical assessment
Results were presented as means ± standard errors of means. The probability P<0.05 was considered significant. Descriptive group data were compared using the unpaired Student’s t-test and Pearson chi-square test.
Results
A total of 44 patients were included in the study. The patients’ demographic data and baseline pulmonary function test results are shown in Table 1. The mean age of patients was 48.5±17.3 (range 10–75) years and the male-to-female ratio was 36:8. The basal (pre-bronchodilator) pulmonary function test results are as follows: mean FVC, 3,025±1,162 mL (76.3%±23.2%); mean FEV1, 1,898±725 mL (59.2%±19.1%); mean FEV1/FVC, 62.8±6.3 mL (range 42%–70%); mean PEF, 3,859±1,779 mL (48.0%±19.7%); and FEF (25%–75%), 1,295±486 mL (35.8%±12.3%). The post-bronchodilator test results and changes in values are shown in Table 2. The reversibility was positive in 26 (59.1%) patients. The absolute change and percentage of change in FEV1 were 318±228 mL and 17.7%±11.9%, respectively. The patients were divided into two groups according to reversibility (reversible and irreversible), and results were shown in Table 3. Both groups were compared with changes according to spirometric results, and only in FEV1, values were statistically different between two groups (Table 4) (P≤0.05).
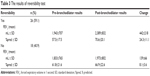  | Table 3 The results of reversibility test |
Discussion
There is no consensus on which bronchodilator drug should be used for the reversibility test. The reversibility test might be performed using metered-dose inhaled bronchodilators, dry powder inhaler, and metered-dose inhaled bronchodilators through an air chamber or nebulizer.2 Although the short-acting beta-2 agonists, salbutamol (SLB) and terbutaline, are the most commonly used bronchodilators for early reversibility test, the fixed combination of long-acting beta-2 agonist (LABA) plus inhaled steroid drugs are used in the treatment of patients with airway obstruction. LABA plus inhaled steroid drugs have also been reported to act in short term. In our previous study, the positive reversibility test with SLB was found in 61.9% of patients.3 Another study that compared the fixed combination of BUD/FOR (320/9 μg) with SLB reported that the positive reversibility test was higher in BUD/FOR group (50%) than in SLB group (20%).4 In the present study, the reversibility was positive in 26 (59.1%) patients. Recent study suggested a new approach for acute exacerbation – SMART (single inhaler maintenance and reliever therapy). This uses the combination inhaler, rather than the short-acting β2 agonist, as the reliever.5–7
We examined the relationship between reversibility and change in values of FVC, FEV1, PEF, and FEF (25%–75%). Only the absolute change and percentage of change in mean FEV1 were statistically significant (P≤0.05). These findings show that FEV1 is a true parameter for the evaluation of reversibility.
We used the short-acting beta-2 agonists (usually SLB) and a combination inhaler containing a corticosteroid plus a LABA for early and late reversibility tests in patients with airway obstruction, respectively. Also, we prescribed a combination inhaler containing a corticosteroid plus a LABA (usually BUD/FOR) for patients with airway obstruction, and trained the patients in the correct usage of inhaler devices. These results authenticate the idea of using a combination inhaler containing a corticosteroid and a LABA for early reversibility test. This study proves that a fixed combination of BUD/FOR can be used in early reversibility testing. Further studies are required to support this result.
Conclusion
The following results are observed regarding the usage of fixed combination of BUD/FOR in early reversibility test. They are fast acting. They can be used for early reversibility test (not only for treatment). The procedure contributes to the patients in the training of using inhaler devices during spirometry. Patients can immediately see the effects of the drugs used in the treatment. It improves the treatment compliance of patients.
Author contributions
HB, SO, and AD designed the study and performed the statistical analysis. FE conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Barnes PJ. Using a combination inhaler (budesonide plus formoterol) as rescue therapy improves asthma control. BMJ. 2007;335:513. | ||
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (Revised Update 2011). Global Initiative for Chronic Obstructive Lung Disease. Available from: www.goldcopd.com | ||
Ozkaya S, Dirican A, Kaya SO, et al. The relationship between early reversibility test and maximal inspiratory pressure in patients with airway obstruction. Int J Chron Obstruct Pulmon Dis. 2014;9:453–456. | ||
Ozkaya S, Dirican A, Tuna T. The effects of long-acting β2-agonists plus inhaled corticosteroids for early reversibility in patients with airway obstruction. J Thorac Dis. 2013;5(4):461–465. | ||
Patel M, Pilcher J, Pritchard A, et al; SMART Study Group. Efficacy and safety of maintenance and reliever combination budesonide–formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013;1(1):32–42. | ||
Pilcher J, Patel M, Smith A, et al; SMART Study Group. Combination budesonide/formoterol inhaler as maintenance and reliever therapy in Māori with asthma. Respirology. 2014;19(6):842–851. | ||
Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013;4:CD007313. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



