Back to Journals » Drug Design, Development and Therapy » Volume 13
The Protective Role of Tanshinone IIA in Silicosis Rat Model via TGF-β1/Smad Signaling Suppression, NOX4 Inhibition and Nrf2/ARE Signaling Activation
Authors Feng F, Cheng P, Zhang H, Li N, Qi Y, Wang H, Wang Y, Wang W
Received 10 September 2019
Accepted for publication 14 November 2019
Published 18 December 2019 Volume 2019:13 Pages 4275—4290
DOI https://doi.org/10.2147/DDDT.S230572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Video abstract presented by Wei Wang.
Views: 177
Feifei Feng,1 Peng Cheng,2 Huanan Zhang,1 Nannan Li,1 Yuxin Qi,3 Hui Wang,1 Yongbin Wang,1 Wei Wang1
1Department of Respiratory Medicine, The Second Hospital of Shandong University, Jinan, Shandong 250033, People’s Republic of China; 2Department of Neural Medicine, The Second Hospital of Shandong University, Jinan, Shandong 250033, People’s Republic of China; 3Department of Respiratory Medicine, Jinan People’s Hospital, Jinan, Shandong 250033, People’s Republic of China
Correspondence: Wei Wang
Department of Respiratory Medicine, The Second Hospital of Shandong University, 247 Beiyuan Street, Jinan, Shandong 250033, People’s Republic of China
Tel +86 17660080618
Email [email protected]
Purpose: Silicosis is an occupational disease caused by inhalation of silica and there are no effective drugs to treat this disease. Tanshinone IIA (Tan IIA), a traditional natural component, has been reported to possess anti-inflammatory, antioxidant, and anti-fibrotic properties. The current study’s purpose was to examine Tan IIA’s protective effects against silica-induced pulmonary fibrosis and to explore the underlying mechanisms.
Methods: 48 male SD rats were randomly divided into four groups (n=12): i) Control group; ii) Silicosis group; iii) Tan IIA group; iv) Silicosis +Tan IIA group. Two days after modeling, the rats of Tan IIA group and Silicosis +Tan IIA group were given intraperitoneal administration 25 mg/kg/d Tan IIA for 40 days. Then, the four groups of rats were sacrificed and the lung inflammatory responses were measured by ELISA, lung damage and fibrosis were analyzed by hematoxylin and eosin (H&E) staining and Masson staining, the expression levels of collagen I, fibronectin and α-smooth muscle actin (α-SMA) were measured by immunohistochemistry. The markers of oxidative stress were measured by commercial kits, and the activity of the TGF-β1/Smad and NOX4, Nrf2/ARE signaling pathways were measured by RT-PCR and Western blotting.
Results: The silica-induced pulmonary inflammtory responses, structural damage and fibrosis were significantly attenuated by Tan IIA treatment. In addition, treatment with Tan IIA decreased collagen I, fibronectin and α-SMA expression, and inhibited TGF-β1/Smad signaling in the lung tissue. The upregulated levels of oxidative stress markers in silicosis rats were also markedly restored following Tan IIA treatment. Furthermore, treatment with Tan IIA reduced NOX4 expression and enhanced activation of the Nrf2/ARE pathway in the lung tissue of silicosis rats.
Conclusion: These findings suggest that Tan IIA may protect lung from silica damage via the suppression of TGF-β1/Smad signaling, inhibition of NOX4 expression and activation of the Nrf2/ARE pathway.
Keywords: silicosis, tanshinone IIA, TGF-β1/Smad, NOX4, Nrf2/ARE
Introduction
Silicosis is an occupational disease caused by long-term exposure to large quantity of free silica dust, which is regarded as incurable for the irreversibility of progressing diffuse nodular pulmonary fibrosis, eventually seriously impairs lung function, leading to respiratory failure and even death.1,2 The prevalence and incidence of silicosis have been rising, particularly in developing countries like India and China. At the same time, the silicosis in the emerging industries has gradually appeared in many countries.3,4 Every year, the direct economic losses caused by silicosis in China amount to more than 8 billion yuan (RMB), and the indirect losses are incalculable. In developed countries, silicosis is also a high-profile occupational health problem.5,6 However, the pathogenesis of silicosis is still unclear and none of the current therapies can prevent disease progression effectively or reverse lung fibrosis. There is yet a pressing need to advancing novel and efficient approaches.7,8
During the past decades, cumulated studies have identified silicosis’ numerous important pathogenic mechanisms.9 After silica-induced lung damage, various kinds of cytokines, inflammatory mediators, proteases and reactive oxygen species (ROS) are released by alveolar epithelium and other resident cells.10 These factors can promote inflammatory cells’ recruitment, resulting in abnormal proliferation of fibroblasts and collagen deposition in lung tissue. Among them, the transforming growth factor β1 (TGF-β1) plays a central role in fibrogenesis, which is widely convoluted in the development of fibrosis by interrupting the homeostasis microenvironment and promoting cell differentiation, migration, invasion or hyperplasia mainly through the TGF-β1/Smad signaling pathway.11–13 It has been demonstrated that oxidative stress is a deleterious factor that is related to the profibrogenic activities of TGF-β1. There is a clear connection between TGF-β1 and oxidative stress during fibrogenesis.14 Fibrosis events that are associated with TGF-β1 are consonant with ROS-producing enzymes’ induction and/or the ROS-scavenging enzymes’ reduction.15,16 In these cases, Nrf2/ARE signaling pathway has been reported to be complicated in the dynamic procedure of fibrosis formation.16
Natural products play a very important role in research and development of drugs. Nevertheless, their potential mechanisms are not yet clear, which hinders drugs’ discovery. Tanshinone IIA (Tan IIA) is the most important active component of the traditional Chinese herb Salvia miltiorrhiza (Danshen), which possesses superior bio-availability and various pharmacological actions, has been reported to possess anti-inflammatory, antioxidant, and anti-fibrosis properties in various organs.17,18 However, there are few studies available on the efficacy of Tan IIA in silicosis, and the molecular mechanisms by which Tan IIA attenuates silica-induced lung fibrosis remain elusive. In the present study, we used the silicosis rat model to study the therapeutic effect of Tan IIA on silicosis and further explored its mechanism.
Materials and Methods
Silica-Induced Silicosis Rat Model
A total of 48 SD male rats (age, 6–8 weeks; weight, 200 ± 20 g) were obtained from Laboratory Animal Center of Shandong University and fed according to the SPF criterion. All animal experimentations in the present research were followed through in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Health’s National Organizations. Silicosis model was established by tracheal intubation method: the rats were anaesthetized by 2% sodium pentobarbital’s intraperitoneal injection (40 mg/kg of body weight, Sigma-Aldrich). Epidural anesthesia catheter with adapter was inserted into the trachea, then 1 mL 50 mg/mL silicon dioxide dust suspension (Sigma-Aldrich, diluted into 50 mg/mL suspension by physiological saline solution, content of free silica ≥ 99%, <5µm particles ≥ 99%, 8000 U/mL penicillin was added before use) and 0.25 mL air were perfused into the trachea. After that, the rat was rotated immediately to distribute the injection evenly in the lungs. The animal use protocol has been examined and approved by the Scientific Research Ethics Committee of the Second Hospital of Shandong University (Permit Number: 20140195).
Animal Grouping and Tan IIA Treatment
After a week of adjustable feeding, the rats were divided into four groups at random (n=12 per group): i) Control group; ii) Silicosis group; iii) Tan IIA group; iv) Silicosis +Tan IIA group. Two days after modeling, the rats of Tan IIA group and Silicosis +Tan IIA group were given intraperitoneal administration 25 mg/kg body weight of Tan IIA daily (Sigma-Aldrich) for 40 days, while the rats of control group and silicosis group were intraperitoneally injected with equal aseptic saline. Six weeks post-modeling, the rats in four groups were anesthetized with 10% chloral hydrate (3.0 mL/kg) by intraperitoneal injection, then the lungs were lavaged three times with 1.5mL saline, and the bronchoalveolar lavage fluid (BALF) was collected for analysis. Subsequently, rats were executed with 10% chloral hydrate (5.0 mL/kg) anesthesia and their lungs were taken for further experiments.
BALF Analysis
The BALF samples were first centrifuged at 4 °C (150 × g, 10mins), then the supernatant was collected and stored at −80°C, and cell particles were suspended in 1 mL PBS. The total number of cells in BALF was counted by hemocytometer. The remaining cells were centrifuged onto the slides. The number of neutrophils, macrophages and lymphocytes were counted by Wright-Giemsa staining.
Measurement of TNF-α, IL-1β and IL-6
TNF-α ELISA Kit (WLE05, Wanleibio), IL-6 ELISA Kit (WLE04, Wanleibio) and IL-1β ELISA Kit (WLE03, Wanleibio) were used to determine the levels of TNF-α, IL-6 and IL-1β in rat lung tissue of the four groups following instructions of the manufacturer. The optical density at 450 nm was measured with a microplate reader (ELX800; Biotek, Inc., USA), then the concentrations of TNF-α, IL-6 and IL-1β were calculated according to the standard curves.
Lung Histological Analyses
The rat lungs were immobilized in 4% paraformaldehyde solution for 48 hrs, imbedded in paraffin and cut into 5 μm dense sections. Fixed sections were stained with hematoxylin and eosin (H&E) with 1% hematoxylin for 6 mins and ponceau acid fuchsin staining solution for 1 min; as for Masson staining, the sections were stained with 1% hematoxylin for 6 mins and ponceau acid fuchsin staining solution for 1 min. An optical microscope (Olympus D72, Japan) was used to examine the slides (100× magnification).
Immunohistochemical Analyses
The paraffin-embedded slices were dewaxed and heated in citrate buffer to repair the antigen. The endogenous peroxidase was then inactivated by 0.3% hydrogen peroxide. After that the sections were blocked in goat serum (SL038, Solarbio) for 10 mins, followed by incubation with anti-collagen I rabbit polyclonal antibody (1:100; Affinity Bioscience Technology, Ltd.; cat. no. AF7001), anti-α-SMA mouse monoclonal antibody (1:100; Wanlei Biological Technology, Ltd.; cat. no. WL02510), or anti-Fibronectin 1 rabbit polyclonal antibody (1:100; Wanlei Biological Technology, Ltd.; cat. no. WL00712a) at 4°C overnights. The next day, the sections were washed with PBS for three times, and then incubated with goat anti-rabbit or mouse secondary antibodies conjugated with horseradish peroxidase (HRP) (1:500; ThermoFisher, USA; cat. no. #31460 and #31430) for 30 mins at 37°C, followed by incubation with HRP-Streptavidin at 37°C for 20 mins and washed with PBS for three times. Positive staining was revealed by DAB color reagent (DA1010, Solarbio) according to the manufacturer’s instructions. Nuclei were restained with hematoxylin. Then, light microscopy (DP73; Olympus Corporation, Tokyo, Japan) was used to analyze the sections (×200 magnification). The integrated optical density (IOD) of collagen I, fibronectin and α-SMA was measured using Image-Pro Plus 4.5 (Media Cybernetics, Inc., USA), and the mean density was calculated by IOD/area.
Western Blot Analysis
Lung tissues’ total proteins were extracted applying Whole Protein Extraction kit (WLA019, Wanlei Biological Technology, Ltd.). Nuclear and cytoplasmic protein were extracted using Nuclear and Cytoplasmic Protein Extraction kit (P0028, Beyotime Biotechnology). Protein concentration was calculated applying the BCA Protein assay kit (P0012S, Beyotime Biotechnology) and samples containing equal amount of protein (40 μg) were divided using 10 or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), then electro-transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, USA). After blocking with 5% non-fat milk made with Tris-buffered saline (containing 0.1% Tween 20), the membranes were incubated with the following specific primary antibodies at 4°C overnight: rabbit anti-TGF-β1 (1:1000, abcam; cat. no. ab-92486), rabbit anti-Smad3 (1:1000, abcam; cat. no. ab-40854), rabbit anti-p-Smad3 (S208) (1:1000, abcam; cat. no. ab138659), rabbit anti-Smad2/3 (1:200; Boster Biological Technology; cat. no. BA1395), rabbit anti-p-Smad2 (S465+S467)/p-Smad3 (S423+S425) (1:500; Wanlei Biological Technology, Ltd; cat. no. WL02305), rabbit anti-Smad7 (1:1000, abcam; cat. no. ab-216428), rabbit anti-NOX-4 (1:200; Boster Biological Technology; cat. no. BM4135), mouse anti-Nrf2 (1:400, Santa Cruz; cat. no. sc-81342), mouse anti Keap-1 (1:200; Santa Cruz; cat. no. sc-514914), mouse anti HO-1 (1:200; Santa Cruz; cat. no. sc-136960), mouse anti NQO-1 (1:200; Santa Cruz, Inc.; cat. no. sc-32793), rabbit β-actin (1:500; Wanlei Biological Technology, Ltd; cat. no. WL01372). Afterwards, the membranes were incubated with HRP-conjugated goat anti-rabbit (1:5000; cat. no. ZB-2305) or goat anti-mouse (1:5000; cat. no. ZB-2301) secondary antibodies (ZSGB-BIO, Beijing, China) at 37°C for 1 hr. The specific bands were displayed applying immobilon western chemiluminescent HRP substrate (WBKLS0100, Millipore, USA) according to the manufacturer’s protocol and analyzed by Quantity One software (version 4.6.6; Bio-Rad, USA). The relative protein levels were quantified through the comparison to the internal control β-actin or Histone H3.
Measurement of Reactive Oxygen Species (ROS), Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GSH-Px)
Lung tissues of each group were accurately weighed and add precooled normal saline according to the ratio of weight (g): volume (mL) = 1:9. Following centrifugation at 2500 rpm at 4°C for 10 mins, the supernatant was gathered, so as to determine the content of ROS, MDA, and the activities of SOD and GSH-Px. All measurements were performed using commercial kits: Reactive oxygen species assay kit (WLA070), Malondialdehyde assay kit (WLA048), Total Superoxide Dismutase assay kit (WLA110), and Glutathione Peroxidase assay kit (WLA107), obtained from Wanlei Biological Technology, Ltd. (Shenyang, China) according to the instructions of the manufacturer.
Quantitative Real-Time Polymerase Chain Reaction Assay
Total RNA of lung tissues from each group was extracted using TRIzol reagent (Invitrogen, USA) according to the protocols of the manufacturer. 1 μg RNA was reverse-transcribed applying a PrimeScript RT reagent Kit (TaKaRa, Japan) in a thermal cycler PCR system (Bio-Rad, USA). The gene-specific primers for mice TGF-β1, Smad2, Smad3, Smad7, collagen I, fibronectin, α-SMA, NOX4, Nrf2, Keap1, HO-1, NQO1 and β-actin are listed in Table 1. Gene expression was determined through ExicyclerTM 96 real-time system (Bioneer, Korean) with SYBR Premix ExTaqII (TaKaRa, Japan). After enzyme activation at 94°C for 5 mins, the amplification protocol is composed of denaturation for 10 s at 94°C, annealing for 20s at 60°C and extension for 30 s at 72°C for 40 cycles, and a last extension at 72°C for 6 mins. Data were assembled and dealt using SDS 2.4 software (Applied Biosystems, USA) and provided as threshold cycle (Ct) values. All values were normalized to the transcription of the endogenous β-actin and we calculated the average fold changes of the expression of each target gene by the 2-ΔΔCT method.
 |
Table 1 The Primer Sequences Amplified Used for RT-PCR |
Statistical Analysis
Unless otherwise specified, all experiments were repeated independently for at least three times. The data are expressed as mean ±SD. Differences between the groups were compared by One-way ANOVA and Newman–Keuls post hoc test applying SPSS version 19.0 software (SPSS Inc., USA). P<0.05 was considered to be a significant statistical difference.
Results
Tan IIA Suppresses Silica-Induced Lung Inflammation
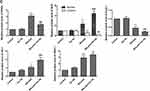
To look into Tan IIA’s anti-inflammatory effects on silicosis, rats were exposed with silica dust through tracheal intubation, and the BALF was collected for counting inflammatory cells (Figure 1A–D). As in comparison with the control group, the number of total cells, neutrophils, macrophages and lymphocytes increased significantly in the silicosis group (P<0.05), while these were markedly reduced by Tan IIA treatment (P<0.05). The levels of Inflammatory factors TNF-α, IL-6, IL-1β levels in the lung tissues were also determined using ELISA kit. As shown in Figure 2, the elevated levels of TNF-α, IL-6 and IL-1β in the silicosis group’ lung tissues were markedly reduced following Tan IIA treatment (P<0.05). These results distinctly suggest that Tan IIA may suppress silica-induced lung inflammation effectively.
Tan IIA Attenuates Silica-Induced Lung Fibrosis
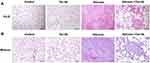
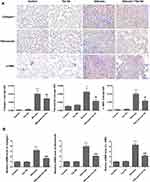
To ascertain Tan IIA’s impacts on silica-induced lung fibrosis, lung histological analyses and immunohistochemical analyses of fibrosis markers were conducted. Histopathological examination showed that in the silicosis group, silica-injured lungs lost normal alveolar structure, alveolar wall became thicker, accompanied by a large number of inflammatory cells infiltration, and formed characteristic silicosis nodules around the bronchial tree and vascular bed, showing diffuse pulmonary fibrosis. However, these destructive effects of SiO2 were alleviated in the silicosis + Tan IIA group compared with the silicosis group. The rats in the control group and Tan IIA group showed no histological changes (Figure 3A). Consonant with H&E staining results, Masson’s staining suggested that silica-induced collagen deposition in the lungs of silicosis rat was decreased by Tan IIA treatment markedly (Figure 3B). In immunohistochemical analyses, the expression of collagen I, fibronectin and α-SMA was observed, as demonstrated in Figure 4A, silica exposure led to a marked rise in expression of collagen I, fibronectin and α-SMA, which were reduced by Tan IIA treatment (P<0.05). Using RT-PCR, the above result was further confirmed (Figure 4B). Collectively, these data indicate that Tan IIA attenuates silica-induced lung fibrosis.
Tan IIA Suppresses Silica-Induced Activation of the TGF-β1/Smad Pathway
Former researches have indicated that TGF-β1 is a key regulator in respiratory diseases,19,20 and TGF-β1/Smad signaling is a major pathway of fibrogenesis which is activated in fibrotic diseases.21,22 Thus, the activities of TGF-β1 and its downstream signaling pathway, Smad2, Smad3 and Smad7 were determined by RT-PCR and Western blot analyses, so as to determine whether TGF-β1/Smad pathway suppression is convoluted in the protective effects of Tan IIA on silica-induced pulmonary fibrosis. Silica exposure led to a marked rise in the mRNA and protein expression levels of TGF-β1 in lung tissues, as shown in Figure 5; however, they were markedly restored by Tan IIA treatment. Moreover, the levels of p-Smad3 and p-Smad2/3 in the silicosis rat lungs were significantly upregulated and were further inhibited by Tan IIA treatment. No significant change was observed in the mRNA and protein expression level of Smad2 and Smad3 in lung tissues. Besides, the mRNA and protein levels of Smad7, which is a negative regulatory factor of TGF-β1/Smad pathway, were markedly downregulated by silica exposure and were further upregulated by Tan IIA treatment (Figure 5A–C). Overall, it can be seen from these data that the TGF-β1/Smad pathway inhibition is connected with Tan IIA’s protection effect on silica-induced pulmonary fibrosis.
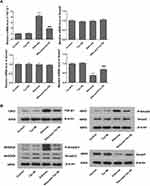 |
Figure 5 Continued. |
Tan IIA Inhibits Silica-Induced Oxidative Stress via Inhibition of NOX4 and Activation of the Nrf2/ARE Signaling Pathway
To assess Tan IIA’s effects on oxidative stress induced by silica-exposure, the ROS, MDA content, and SOD, GSH-Px activities in the lung tissues of four groups were determined by commercial kits. The ROS and MDA content were markedly raised in the silicosis group (P<0.05), which were accompanied by a significant reduction in SOD and GSH-Px activities (P<0.05), as shown in Figure 6A–D. These effects, however, were significantly attenuated by Tan IIA treatment.
Since oxidative stress and TGFβ1-mediated pro-fibrosis signal are the principal factors that promote fibrosis.15,16 TGF-β1 advances ROS formation mainly via induction of ROS-producing enzyme NOX4 expression23 and Nrf2 is a key defense factor against oxidative stress and a regulator of TGF-β1 signal transduction.24 Former researches also indicate that Tan IIA is able to activate Nrf2 signaling and enhance the expression of Nrf2 downstream protein.18,25 Thus, to determine whether it is connected with Tan IIA’s protective effects on silica-induced oxidative impairment to the lungs, the expression of NOX4 and activity of Nrf2/ARE signaling pathway, including Nrf2 and its inhibitor Keap-1, its downstream proteins HO-1, NQO-1 were examined by RT-PCR and Western blot. The results indicated that (Figure 7A–C), in comparison with control group, the mRNA and protein levels of NOX4 were considerably raised in the silicosis group (P<0.05), while they were restored by Tan IIA treatment (P<0.05). The mRNA of Nrf2 and its downstream proteins HO-1 and NQO-1 were significantly increased in the silicosis group (P<0.05), and were further upregulated following Tan IIA treatment (P<0.05). (Figure 7A). Because Nrf2 nuclear translocation is a necessary step for Nrf2 activation, nuclear Nrf2 accumulation and cytoplasmic Nrf2 expression were assessed. As shown in Figure 7B and C, nuclear Nrf2 expression was upregulated whereas cytoplasmic Nrf2 was decreased in the silicosis group (P<0.05), which were further enhanced by Tan IIA treatment (P<0.05). In accordance with Nrf2, the protein expression levels of its downstream proteins HO-1 and NQO-1 were also significantly upregulated in lung tissues from the silicosis group (P<0.05), which were also further enhanced by Tan IIA treatment (P<0.05) (Figure 7B and C). And contrary to Nrf2, the mRNA and protein expression of its inhibitor Keap-1 was downregulated in the silicosis group (P<0.05), and this effect was further strengthened by Tan IIA treatment (P<0.05) (Figure 7A–C). These data suggest that Tan IIA safeguards lungs from silica-induced oxidative stress damage, at least partly via NOX4 inhibition and Nrf2/ARE signaling pathway activation.
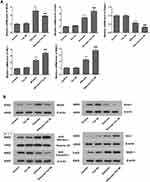 |
Figure 7 Continued. |
Discussion
Silicosis is a common occupational lung disease that is caused by inhalation of large amounts of crystalline silica dust. It is characterized by the formation of a large number of inflammatory and scar-forming nodular fibrosis in the lungs. Despite the work of many previous studies, the treatment of silicosis is still a worldwide problem. At present, there is no effective non-drug or drug treatment to reverse or slowdown the progress of silicosis. Therefore, the treatment of silica-induced lung damage and fibrosis continues to be a region of pressing medical study need. In recent years, Chinese medicine has shown great potential in the treatment of silicosis, a lot of natural plant extractions have come out to be promising ways for plenty of inflammatory and fibrosis disorders’ therapeutic treatment.
As the most important active component of the traditional Chinese herb Salvia miltiorrhiza (Danshen), Tanshinone IIA (Tan IIA) has been proved to have anti-inflammatory, anti-inflammatory, antioxidant, and anti-fibrosis effects in various diseases.17,18,25 However, there are few studies available on the efficacy of Tan IIA in silicosis, and the exact mechanisms remain largely undefinable. In the present research, a silicosis rat model was induced through silica particles’ intratracheal administration. The results demonstrated that Tan IIA effectively suppressed lung inflammation and fibrosis in silicosis rats. In addition, the overexpression of collagen I, fibronectin and α-SMA levels in silicosis rat were reversed by Tan IIA treatment. Mechanism Research found that Tan IIA treatment suppressed silica-induced TGF-β1/Smad pathway’s activation. Moreover, Tan IIA restrained oxidative stress in silicosis rats’ lungs, alongside decreased NOX4 expression and increased Nrf2/ARE pathway’s activation. In general, these results indicated that Tan IIA may keep lung damage from silica exposure via the suppression of TGF-β1/Smad signaling, inhibition of NOX4 and activation of the Nrf2/ARE pathway. Tan IIA may be thus considered as an effective method to control silicosis.
Previous studies have confirmed that silicosis is a complex chronic inflammatory process involving a variety of cells (alveolar macrophages, alveolar epithelial cells, fibroblasts, lymphocytes, etc.), cytokines and a variety of mediators. The present study demonstrates that in silicosis rats, the lungs lost normal alveolar structure, formed characteristic silicosis nodules and showed diffuse pulmonary fibrosis, accompanied by an accumulation of large number of inflammatory cells in BALF, as well as significantly upregulation of pro-inflammatory factors, including TNF-α, IL-6 and IL-1β in lung tissues. However, Tan IIA effectively alleviated silica-induced destruction of lung structure, collagen accumulation and inflammatory responses. Pulmonary fibrosis can be assessed by determining the collagen I, fibronectin and α-SMA levels in lung tissue. Our study detected a marked increase in collagen I, fibronectin and α-SMA mRNA expression and protein deposition in silica-exposure rats; these effects, however, were all reduced by Tan IIA treatment significantly, which indicate that Tan IIA possesses significantly protective effects on silica-induced lung damage and pulmonary fibrosis in rats.
TGF-β1 is a powerful key fibrogenic cytokine. Many studies have confirmed that TGF-β1 plays an important regulatory role in cell proliferation,11 differentiation,12 migration,13 immune regulation,27 and extracellular matrix (ECM) transformation28 in fibrotic diseases, and participates in tissue repair and fibrosis. The role of TGF-β1 in promoting fibrosis is mainly mediated by phosphorylating its downstream Smad proteins.11,14 TGF-β1/Smad signaling is identified as the main pathway of pulmonary fibrosis. The binding of TGF-β1 with its receptor II (TβRII) activates TGF-β1 receptor I (TβRI)’s kinase I. TβRI was phosphorylated and then phosphorylate Smad2 and Smad3, phosphorylated Smad2 and Smad3 afterwards bind to Smad 4 to constitute a Smad complex. The complex is then transferred to the nucleus to regulate target genes’ transcription, including collagen I, fibronectin, α-SMA, etc. Smad7 is considered to be an inhibitory Smad, which regulates the activation and function of Smad2, Smad3 negatively. And as a critical factor that was responsible for fibrosis, Smad3 is proved to play a more important role in the information transmission of TGF-β1 than Smad2.26,27 The present study revealed that the expression of TGF-β1, p-Smad2/3 and p-Smad3 was significantly upregulated in the rat model of silica-induced pulmonary fibrosis, while the Smad7 was downregulated, confirming that TGF-β1/Smad signaling pathway was activated after silica exposure. However, these effects were inhibited by Tan IIA treatment, indicating that Tan IIA can protect silica-induced pulmonary fibrosis via blocking TGF-β1/Smad signaling pathway.
Lung injury leads to the elevation of both TGF-β1 and oxidative stress levels. Growing evidence suggests that oxidative stress and ROS production are associated with activation and production of a lot of cytokines and pro-fibrotic growth factors.28 Although the TGF-β1/Smad signaling is fibrogenesis’ principal driving force, there are some pro- and anti-fibrogenic factors appear to interact directly with it.29 Previous studies have demonstrated that oxidative stress is a deleterious factor that is related to the profibrogenic activities of TGF-β1. There is a clear relationship between TGF-β1 and oxidative stress signaling during the whole fibrosis process.14 TGF-β1 raises ROS levels in lung, while ROS that is generated in turn excites myofibroblast differentiation and TGF-β1-associated fibroblast activation. Fibrosis events that are associated with TGF-β1 are consonant with ROS-producing enzymes’ induction and/or the ROS-scavenging enzymes’ reduction.15,16 In the present study, silica-exposure promotes the production of ROS, and raised the level of MDA significantly, which is the end product of lipid peroxidation, as well as reduced the antioxidant enzymes’ activities, including SOD and GSH-Px. These alterations, however, were significantly attenuated by Tan IIA treatment. These results indicated that Tan IIA may restrain silica-induced lung injury by lessening oxidative stress, which were in accordance with those of preceding researches, indicating that Tan IIA has antioxidant effect.17,18,25
Over the inflammatory reaction, oxidative stress that is characterized by a rise in ROS activates an effective antioxidant system, which determines the redox balance. As extracellular ROS production’s main enzymatic source, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4) elicits ROS output and potentiates myofibroblast activation in reply to TGF-β1.30 TGF-β1 promotes ROS formation principally via induction of NOX4 expression and activity in lots of cell types.15 The increase of NOX4 is in balance with the rise of antioxidant redox-sensitive protein E2-related factor 2 (Nrf2)/antioxidant response element (ARE) signaling, promoting inflammatory process’ decomposition and local tissue repair.31
Nuclear factor erythroid 2-like 2 (Nrf2) is a cellular sensor for oxidation reaction, and as the central regulator of antioxidant and detoxifying systems, it protects cells and tissues against oxidative stress through regulation of antioxidant response element (ARE)-mediated induction of antioxidant enzymes and diverse phase II detoxification.32,33 Nrf2 is principally controlled by the binding of cytosolic protein Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and subsequent proteasome degradation. In the presence of ROS, Nrf2 was separated from its bound with keap1, transferred to the nucleus and bounds to ARE, a small Maf protein, inducing the expression of a series of antioxidant genes, including heme oxygenase-1 (HO-1) and NADPH quinone oxidoreductase 1 (NQO1).15,16,30 It was proved that activation of the Nrf2 signaling relieved the lung impairment and suspended lung fibrosis’ progression.34–36 Antioxidants’ supplementation appears to be capable to modulate the plasticity’s degree and severity of fibrosis.16,17,30
In this research, we demonstrated that Tan IIA activated Nrf2/ARE signaling, which was implied by raised levels of Nrf2 and its downstream antioxidant enzymes HO-1 and NQO1, and reduced level of keap-1 in Tan IIA-treated silicosis rats, which suggested that Tan IIA’s antioxidative effect may contribute to its antifibrotic activity.
Among the enzymes that produce ROS, NOX seems to be a key factor in promoting fibrosis.28,37 ROS derived from NOX is associated with fibrosis of multiple organs, including the lung. Of the NOX enzymes, the characteristic of NOX4 is that its activity is mainly regulated by its expression level. In addition to its dimerization partner p22phox, no further regulatory subunits are needed, while the regulation of other NOX4 enzymes is more complicated. Therefore, under the condition of promoting fibrosis, NOX4 may contribute more to ROS production. TGF-β1 promotes ROS formation mainly by inducing the expression and activity of NOX4 in various cell types. NOX4 is upregulated in fibrosis, and its genetic or pharmacological inhibition decreases ECM deposition and TGF-β1-dependent fibrosis-promoting response significantly.38,39 Our result suggested that Tan IIA reduced the upregulated mRNA and protein levels of NOX4 in silicosis rats. Thus, the antifibrotic effect of Tan IIA may also be attributed to NOX4 inhibition induced decrease in oxidative stress.
Conclusion
In summary, the present research reported a protective function of Tan IIA in silicosis rats. Further, suppression of TGF-β1/Smad signaling, inhibition of NOX4 and activation of the Nrf2/ARE pathway are possible mechanisms through which Tan IIA protects against silica-induced lung impairment and fibrosis. Tan IIA might be used as a potential therapeutic drug in silicosis’ treatment.
Ethics Approval and Consent to Participate
All of the animal procedures, including housing, care and experimental protocols, were approved by the Scientific Research Ethics Committee of the Second Hospital of Shandong University (Permit Number: 20140195).
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao JQ, Li JG, Zhao CX. Prevalence of pneumoconiosis among young adults aged 24–44 years in a heavily industrialized province of China. J Occup Health. 2019;61(1):73–81. doi:10.1002/1348-9585.12029
2. Hegde B, Bodduluri SR, Satpathy SR, et al. Inflammasome-independent leukotriene B4 production drives crystalline silica-induced sterile inflammation. J Immunol. 2018;200(10):3556–3567. doi:10.4049/jimmunol.1701504
3. Hoy RF, Baird T, Hammerschlag G, et al. Artificial stone-associated silicosis: a rapidly emerging occupational lung disease. Occup Environ Med. 2018;75(1):3–5. doi:10.1136/oemed-2017-104428
4. Mandrioli D, Schlünssen V, Ádám B, et al. WHO/ILO work-related burden of disease and injury: protocol for systematic reviews of occupational exposure to dusts and/or fibres and of the effect of occupational exposure to dusts and/or fibres on pneumoconiosis. Environ Int. 2018;119:174–185. doi:10.1016/j.envint.2018.06.005
5. Ferrante P. Asbestosis and silicosis hospitalizations in Italy (2001–2015): results from the National Hospital Discharge Registry. Eur J Public Health. 2019;29(5):876–882. doi:10.1093/eurpub/ckz003
6. Reilly MJ, Timmer SJ, Rosenman KD. The burden of silicosis in Michigan: 1988–2016. Ann Am Thorac Soc. 2018;15(12):1404–1410. doi:10.1513/AnnalsATS.201802-117OC
7. Xu Q, Liu Y, Pan H, et al. Aberrant expression of miR-125a-3p promotes fibroblast activation via Fyn/STAT3 pathway during silica-induced pulmonary fibrosis. Toxicology. 2019;414:57–67. doi:10.1016/j.tox.2019.01.007
8. Hou X, Summer R, Chen Z, et al. Lipid uptake by alveolar macrophages drives fibrotic responses to silica dust. Sci Rep. 2019;9(1):399. doi:10.1038/s41598-018-36875-2
9. Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;11(2):169–173. doi:10.1097/01.mcp.0000152998.11335.24
10. Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med. 2003;34(12):1507–1516. doi:10.1016/S0891-5849(03)00149-7
11. Li PF, He RH, Shi SB, et al. Modulation of miR-10a-mediated TGF-β1/Smads signaling affects atrial fibrillation-induced cardiac fibrosis and cardiac fibroblast proliferation. Biosci Rep. 2019;39(2):BSR20181931. doi:10.1042/BSR20181931
12. Lu Y, Zhang T, Shan S, et al. MiR-124 regulates transforming growth factor-β1 induced differentiation of lung resident mesenchymal stem cells to myofibroblast by repressing Wnt/β-catenin signaling. Dev Biol. 2019;449(2):115–121. doi:10.1016/j.ydbio.2019.02.010
13. Bellaye PS, Shimbori C, Upagupta C, et al. Lysyl oxidase-like 1 protein deficiency protects mice from adenoviral transforming growth factor-β1-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018;58(4):461–470. doi:10.1165/rcmb.2017-0252OC
14. Qin T, Yin S, Yang J, et al. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFβ signaling. Toxicol Appl Pharmacol. 2016;304:1–8. doi:10.1016/j.taap.2016.05.009
15. Huang LS, Jiang P, Feghali-Bostwick C, Reddy SP, Garcia JGN, Natarajan V. Lysocardiolipin acyltransferase regulates TGF-β mediated lung fibroblast differentiation. Free Radic Biol Med. 2017;112:162–173. doi:10.1016/j.freeradbiomed.2017.07.023
16. Cho HY, Kleeberger SR. Noblesse oblige: NRF2 functions in the airways. Am J Respir Cell Mol Biol. 2014;50(5):844–847. doi:10.1165/rcmb.2014-0116PS
17. Zhu S, Wei W, Liu Z, Yang Y, Jia H. Tanshinone-IIA attenuates the deleterious effects of oxidative stress in osteoporosis through the NF-κB signaling pathway. Mol Med Rep. 2018;17(5):6969–6976. doi:10.3892/mmr.2018.8741
18. Tsai YT, Loh SH, Lee CY, et al. Tanshinone IIA inhibits high glucose-induced collagen synthesis via nuclear factor erythroid 2-related factor 2 in cardiac fibroblasts. Cell Physiol Biochem. 2018;51(5):2250–2261. doi:10.1159/000495870
19. Aschner Y, Downey GP. Transforming growth factor-β: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol. 2016;54(5):647–655. doi:10.1165/rcmb.2015-0391TR
20. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338. doi:10.1038/nrneph.2016.48
21. Hill CS. Transcriptional control by the SMADs. Cold Spring Harb Perspect Biol. 2016;8(10):a022079. doi:10.1101/cshperspect.a022079
22. Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64(3):157–167. doi:10.1369/0022155415627681
23. Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi:10.1016/j.bbadis.2016.11.005
24. Wei J, Zhu H, Lord G, et al. Nrf2 exerts cell-autonomous antifibrotic effects: compromised function in systemic sclerosis and therapeutic rescue with a novel heterocyclic chalcone derivative. Transl Res. 2017;183(183):71–86.e1. doi:10.1016/j.trsl.2016.12.002
25. Guo Z, Yan M, Chen L, et al. Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Exp Ther Med. 2018;16(4):3333–3344. doi:10.3892/etm.2018.6614
26. Katz LH, Likhter M, Jogunoori W, Belkin M, Ohshiro K, Mishra L. TGF-β signaling in liver and gastrointestinal cancers. Cancer Lett. 2016;379(2):166–172. doi:10.1016/j.canlet.2016.03.033
27. Kobayashi T, Liu X, Wen FQ, et al. Smad3 mediates TGF-beta1-induced collagen gel contraction by human lung fibroblasts. Biochem Biophys Res Commun. 2006;339(1):290–295. doi:10.1016/j.bbrc.2005.10.209
28. Richter K, Kietzmann T. Reactive oxygen species and fibrosis: further evidence of a significant liaison. Cell Tissue Res. 2016;365(3):591–605. doi:10.1007/s00441-016-2445-3
29. Latella G. Redox imbalance in intestinal fibrosis: beware of the TGFβ-1, ROS, and Nrf2 connection. Dig Dis Sci. 2018;63(2):312–320. doi:10.1007/s10620-017-4887-1
30. Hecker L, Cheng J, Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci. 2012;69:2365–2371. doi:10.1007/s00018-012-1012-7
31. Kim J, Cha YN, Surh YJ. A protective role of nuclear factorerythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690(1–2):12–23. doi:10.1016/j.mrfmmm.2009.09.007
32. Paunkov A, Chartoumpekis DV, Ziros PG, Sykiotis GP. A bibliometric review of the Keap1/Nrf2 pathway and its related antioxidant compounds. Antioxidants (Basel). 2019;8(9):E353. doi:10.3390/antiox8090353
33. Yu H, Chen B, Ren Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):3657–3663. doi:10.1080/21691401.2019.1657879
34. Kikuchi N, Ishii Y, Morishima Y, et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res. 2010;11:31. doi:10.1186/1465-9921-11-31
35. Liu Y, Lu F, Kang L, Wang Z, Wang Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 2017;17(1):63. doi:10.1186/s12890-017-0405-7
36. Xu Y, Tai W, Qu X, et al. Rapamycin protects against paraquat-induced pulmonary fibrosis: activation of Nrf2 signaling pathway. Biochem Biophys Res Commun. 2017;490(2):535–540. doi:10.1016/j.bbrc.2017.06.074
37. Liu RM, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi:10.1016/j.redox.2015.09.009
38. Sato N, Takasaka N, Yoshida M, et al. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res. 2016;17(1):107. doi:10.1186/s12931-016-0420-x
39. Kim Y, Park SY, Jung H, Noh YS, Lee JJ, Hong JY. Inhibition of NADPH oxidase 4 (NOX4) signaling attenuates tuberculous pleural fibrosis. J Clin Med. 2019;8(1):E116. doi:10.3390/jcm8010116
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.