Back to Journals » Drug Design, Development and Therapy » Volume 14
The PPARγ Agonist Rosiglitazone Enhances the Radiosensitivity of Human Pancreatic Cancer Cells
Authors Wang Z , Shen W, Li X, Feng Y, Qian K, Wang G, Gao Y , Xu X, Zhang S, Yue L, Cao J
Received 28 January 2020
Accepted for publication 3 July 2020
Published 31 July 2020 Volume 2020:14 Pages 3099—3110
DOI https://doi.org/10.2147/DDDT.S242557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Zhenyu Wang1 *, Wenhao Shen1 *, Xugang Li,2 Yang Feng,1 Kun Qian,1 Gaoren Wang,3 Yiying Gao,1 Xiaohui Xu,4 Shuyu Zhang,1 Ling Yue,1 Jianping Cao1
1School of Radiation Medicine and Protection (SRMP) of Soochow University, State Key Laboratory of Radiation Medicine and Protection, Suzhou 215123, People’s Republic of China; 2Department of Radiotherapy, Anshan Cancer Hospital, Anshan 114036, People’s Republic of China; 3Nantong Tumor Hospital, Affiliated Tumor Hospital of Nantong University, Nantong 226361, People’s Republic of China; 4Department of General Surgery, The First People’s Hospital of Taicang, Taicang Affiliated Hospital of Soochow University, Suzhou 215400, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianping Cao; Ling Yue Tel +86-512-65880037
Email [email protected]; [email protected]
Purpose: As radiation therapy is widely used for the management of pancreatic cancer, identifying novel targets to improve the radiosensitivity of cancer cells is beneficial. Rosiglitazone, a specific peroxisome proliferator-activated receptor γ (PPARγ) agonist, has an inhibitory effect on various types of cancer cells. The purpose of this paper is to investigate the effect of rosiglitazone on the radiosensitivity of pancreatic cancer cells and the potential mechanism.
Materials and Methods: PPARγ expression in pancreatic cancer and adjacent tissues was evaluated using immunohistochemistry analysis. The viability, migration and invasion ability of PANC1 and PaTu8988 cells were detected using MTT assay, scratch-wound assay and transwell invasion assay. The effect of rosiglitazone on radiosensitivity of the cells was determined using the clonogenic assay. PANC1 cells were inoculated into BALB/c mice to establish tumors. Microarray was used to investigate changes of genes involved.
Results: Higher PPARγ expression was demonstrated in pancreatic cancer tissues compared with para-carcinoma tissues. Rosiglitazone inhibited the cell viability and enhanced the radiation-induced anti-migration and anti-invasion effect. Rosiglitazone potentiated the radiosensitivity of pancreatic cancer cells and PANC1 xenografts. Microarray analysis revealed that rosiglitazone plus radiation altered the expression of multiple genes and affected multiple pathways.
Conclusion: Rosiglitazone enhances the radiosensitivity of human pancreatic cancer cells in vitro and in vivo via complex mechanisms.
Keywords: ionizing radiation, pancreatic cancer, peroxisome proliferator-activated receptor γ, rosiglitazone, radiosensitivity
Introduction
Pancreatic cancer is a fatal disease with high mortality and poor prognosis. As no detection is effective and no symptoms or signs are recognizable in the early stage of this complex disease, patients are diagnosed late in their disease, often after their cancer has metastasized to other organs.1 In the United States, pancreatic cancer is the fourth leading cause of cancer-related deaths in 2017,2 and the estimated numbers of new cancer cases and deaths have gradually increased in the past 5 years. In China, the incidence and mortality of pancreatic cancer have risen slightly since 2006 and may increase faster in the next few years.3 Radiotherapy is an effective cancer treatment that also plays an important role in preventing recurrence and improving the resectability of pancreatic tumors. To improve patient outcome, radiotherapy can be used as a single modality or in combination with other treatments.4,5 Even though radiotherapy technology is continually evolving, enhancing the radiosensitivity of irradiated tumors with drug-radiotherapy combination offers an alternative strategy, and it is therefore advisable to screen and define more novel drugs in combination with radiotherapy for better clinical outcomes.5
Peroxisome proliferator-activated receptors (PPARs) are nuclear transcription factors and belong to the nuclear hormone receptor super family. Three PPAR subtypes have been identified, PPARα, PPARβ, and PPARγ. The three PPAR subtypes share a high degree of sequence homology but differ in physiological and pharmacological functions due to their ligands specificity and distinct tissue distribution.6,7 PPARγ may have a primary role in anti-inflammatory processes, adipocyte differentiation, and glucose metabolism. However, more and more studies have shown that PPARγ is expressed in a variety of tumor cells, and the activation of PPARγ may be involved in antineoplastic processes.8,9 Rosiglitazone is a representative PPARγ agonist used as a common anti-diabetic drug, and it has also been suggested to be responsible for other biological effects, including bone formation and resorption, neuroprotection, and anti-inflammation by activating PPARγ.9–12 Recently, studies have indicated that rosiglitazone may play an inhibitory role in various types of cancer cells, such as breast cancer,13 hepatocarcinoma,14 lung carcinoma15 and neuroblastoma.16 However, there is no report of the effect and underlying mechanisms of rosiglitazone on the radiosensitivity of pancreatic cancer cells.
This study aimed to examine the effect of the PPARγ agonist rosiglitazone on irradiated pancreatic cancer cells, with an emphasis on radiosensitivity.
Materials and Methods
Tissue Samples and Immunohistochemistry
Eighteen samples of paired pancreatic cancer and para-carcinoma tissues without radiation therapy were obtained at the Fujian Medical University Union Hospital between 2010 and 2012 and had been kept in the hospital. The use of the human tissue samples has been reported in our previous paper.17 The samples provider was also affiliated to Fujian Medical University Union Hospital. Therefore, a waiver of informed consent was approved by the Ethics Committee of Fujian Medical University Union Hospital (Fuzhou, China) when these residual tissue samples were applied for the secondary use in our study. The data analysis was anonymized to protect the privacy and confidentiality of participants. All the samples were prepared into paraffin section for PPARγ expression examination using immunohistochemistry. Paraffin sections were deparaffinized routinely, and treated with 3% H2O2 at 37°C after the slides were washed 3 times with PBS. Then, heat-mediated antigen retrieval in citrate buffer was performed. Following incubation with the rabbit antibody against PPARγ (Abcam, Cambridge, UK) at 4°C overnight, paraffin sections were incubated with goat anti-rabbit biotinylated secondary antibody (Beyotime, Nantong, China), detected, stained, dehydrated and mounted. Five microscopic vision fields were counted and scored according to the degree of staining and the percentage of stained cells in each section.
Cell Culture and Irradiation
The human pancreatic cancer cell lines PANC1 and PaTu8988 were confirmed by STR as described as before,17 and maintained in dulbecco’s modified eagle medium (DMEM, HyClone, Logan, UT, USA) supplemented with 10% fetal bovineserum (FBS, HyClone, Logan, UT, USA) and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) in a 37°C incubator with 5% CO2. Cells were treated with or without rosiglitazone (Sigma, St Louis, MO, USA), which was dissolved in DMSO (dimethylsulfoxide, Sigma, St Louis, MO, USA) and 48 h later, exposure in ionizing radiation to cells was performed by an X-ray linear accelerator (RadSource, Suwanee, GA, USA) at a fixed dose rate of 1.15 Gy/min. The use of the cell lines was approved by the Ethics Committee of Soochow University.
Cell Viability Assay
Cells were seeded in 96-well plates at 2 × 103 cells/well and exposed to rosiglitazone for 48 h before irradiation. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was used to measure the viability of treated cells. Briefly, 10 µL MTT solution (5 mg/mL) was added to the cells that were treated with rosiglitazone or irradiation and the cells were maintained for 4 hrs at 37°C. After the medium was replaced by 100 µL of DMSO, the absorbance at 492 nm was measured with a spectrophotometric plate reader (Biotek, Winooski, VT, USA), and cell viability rate was calculated with the following formula: cell viability rate = (OD in experimental group/OD in control group) × 100%.
Scratch-Wound Assay
Cells were plated onto 6-well plates to create a confluent monolayer. After incubation for 24 hrs at 37°C, the cells were divided into 4 groups: control group with DMSO treatment, rosiglitazone group, irradiation group, and rosiglitazone plus irradiation group. The cell monolayer was scraped in a straight line with a p200 pipet tip to create a scratch after treatment, and images of the scratch were acquired with a microscope (Olympus, Tokyo, Japan) immediately. Then, the cells were cultured in DMEM with 2% FBS for 24 hrs following three times washing with PBS, and the distance between one side of scratch and the other was observed and captured again.
Transwell Invasion Assay
Polycarbonate transwell membrane filters (pore size 8 μm, Corning Costar, NY, USA) were coated with 50 μL matrigel on the upper surface and incubated for 5 hrs at 37°C. Cells were divided into 4 groups and treated with rosiglitazone or irradiation as previously mentioned. After 24 hrs of the irradiation, the upper chamber was loaded with 1 × 105 cells in 100 µL serum-free DMEM with 0.2% BSA, and the lower part of chamber was supplied with 600 µL DMEM containing 10% FBS. Following 24 hrs incubation at 37°C, the non-invading cells were removed with a cotton swab, while the migrated cells on the other side of the membrane were fixed with methanal, stained with crystal violet, and counted in 10 random selected fields under the microscope.
Clonogenic Assay
Cells were seeded onto 6-well plates with different densities (200–1000) depending on the dose of irradiation. After 24 hrs treatment with or without rosiglitazone, cells were irradiated with 0, 2, 4, 6 or 8 Gy X-ray irradiation. At the end of irradiation, the medium was refreshed immediately and cells were kept in culture for 10 days. Following twice washing with PBS, the cells were fixed with methanol for 15 min and stained in Giemsa for 30 min. The colonies containing 50 cells or more were counted. The surviving fraction (SF) was calculated as: Number of colonies/(cells inoculated × plating efficiency). According to the multi-target, single-hit model, the survival curve was plotted and the radiation sensitivity enhancement ratio (SER) was measured.18
Xenograft Models
Male out-bred BALB/c nude mice of 4-weeks old (Shanghai SLAC Laboratory Animal, shanghai, China) were maintained in specific pathogen-free conditions. Mice were injected subcutaneously with PANC1 cells (1×106 cells in PBS/per mouse) into the hind limb. When tumors grew to approximately 150 mm3(9 days after injection), mice were randomly divided into four groups (n=5): DMSO group, rosiglitazone group, irradiation group and irradiation plus rosiglitazone group. Mice were irradiated at 2Gy/min, resulting in a total dose of 10 Gy of X-ray with an X-ray linear accelerator (Clinac 2100 EX, Varian Medical Systems, Palo Alto, CA, USA). Mice in the third and fourth groups were injected intraperitoneally with rosiglitazone (40 µM) for 4 consecutive days. All mice were killed 21 days after the first inoculation, and tumor size was measured with a caliper. Tumor volumes in mm3 were calculated by the formula: tumor volume = (length (L) × (width (W)2) × 0.52.18 The animal studies were in accordance with the Guide for the Care and Use of Laboratory Animals,19 and approved by the Animal Experimentation Ethics Committee of Soochow University.
Microarray
PANC1 cells were treated with DMSO (as control) or rosiglitazone for 48 hrs prior to 4 Gy irradiation, and subsequently, the medium was refreshed. 24hrs later total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA). Microarray-based mRNA expression profiling was carried out with the Roche-NimbleGen (135 K array) Array (Roche, WI) as described in the previous study.20 Genes with > 2 fold-change were subjected to Gene Ontology (GO) analysis and classified into three categories: biological process, cellular component and molecular function.
Statistical Analysis
Statistical analysis was conducted with SPSS software (Release 19.0, SPSS Inc.). Data were presented as the mean ± SEM of at least three individual experiments. Student’s t-test was used to compare the difference between the two groups. One-way analysis of variance (ANOVA) was applied when more than two groups were compared. Differences were considered significant at P< 0.05 and highly significant at P< 0.01.
Results
PPARγ Expression Was Enhanced in Pancreatic Cancer Tissues
We detected the PPARγ expression in 18 pairs of paraffin sections of pancreatic cancer and para-carcinoma tissues with immunohistochemistry assays. Compared to the para-carcinoma tissues, cancer tissues were stained much darker, suggesting higher expression of PPARγ in cancer tissues (Figure 1A and B). Moreover, we found that the expression of PPARγ, a nuclear hormone receptor, was especially concentrated in the cytoplasm. The sections were scored, and the scores of cancer tissues were significantly higher than the normal ones, as shown in Figure 1C (P< 0.01).
Rosiglitazone Inhibited the Growth of Pancreatic Cancer Cells
Since PPARγ was highly expressed in pancreatic cancer cells, its agonist rosiglitazone was used to activate PPARγ to modulate cell radiosensitivity. PANC1 and PaTu8988 cells were incubated with different concentrations of rosiglitazone for 48 hrs, and the MTT assay revealed the viability of the cells was inhibited in a dose-dependent manner (Supplementary Figure S1). For measuring the effect of rosiglitazone in combination with radiation on pancreatic cancer cell viability, the dose of 40 µM rosiglitazone, which resulted in a viability around 80%, was used in the pretreatment before the exposure to ionizing radiation, and the MTT assay was performed again to assess the viability of pancreatic cancer cell. As shown in Figure 2A and B, 40 rosiglitazone plus 4 Gy irradiation inhibited cell viability significantly compared with rosiglitazone or radiation alone, demonstrating the enhancement of antigrowth effects by rosiglitazone. Considering the cytotoxicity and radiosensitization of rosiglitazone, 40 µM rosiglitazone was chosen for further experiments.
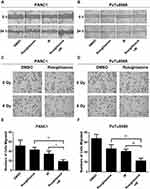
Rosiglitazone Suppressed the Migration and Invasion of Irradiated Pancreatic Cancer Cells
To investigate whether rosiglitazone could affect the radiation-induced anti-migration and anti-invasion effects in PANC1 and PaTu8988 cells, scratch-wound assays and transwell invasion assays were performed. Compared with the control group (DMSO-treated group), the scratches in the rosiglitazone group and the radiation group were modestly wider, suggesting that migration ability was not suppressed severely by rosiglitazone alone or radiation alone. However, the migration distance in the rosiglitazone plus radiation group was shorter than that in the rosiglitazone group or radiation group, which means rosiglitazone increased the anti-migration effect induced by radiation in pancreatic cancer cells in some ways (Figure 3A and B). The roles of rosiglitazone and radiation on invasion are shown in Figure 3C–F. Compared with the rosiglitazone group or radiation group, the number of cells that migrated through the Matrigel-coated membrane in the rosiglitazone plus radiation group was decreased significantly (P < 0.05) or marked significantly (P < 0.01). The results indicated that rosiglitazone suppressed the radiation-impaired anti-invasion ability of PANC1 and PaTu8988 cells.
Rosiglitazone Enhanced the Radiosensitivity of Pancreatic Cancer Cells
The effect of rosiglitazone on the radiosensitivity of pancreatic cancer cells was detected by clonogenic assay. According to the single-hit multimarket model,21 the survival curves of cells and main parameters were obtained (Figure 4A and B). The survival fraction of PANC1 cells was downregulated by increasing radiation, especially in the rosiglitazone group (P< 0.01). The difference between the rosiglitazone group and the DMSO group was significant (P< 0.01). The mean lethal dose (D0) of DMSO and rosiglitazone in PANC1 cells were 2.15 Gy and 1.44 Gy, respectively, and the quasi-threshold doses (Dq) were 2.67 Gy and 1.68 Gy, respectively. The sensitizing enhancement ratio (SER) of PANC1 cell was 1.49 (Figure 4A). In PaTu8988 cells, similar results were observed. Radiation has been shown to cause a dose-dependent decrease in survival fraction (P< 0.01), and the decrease in the rosiglitazone group was more severe than that in the DMSO group (P< 0.01). In the DMSO and rosiglitazone groups of PaTu8988 cells, the values of D0 were 1.50 Gy and 1.12 Gy, respectively, and the values of Dq were 1.25 Gy and 1.18 Gy, respectively. The sensitizing enhancement ratio (SER) of PaTu8988 cells was 1.34 (Figure 4B). These results suggested rosiglitazone sensitized pancreatic cancer cells to radiation.
Rosiglitazone Enhanced the Radiosensitivity of PANC1 Cell via Complex Mechanisms
To explore the mechanisms for rosiglitazone-mediated radiosensitization, microarray analysis of radiation alone and radiation plus rosiglitazone was performed. As shown in Figure 5A, thousands of genes displayed differential expression (2.0-fold or greater between the two groups). The top 30 genes in expression change were listed and mapped (Table 1 and Figure 5B). The most upregulated genes in the radiation plus rosiglitazone group included FABP4, DHRS9, and SLAMF7, while the most downregulated genes included SLC39A7, PSG5, and TRPV1. Gene Ontology (GO) analysis for modulated genes demonstrated that rosiglitazone affected molecular function, biological processes, and cellular components in many aspects (Figure 5C-H). In terms of molecular function, upregulated genes were involved in binding, nucleic acid binding transcription factor activity, catalytic activity and so on, while downregulated genes were mainly related to transporter activity, structural molecule activity, binding, etc. Upregulated genes were implicated in many biological processes, including the metabolic process, responses to stimuli, and cellular processes (eg, intercellular communication), whereas downregulated genes were most related to localization, biological regulation, cellular process, such as intracellular communication or cell cycle regulation, and so forth. For cellular component, most affected genes were located in the cell part. However, the genes increased participated in the component parts of the membrane and extracellular matrix, and the location of genes decreased were mostly the membrane and organelles. Overall, the results above indicated that complex mechanisms were involved in the rosiglitazone-modulated radiosensitization of pancreatic cancer cells.
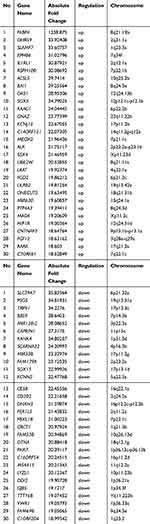 |
Table 1 Micorarray Analysis of Gene Expression Changes Between Radiation and Rosiglitazone Plus Radiation in PANC1 Cells (Top 30) |
Irradiation Combined with Rosiglitazone Inhibited the Growth of PANC1 Xenografts
To investigate the effect of rosiglitazone on the xenograft growth of PANC1 cells in nude mice, tumor growth delay assays were performed. As shown in Figure 6A and B, compared with the control group (DMSO group), the tumor volume of mice treated with radiation alone was reduced by 76.6%, and the tumor volume of mice treated with radiation plus rosiglitazone was reduced by 40.7%. These results indicated that rosiglitazone can enhance the radiosensitivity of pancreatic cancer in vivo.
Discussion
Radiation therapy is widely used for the management of various types of cancer, including pancreatic cancer.4,22 Double-strand DNA breaks are considered as the major cause responsible for ionizing radiation-induced cancer cell death.23 Improving the radiosensitivity by drug-radiotherapy combinations is a pragmatic strategy for cancer management.5 PPARγ is expressed in many types of tumor cells, including colonic tumors,24 lung tumors,25 thyroid carcinoma,26 etc., and it has been shown to have antineoplastic effects. Rosiglitazone, an agonist of PPARγ, could suppress tumor development by activating PPARγ.8 However, little is known about the radiosensitization effect of rosiglitazone on pancreatic cancer cells. To investigate the question, surgically resected human pancreatic adenocarcinomas, human pancreatic carcinoma cell lines, and nude mice xenograft tumor models were used in the present study.
First, we examined the expression of PPARγ in resected pancreatic cancer. Previous research showed that a majority of pancreatic adenocarcinoma cells express PPARγ.27 In the present study, we also observed the expression of PPARγ in pancreatic cancer tissues, and furthermore demonstrated a significant increase of PPARγ expression compared to the para-carcinoma tissues. Thus, PPARγ could be considered as a diagnostic marker for pancreatic cancer. The immunohistochemistry assay showed that PPARγ was located in the cytoplasm rather than the nucleus, so targeting PPARγ nuclear translocation may be a potential strategy for treatment considering the inhibitory role PPARγ played in tumor development.
Next, assays related to radiosensitivity were performed to analyze the radiosensitizing effect of rosiglitazone, and we found that rosiglitazone at a dose of 40 µM sensitized the pancreatic cancer cells to radiation in metastatic effect, invasive ability, clonogenic assay and xenograft studies. Rosiglitazone has been reported to exert an antitumor effect in various cancer, including colon cancer,28 prostate cancer,29 and human lung carcinoma cells.15 Given its ability of reducing tumor growth and metastasis in human cancer cells, studies have put emphases on the radiosensitization properties of rosiglitazone, and in accordance with our report, rosiglitazone enhances the radiosensitivity of human colorectal cancer cells30 and cervical cancer cells.31 This study reveals the radiosensitizing effect of rosiglitazone on pancreatic cancer cells, which expands the utilization of rosiglitazone in radiosensitization and offers a theoretical basis for its potential use in radiotherapy.
Finally, we investigated the underlying mechanism of how rosiglitazone modulated the radiosensitivity of pancreatic cancer cells to exert the antitumor and anti-metastatic effects. A variety of molecular mechanisms regarding the antitumor effect of rosiglitazone have been reported. For example, a microarray in colon cancer cells indicated β-PIX protein, which belongs to a family of cytoplasmic proteins, could be involved in the rosiglitazone-mediated inhibition of cell migration;32 another study suggested KLF6 was altered by rosiglitazone in colon cancer cells.33 In lung carcinoma cells, both PPARγ-dependent and PPARγ-independent signal pathways were related to the suppressive role of rosiglitazone.15 Rosiglitazone increased PPARγ expression and its nuclear translocation in this study, which suggested the activation of PPARγ-dependent pathways. Microarray analysis further showed that rosiglitazone modulated multiple genes that function in multiple pathways in pancreatic cancer cells. FABP4, the most rosiglitazone-upregulated mRNA, belongs to the fatty acid-binding protein family and controls fatty acid uptake, transport, and metabolism.34 It has been reported that FABP4 suppressed the proliferation and invasion of hepatocellular carcinoma cells via the phosphorylation of Stat3 through the Ras-p-Stat3 signaling pathway.35 There is also evidence that FABP4 interacted with PPARγ, and consistent with the present report, rosiglitazone increased FABP4 mRNA expression in human prostate cancer cells.36,37 Among the differentially expressed genes, SLC39A7 (Zip7) was downregulated by the highest fold-change. Zip7 was reported to be involved in the release of zinc from the endoplasmic reticulum and in modulating cell signaling related to proliferation.38 Another study suggested that intracellular zinc pools regulated by Zip7 could result in alteration of the Bcl-2/Bax ratio, hence having impact on apoptosis of cervical cancer cell.39,40 The results obtained in our microarray experiments suggested that differentially expressed genes-mediated many signal pathways that were related to the radiosensitization of rosiglitazone.
Conclusion
We demonstrated that 40 µM rosiglitazone inhibited the radiation-induced migration and invasion of pancreatic cancer cells, and enhanced radiosensitivity in vitro and in vivo. PPARγ should be the principal target of rosiglitazone considering it augmented expression in the pancreatic cancer tissues, yet more complex mechanisms may be involved in the radiosensitization effects of rosiglitazone. These findings demonstrate that rosiglitazone has the potential to be a radiosensitizer in radiotherapy in pancreatic cancer.
Acknowledgments
We thank Jianyuan Song, who worked at Fujian Medical University Union Hospital for providing the human samples of paired pancreatic cancer and para-carcinoma tissues. This work is supported by the National Natural Science Foundation of China (81803166, 81673100, 81872552, 81602101 and 81703022), China Postdoctoral Science Foundation (2018M632368), Jiangsu Province Key Youth Talents Project (QNRC2016262) and Collaborative Innovation Center of Radiological Medicine of Jiangsu Higher Education Institutions. Zhenyu Wang and Wenhao Shen are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in Pancreatic Cancer. CA Cancer J Clin. 2013;63(5):318–348. doi:10.3322/caac.21190
2. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
3. Lin QJ, Feng Y, Chen J, et al. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21(26):7988–8003. doi:10.3748/wjg.v21.i26.7988
4. Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20(32):11142–11159. doi:10.3748/wjg.v20.i32.11142
5. Sharma RA, Plummer R, Stock JK, et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13(10):627–642. doi:10.1038/nrclinonc.2016.79
6. Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPARα), beta (PPARβ), and gamma (PPARγ) in rodent and human development. ReprodToxicol. 2009;27(34):246–257.
7. Youssef J, Badr M. Peroxisome proliferator-activated receptors and cancer: challenges and opportunities. Br J Pharmacol. 2011;164(1):68–82. doi:10.1111/j.1476-5381.2011.01383.x
8. Schmidt MV, Brüne B, Von Knethen A. The nuclear hormone receptor PPARγ as a therapeutic target in major diseases. Scientific World J. 2010;10:2181–2197. doi:10.1100/tsw.2010.213
9. Teresi RE, Waite KA. PPARγ, PTEN, and the fight against cancer. PPAR Res. 2008;932632.
10. Cho ES, Kim MK, Son YO, et al. The effects of rosiglitazone on osteoblastic differentiation, osteoclast formation and bone resorption. Mol Cells. 2012;33(2):173–181. doi:10.1007/s10059-012-2240-z
11. Luo Y, Yin W, Signore AP, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. J Neurochem. 2006;97(2):435–448. doi:10.1111/j.1471-4159.2006.03758.x
12. Lin CF, Young KC, Bai CH, et al. Rosiglitazone regulates anti-inflammation and growth inhibition via PTEN. Biomed Res Int. 2014;2014:787924.
13. Bonofiglio D, Cione E, Qi H. Combined low doses of PPARγ and RXR ligands trigger an intrinsic apoptotic pathway in human breast cancer cells. Am J Pathol. 2009;175(3):1270–1280. doi:10.2353/ajpath.2009.081078
14. Zhang W, Wu N, Li Z, Wang L, Jin J, Zha XL. PPAR gamma activator rosiglitazone inhibits cell migration via upregulation of PTEN in human hepatocarcinoma cell line BEL-7404. Cancer Biol Ther. 2006;5(8):1008–1014. doi:10.4161/cbt.5.8.2887
15. Han SW, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Mol Cancer Ther. 2006;5(2):430–437. doi:10.1158/1535-7163.MCT-05-0347
16. Krieger-Hinck N, Schumacher U, Müller A, Valentiner U. The effect of the PPAR-gamma agonist rosiglitazone on neuroblastoma SK-N-SH cells in a metastatic xenograft mouse model. Oncol Res. 2010;8(8):387–393.
17. Xue J, Zhu W, Song J, et al. Activation of PPARα by clofibrate sensitizes pancreatic cancer cells to radiation through the Wnt/β-catenin pathway. Oncogene. 2018;37(7):953–962. doi:10.1038/onc.2017.401
18. Song J, Shen W, Xue J, et al. Fenofibrate enhances the radiosensitivity of human pancreatic cancer cells in vitro and in vivo. Int J Clin Exp Med. 2017;10(11):15168–15177.
19. Guide for the Care and Use of Laboratory Animals. National Research Council.
20. Xu C, Chen Y, Zhang H, et al. Integrated microRNA-mRNA analyses reveal OPLL specific microRNA regulatory network using high-throughput sequencing. Sci Rep. 2016;6:21580. doi:10.1038/srep21580
21. Spring E, Holmberg P. Evaluation of experimental irradiation fractionation with the single-hit, multi-target model. Acta Radiol Ther Phys Biol. 1968;7(4):297–306. doi:10.3109/02841866809133203
22. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–199. doi:10.7150/ijms.3635
23. Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: A direct and indirect lighting up. Radiother Oncol. 2013;108(3):362–369. doi:10.1016/j.radonc.2013.06.013
24. Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med. 1998;4(9):1046–1052. doi:10.1038/2030
25. Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor γ in non-small cell lung cancer. Cancer Res. 2000;60(4):1129–1138.
26. Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for peroxisome proliferator-activated receptor γ inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86(5):2170–2177. doi:10.1210/jcem.86.5.7493
27. Motomura W, Okumura T, Takahashi N, Obara T, Kohgo Y. Activation of peroxisome proliferator-activated receptor γ by troglitazone inhibits cell growth through the increase of p27Kip1 in human Pancreatic carcinoma cells. Cancer Res. 2000;60(19):5558–5564.
28. Dai Y, Qiao L, Chan KW, et al. Loss of XIAP sensitizes rosiglitazone-induced growth inhibition of colon cancer in vivo. Int J Cancer. 2008;122(12):2858–2863. doi:10.1002/ijc.23443
29. Qin L, Gong C, Chen AM, et al. Peroxisome proliferator-activated receptor γ agonist rosiglitazone inhibits migration and invasion of prostate cancer cells through inhibition of the CXCR4/CXCL12 axis. Mol Med Rep. 2014;10(2):695–700. doi:10.3892/mmr.2014.2232
30. Chiu SJ, Hsaio CH, Tseng HH, et al. Rosiglitazone enhances the radiosensitivity of p53-mutant HT-29 human colorectal cancer cells. Biochem Biophys Res Commun. 2010;394(3):774–779. doi:10.1016/j.bbrc.2010.03.068
31. An Z, Yu JR, Park WY. Rosiglitazone enhances radiosensitivity by inhibiting repair of DNA damage in cervical cancer cells. Radiat Environ Biophys. 2017;56(1):89–98. doi:10.1007/s00411-016-0679-9
32. Cerbone A, Toaldo C, Minelli R, et al. Rosiglitazone and AS601245 decrease cell adhesion and migration through modulation of specific gene expression in human colon cancer cells. PLoS One. 2012;7(6):e40149. doi:10.1371/journal.pone.0040149
33. Rojas V, Laime D. Rosiglitazone role in the expression of KLF6 Caco-2 colon cancer cells. Int J Morphol. 2017;35(1):259–264. doi:10.4067/S0717-95022017000100042
34. Tang Z, Shen Q, Xie H, et al. Elevated expression of FABP3 and FABP4 cooperatively correlates with poor prognosis in non-small cell lung cancer (NSCLC). Oncotarget. 2016;7(29):46253–46262. doi:10.18632/oncotarget.10086
35. Zhong CQ, Zhang XP, Ma N, et al. FABP4 suppresses proliferation and invasion of hepatocellular carcinoma cells and predicts a poor prognosis for hepatocellular carcinoma. Cancer Med. 2018;7(6):2629–2640. doi:10.1002/cam4.1511
36. Tan NS, Shaw NS, Vinckenbosch N, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22(14):5114–5127. doi:10.1128/MCB.22.14.5114-5127.2002
37. Olokpa E, Bolden A, Stewart LV. The androgen receptor regulates PPARγ expression and activity in human Prostate Cancer cells. J Cell Physiol. 2016;231(12):2664–2672. doi:10.1002/jcp.25368
38. Taylor KM, Morgan HE, Smart K, et al. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13(78):396–406. doi:10.2119/2007-00040.Taylor
39. Taylor KM, Hiscox S, Nicholson RI, Hogstrand C, Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci Signal. 2012;5(210):ra11. doi:10.1126/scisignal.2002585
40. Wei Y, Dong J, Li F, Wei Z, Tian Y. Knockdown of SLC39A7 suppresses cell proliferation, migration and invasion in cervical cancer. Excli J. 2017;16:1165–1176. doi:10.17179/excli2017-690
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.