Back to Journals » Drug Design, Development and Therapy » Volume 14
The Inhibitory Effects of Juglanin on Adipogenesis in 3T3-L1 Adipocytes
Authors Wang G, Wu B, Xu W, Jin X, Wang K, Wang H
Received 1 April 2020
Accepted for publication 23 September 2020
Published 2 December 2020 Volume 2020:14 Pages 5349—5357
DOI https://doi.org/10.2147/DDDT.S256504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Qiongyu Guo
Guang Wang,1,* Bing Wu,2,* Wenzhou Xu,3 Xuefei Jin,4 Kun Wang,5 Heyuan Wang6
1Department of Intensive Care Unit, The First Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China; 2Department of Neurosurgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China; 3Department of Periodontology, School and Hospital of Stomatology, Jilin University, Changchun, Jilin 130033, People’s Republic of China; 4Department of Urology, China-Japan Union Hospital of Jilin University, Jilin Key Laboratory of Urologic Oncology, Changchun, Jilin 130033, People’s Republic of China; 5Department of Obstetrics and Gynecology, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China; 6Department of Endocrinology and Metabolism, The First Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Heyuan Wang
Department of Endocrinology and Metabolism, The First Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China
Tel/Fax +86-431-88782222
Email [email protected]
Introduction: Deregulation of adipogenesis plays an important role in obesity and other metabolism disorders. PPAR, C/EBP and SREBP1c are key transcriptional factors involved in adipogenesis and lipogenesis. Juglanin is a natural compound belonging to flavonoids, and it has been reported that juglanin has a potent inhibitory effect on inflammation and certain type of cancers. However, the effects of juglanin in adipogenesis have not been reported before.
Materials and Methods: 3T3-L1 preadipocytes were incubated with differentiation induction medium in the presence or absence of 0.5, 2.5, or 5 μM juglanin for an 8-day differentiation period. The lipid droplets accumulated in the cytoplasm were monitored by Oil Red O staining on days 0, 2, 5, and 8. The regulatory effects of juglanin on adipogenesis-related genes and proteins were investigated by real-time polymerase chain reaction and Western blot analysis.
Results: Juglanin significantly decreased lipid accumulation in differentiated adipocytes. Our findings show that juglanin reduced the expression of C/EBPα, C/EBPβ, and SREBP-1c without affecting PPARα or PPARγ expression. Additionally, juglanin increased the activation of the SIRT1/AMPK signaling pathway through the phosphorylation of AMPKα. Finally, we performed an AMPK inhibitor experiment, which revealed that the inhibitory effects of juglanin on adipogenesis are mediated through AMPK.
Discussion: Juglanin can prevent adipogenesis by suppressing lipid accumulation and the differentiation of preadipocytes. The mechanism of juglanin regulating adipogenesis requires further investigation. Future clinical study in vivo could shed more light on its implication in modulating obesity and metabolic disorders.
Keywords: Juglanin, adipogenesis, obesity, lipid metabolism, AMPK
Introduction
Globalization has brought with it an increase in high-fat western-style diets and sedentary lifestyles, which has resulted in a rapid increase in the rates of obesity and metabolism-related disorders, even among young children.1 In normal physiology, adipose tissue functions as energy storage and serves to regulate energy metabolism through the secretion of adipokines and other signaling molecules. However, excessive differentiation of fibroblast-like preadipocytes into mature adipocytes, such as in many obese patients, has been shown to promote a wide range of diseases including cancers, type II diabetes, and osteoarthritis, among others.2,3 Current concept of therapeutic intervention is to keep the energy balance positive but prevent excessive adipogenesis, ectopic fat deposition, and associated lipotoxicity. Therefore, therapies that can inhibit adipogenesis are viewed as of great value to global healthcare. As the causative factors behind obesity are complex and multitudinous, ongoing research into the mechanisms of obesity and adipogenesis is required. Various studies have suggested modulating the expression of adipogenesis-related genes and transcription factors as a promising therapeutic strategy. Members of the peroxisome proliferator-activated receptor (PPAR) and CCAAT-enhancer-binding protein (C/EBP) families play a significant role in adipocyte differentiation. For example, the transcription factors PPARγ and C/EBPα are considered to be essential master regulators of adipogenesis.4 Sterol regulatory-element binding proteins (SREBPs) are a family of transcription factors that regulate lipid and fatty acid synthesis, energy storage, and act as intercellular signaling nodes of convergence/divergence.5 Downregulating the activation of these pathways has been suggested as a potential treatment to prevent or reverse obesity.6
Sirtuin 1 (SIRT1) is a conserved protein NAD+-dependent deacetylase. In fasting conditions, SREBP1 is deacetylated by SIRT1 which results in reduced lipogenesis.7,8 Activation of the SIRT1/AMP-activated mitogen protein kinase (AMPK) pathway has also been shown to exert an inhibitory effect on adipogenesis. For example, resveratrol treatment was shown to inhibit adipogenesis via increased SIRT1/AMPKα signaling.9 Mango extract has also been shown to downregulate adipogenesis via SIRT1/AMPK upregulation.10 Interestingly, the role of natural flavonoid compounds such as resveratrol and delphinidin in downregulating adipogenesis through SIRT1/AMPK signaling has yielded some promising results.11–13 Previous research has shown that while not all flavonoids are anti-adipogenic, many can reduce adipogenesis via the upregulation of AMPK and Wnt signaling.14 Juglanin is a naturally occurring flavonoid compound extracted from the husks of green walnuts (Juglans mandshurica) and Polygonum aviculare. Juglanin (kaempferol-3-O-α-L-arabinofuranoside) has a molecular weight of 418.35 Da and has been assigned the molecular formula C20H18O10 by the Royal Society of Chemistry, UK (Figure 1). The compound has been shown to exert powerful anti-cancer anti-inflammatory, and anti-oxidant effects.15,16 However, the potential of juglanin to regulate adipogenesis has not been thoroughly studied. Thus, the aim of the present study was to investigate the effects of juglanin on adipogenesis by measuring lipid accumulation, differentiation, and the expression of proadipogenic transcription factors including PPAR and C/EBPs. We also determined the involvement of the SIRT1/AMPK signaling pathway. Our findings provide a basis for further research on the potential of juglanin to treat or prevent obesity and lipid metabolism disorders.
 |
Figure 1 Molecular structure of juglanin. |
Materials and Methods
Cell Culture
The 3T3-L1 pre-adipocyte cell line used in our study was purchased from ATCC, USA. Briefly, the 3T3-L1 cells were cultured in DMEM (Invitrogen-Gibco, USA) containing 10% heat-inactivated fetal bovine serum (Invitrogen-Gibco, USA) and antibiotics. The cells were then incubated in a 5% CO2 atmosphere at 37°C and subcultured twice weekly.
Adipocyte Differentiation and Treatment
For our differentiation experiment, 3T3-L1 pre-adipocytes were seeded into 6-well plates and incubated in DMEM with 0.5 mM 1-isobutyl-3-methylxanthine, 1 mM dexamethasone, and 10 mg/mL insulin for 48 h. For the treatment experiment, the cell culture media was replaced with juglanin containing media supplied with 10 mg/mL insulin for another 48 h. The medium was replaced with insulin-free DMEM every 2 days through day 8. On the 8th day, lipid accumulation was measured. Our experimental design consisted of an 8-day differentiation period during which 3T3-L1 preadipocytes were incubated with differentiation induction medium in the presence or absence of 0.5, 2.5, or 5 µM juglanin and 24-hour treatment window after 8-day differentiation (Figure 2).
 |
Figure 2 Experimental design. 3T3-L1 preadipocytes were induced to differentiate with induction medium in the presence or absence of juglanin (0.5, 2.5, 5 μM) for 8 days. |
Real-Time PCR Analysis
Total RNA was extracted from 3T3-L1 adipocytes using TRIzol reagent (Life Technologies, Carlsbad, CA, United States), and the cDNA was synthesized using a cDNA synthesis kit from Life Technologies. Then, real-time PCR using an SYBR Green MasterMix kit (Bio-Rad Laboratories, Hercules, CA, United States) and a spectrofluorometric thermal cycler (iCycler; Bio-Rad Laboratories) were used to amplify the specific DNA.
Western Blot Analysis
After the indicated treatment, the cells were lysed with protein lysis buffer (Sigma-Aldrich, USA). Protein samples of equal amounts were separated onto 8–10% SDS-PAGE gels followed by transference onto polyvinylidene fluoride membranes (PVDF; Millipore, Billerica, MA, United States). The PVDF membranes were blocked with non-fat powdered skim milk and incubated with primary antibodies overnight at 4°C. The membranes were then incubated with secondary antibodies, and the resulting fluorescent signal was detected using Luminol/Enhancer solution (Millipore). The BioSpectrum 600 system (UVP, Upland, CA, United States) was used to visualize the protein bands.
Oil Red O Staining
After the indicated treatment, the differentiated 3T3-L1 cells were fixed with formalin as previously described17 and stained with oil red O dye. The oil droplets were observed through a microscope (Olympus). After the cell culture plates were exposed to isopropanol, lipid accumulation was indexed by measuring the absorbance at 490 nm using a microplate reader (Multiskan FC, Thermo Fisher Scientific).
Statistical Analysis
The results of the experiments are provided as means ± SEM (standard error of the mean). Statistical analysis of inter-group comparisons was performed using one-way ANOVA followed by Bonferroni's post hoc test. Values of P <0.005 were considered to represent statistical significance.
Results
Juglanin Reduces Adipogenesis
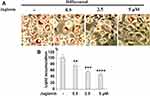
We performed an 8-day differentiation induction of 3T3-L1 preadipocytes in the presence or absence of 0.5, 2.5, or 5 µM juglanin. As shown by the results of Oil Rd O staining in Figure 3, treatment with the three respective doses of juglanin resulted in only 75.6%, 56.3%, and 47.5% adipogenesis, as compared to the control, already indicating a strong inhibitory effect of juglanin on adipogenesis in the 3T3-L1 cell model.
Juglanin Reduces Adipogenic Gene Expression
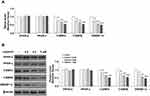
Next, we measured the expression of the two downstream adipogenic genes fatty acid-binding protein 4 (FABP4) and glucose transporter 4 (GLUT4) to determine the effects of juglanin over the 8-day differentiation period. The expression levels of Fabp4 and Glut4 have been used in previous studies to determine the degree of differentiation of preadipocyte into mature adipocytes.18 Measurements were taken on days 0, 2, 5, and 8. As shown in Figure 4A, the expression of Fabp4 remained near baseline on day 2 but was increased 12- and 16-fold on days 5 and 8, respectively. However, the presence of juglanin mitigated these increases to only 5.5- and 4.5-fold on days 5 and 8. As shown in Figure 4B, the expression of Glut4 steadily increased from day 2 onward from 5.5- to 26-fold. Remarkably, juglanin suppressed Glut4 expression to baseline values until day 8 at which point only a negligible increase of 2-fold was observed. Thus, juglanin strongly inhibits adipocyte differentiation.
Adipocytokines, adipokines, adiponectin and leptin are known to be induced in the differentiation of 3T3-L1 cells and regulate adipocyte function. Results in Supplementary Figure 1 demonstrates that juglanin inhibited the gene expression of adiponectin and leptin during differentiation process of 3T3-L1 cells.
Juglanin Inhibits the Expression of Pro-Adipogenic Transcription Factors
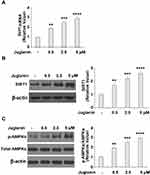
PPAR and C/EBP are key regulatory transcription factors involved in adipogenesis. Here, we employed Western blot analysis to analyze the protein expression levels of PPARα, PPARγ, C/EBPα, and C/EBPβ, as well as SREBP-1c. SREBP-1c plays a role in lipid synthesis, liver steatosis, insulin signaling, and lipid homeostasis.19 As shown in Figure 5A and B, in mature adipocytes stimulated with 0.5, 2.5, and 5 µM juglanin, the expression of PPARα and PPARγ remained consistent with baseline in each dosage group at both the mRNA and protein levels. However, the expression levels of C/EBPα, C/EBPβ, and SREBP-1c were all dose-dependently reduced to about half by the 5 µM dose of juglanin at both the mRNA and protein levels. Thus, juglanin reduces the expression of C/EBPα, β, and SREBP-1c in mature 3T3-L1 adipocytes independent of PPAR signaling.
The Effects of Juglanin are Mediated Through the SIRT1/AMPKα Pathway
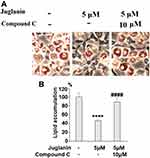
Next, we determined whether the anti-adipogenic effects of juglanin were mediated through the SIRT1/AMPK pathway. In differentiated adipocytes treated with the three doses of juglanin, we observed a dose-dependent increase in the expression of SIRT1 and the phosphorylation of AMPKα to 2.6- and 2.9-fold, respectively, as shown in Figure 6A-C. Finally, we employed the specific AMPK inhibitor compound C to confirm whether the effects of juglanin in adipogenerating cells are dependent on AMPK signaling. Here, we found that treatment with 5 µM juglanin alone reduced lipid accumulation by over 53%, there was only an 11% reduction when 10 µM compound C was added (Figure 7A and B). Thus, the preventative effects of juglanin against lipid accumulation require AMPK signaling.
Discussion
Obesity and other metabolism disorders are quickly becoming a global health concern. Excess adipose tissue can lead to an oversupply of nutrition, which causes the deposition of glycogen and triglycerides in tissues, including liver, muscle, and adipose.20 Flavonoids are secondary plant metabolites that have been receiving increasing attention due to their powerful physiological effects. So far, over 6000 flavonoids have been discovered, exerting a broad spectrum of beneficial effects.21 Indeed, a wide body of research has demonstrated the benefit of flavonoids and other phenolic compounds in preventing or reversing obesity.22 A contemporary study demonstrated the link between dietary flavonoid intake and reduced abdominal obesity in women.23 Another 2020 study found that flavonoid intake can improve intestinal inflammation and gut immune health,24 which has been shown to be a major factor in obesity.25 Thus, flavonoids are likely to provide valuable resources for safe and effective treatments for metabolic disorders such as obesity.
In the present study, we investigated the potential of the flavonoid juglanin to treat obesity and lipid disorders by suppressing adipogenesis. To date, there have been few studies on the effects of juglanin on adipogenesis. As of 2019, 9 juglanin diarylheptanoids had been isolated from the husks of Juglans regia L. (green walnut), named juglanin A-I, respectively.26 Interestingly, previous research has suggested that juglanin may exert anti-obesity effects by binding to the serotonin receptor 5-H2TC, which is involved in appetite, satiety, and eating habits.27 Here, we measured the effects of juglanin on adipogenesis and found that it reduced lipid accumulation by about half over an 8-day period, which was further confirmed by reduced differentiation of preadipocytes into adipocytes, as determined by the expression levels of the adipocyte marker genes Fabp4, Glut4, adiponectin, and leptin. GLUT4 is expressed in both skeletal muscle and adipose tissues.28 GLUT4 is up-regulated when during adipocyte differentiation and is an important marker of mature adipocytes.29 GLUT4 controls glucose uptake in adipose and contributes to insulin resistance in type 2 diabetes.30 Therefore, the expression levels of GLUT4 are often monitored in the differentiation of 3T3-L1 cells. Adipocyte-specific knockout of GLUT4 in mice results in insulin resistance in muscle and liver, suggesting that GLUT4 in adipose plays an important role in glucose transport in the adipose.31 Conversely, GLUT4 overexpression in animals causes enhanced lipogenesis and promotes lipids involved in metabolism and inflammation.32 Our data showed that the presence of juglanin significantly reduced GLUT4 expression in the course of 3T3L1 differentiation, indicating this compound might have an inhibitory effect on glucose transport. Therefore, we hypothesize that juglanin could inhibit GLUT4 expression in adipocytes and skeletal muscle cells. PPAR and C/EBP are recognized as major players in obesity and adipogenesis. Inhibiting the expression of these two important transcription factors has been shown to reduce adipocyte differentiation in 3T3-L1 preadipocytes.33 While previous research found that walnut polyphenols increased PPARα expression,34 we found that juglanin reduced the increase in the expression of both C/EBPα and C/EBPβ without affecting PPARα or PPARγ expression in 3T3-L1 cells, thereby indicating an alternate mechanism of action. The transcripts of PPARγ gene produce two protein isoforms, the shorter PPARγ1, and the longer PPARγ2. PPARγ1 is expressed in various tissues including adipose, PARγ2 is only expressed in adipocytes. Both PPARγ1 and PPARγ2 are induced during adipogenesis. PPARγ1 is the dominant form and is induced in the early phase. PPARγ2 plays a minor role and is induced relatively late.35 The expression of PPARγ during adipogenesis could be regulated at transcriptional and epigenetic levels.36 We hypothesize the reduction of PPARγ protein by juglanin could be due to its effect on epigenetic modification, such as enhanced methylation or weakened acetylation, but this assumption needs to be tested in our future experiments. We also found that juglanin significantly reduced the expression of SREBP-1c, which is induced by high insulin levels to increase the synthesis of fatty acids.37
Activation of the SIRT1/AMPK pathway plays a key role in regulating lipid synthesis and accumulation, adipogenesis, and mitochondrial biogenesis.9,38,39 Thus, substances that can induce the phosphorylation and nuclear translocation of these two transcription factors are of considerable interest. The flavonoid fisetin has recently been shown to prevent nonalcoholic fatty liver disease and steatosis in mice by increasing the phosphorylation of SIRT1 and AMPKα.40 Another study showed that a flavonoid derived from hops could inhibit adipogenesis through AMPK signaling.41 In this study, we found that juglanin indeed enhanced SIRT1/AMPKα signaling, which was further confirmed in our AMPK inhibition experiment using compound C as mediated by AMPK. The molecular mechanism of our study is shown in Figure 8. It should be noted that multiple cellular signals are involved in adipogenesis. The suppression of WNT signaling is essential in the initiation of adipogenesis, suggesting that the WNT pathway inhibits adipogenesis too.42 Additionally, bone morphogenetic proteins (BMPs) and several other cellular signaling pathways are also required in the process.43
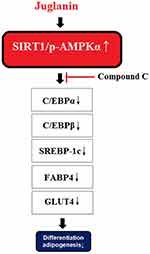 |
Figure 8 A graphical representation of the underlying mechanism. |
Together our findings indicate a promising role of juglanin in inhibiting adipogenesis and lipid accumulation by suppressing adipocyte differentiation via increased expression of SIRT1/AMPK. However, there are several limitations to the current study. 3T3-L1 cells used in this study have been extensively used as a convenient in vitro model to investigate adipogenesis. Firstly, 3T3-L1 cells have been reported to be prone to undergoing spontaneous transformations and lose the potential of differentiation when passaged extensively. Also, the cell line is originally isolated from a single clone from mouse embryo tissues, it cannot recapitulate the characteristics of primary adipocytes from adipose tissue.44 Adipocyte differentiation is a complex process driven by a multitude of factors, including hormones and a cascade of transcriptional and cell-cycle proteins in the adipose.45,46 The future in vivo experiment to test juglanin is warranted for a better understanding of the mechanism of its inhibition on adipogenesis in the context of their surrounding cells in adipose tissue. The research on juglanin is not abundant but has demonstrated a favorable safety profile of juglanin in vivo.15 Thus, clinical trials involving humans are a logical next step. Taking these into consideration, the results of the present study provide a basis for further research into the physiological effects of juglanin.
Acknowledgments
This work was supported by Jilin Provincial Department of Science and Technology (3D517DE33428) and Jilin Provincial Department of Science and Technology (3D518Z843428).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. doi:10.1542/peds.2017-3459
2. Park YK, Obiang‐Obounou BW, Lee KB, Choi JS, Jang BC. AZD1208, a pan‐Pim kinase inhibitor, inhibits adipogenesis and induces lipolysis in 3T3‐L1 adipocytes. J Cell Mol Med. 2018;22(4):2488–2497. doi:10.1111/jcmm.13559
3. Smith KB, Smith MS. Obesity statistics. Prim Care. 2016;43(1):121–135. doi:10.1016/j.pop.2015.10.001
4. Lee JE, Schmidt H, Lai B, Ge K. Transcriptional and epigenomic regulation of adipogenesis. Mol Cell Biol. 2019;39(11):e00601–18. doi:10.1128/MCB.00601-18
5. Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology—divergent pathophysiology. Nat Rev Endocrinol. 2017;13(12):710.
6. Liang YC, Yang MT, Lin CJ, Chang CL, Yang WC. Bidens pilosa and its active compound inhibit adipogenesis and lipid accumulation via down-modulation of the C/EBP and PPARγ pathways. Sci Rep. 2016;6:24285. doi:10.1038/srep24285
7. Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metabol. 2014;25(3):138–145. doi:10.1016/j.tem.2013.12.001
8. Walker AK, Yang F, Jiang K, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24(13):1403–1417. doi:10.1101/gad.1901210
9. Liu X, Zhao H, Jin Q, et al. Resveratrol induces apoptosis and inhibits adipogenesis by stimulating the SIRT1-AMPKα-FOXO1 signalling pathway in bovine intramuscular adipocytes. Mol Cell Biochem. 2018;439(1–2):213–223. doi:10.1007/s11010-017-3149-z
10. Fang C, Kim H, Noratto G, Sun Y, Talcott ST, Mertens-Talcott SU. Gallotannin derivatives from mango (Mangifera indica L.) suppress adipogenesis and increase thermogenesis in 3T3-L1 adipocytes in part through the AMPK pathway. J Funct Foods. 2018;46:101–109.
11. Liou CJ, Wei CH, Chen YL, Cheng CY, Wang CL, Huang WC. Fisetin protects against hepatic steatosis through regulation of the Sirt1/AMPK and fatty acid β-oxidation signaling pathway in high-fat diet-induced obese mice. Cell Physiol Biochem. 2018;49(5):1870–1884. doi:10.1159/000493650
12. Fischer-Posovszky P, Kukulus V, Tews D, et al. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am J Clin Nutr. 2010;92(1):5–15. doi:10.3945/ajcn.2009.28435
13. Park M, Sharma A, Lee HJ. Anti-adipogenic effects of delphinidin-3-o-β-glucoside in 3T3-L1 preadipocytes and primary white adipocytes. Molecules. 2019;24(10):1848. doi:10.3390/molecules24101848
14. Khalilpourfarshbafi M, Gholami K, Murugan DD, Sattar MZ, Abdullah NA. Differential effects of dietary flavonoids on adipogenesis. Eur J Nutr. 2019;58(1):5–25.
15. Sun Z-L, Dong J-L, Wu J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed Pharmacother. 2017;85:303–312. doi:10.1016/j.biopha.2016.11.030
16. Chen L, Xiong YQ, Xu J, Wang JP, Meng ZL, Hong YQ. Juglanin inhibits lung cancer by regulation of apoptosis, ROS and autophagy induction. Oncotarget. 2017;8(55):93878. doi:10.18632/oncotarget.21317
17. Liou CJ, Lai XY, Chen YL, Wang CL, Wei CH, Huang WC. Ginkgolide C suppresses adipogenesis in 3T3-L1 adipocytes via the AMPK signaling pathway. Evid Based Compl Altern Med. 2015;2015.
18. Greenwood-Goodwin M, Teasley ES, Heilshorn SC. Dual-stage growth factor release within 3D protein-engineered hydrogel niches promotes adipogenesis. Biomater Sci. 2014;2(11):1627–1639. doi:10.1039/C4BM00142G
19. Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res Paediatr. 2007;68(2):72–82. doi:10.1159/000100426
20. Liou CJ, Wu SJ, Chen LC, Yeh KW, Chen CY, Huang WC. Acacetin from traditionally used Saussurea involucrata Kar. et Kir. suppressed adipogenesis in 3T3-L1 adipocytes and attenuated lipid accumulation in obese mice. Front Pharmacol. 2017;8:589.
21. Lalani S, Poh CL. Flavonoids as antiviral agents for Enterovirus A71 (EV-A71). Viruses. 2020;12(2):184. doi:10.3390/v12020184
22. Kawser Hossain M, Abdal Dayem A, Han J, et al. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci. 2016;17(4):569. doi:10.3390/ijms17040569
23. Kim SA, Kim J, Jun S, Wie GA, Shin S, Joung H. Association between dietary flavonoid intake and obesity among adults in Korea. Appl Physiol Nutri Metabol. 2020;45(2):203–212. doi:10.1139/apnm-2019-0211
24. Pei R, Liu X, Bolling B. Flavonoids and gut health. Curr Opin Biotechnol. 2020;61:153–159. doi:10.1016/j.copbio.2019.12.018
25. Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr. 2011;31:15–31. doi:10.1146/annurev-nutr-072610-145146
26. Yang H, Ma Y, Gao C, et al. Five novel diarylheptanoids from green walnut husks (Juglans regia L.). Fitoterapia. 2019;134:221–225. doi:10.1016/j.fitote.2019.03.002
27. Yuen H, Hung A, Yang AW, Lenon GB. Mechanisms of action of cassiae semen for weight management: a computational molecular docking study of serotonin receptor 5-HT2C. Int J Mol Sci. 2020;21(4):1326. doi:10.3390/ijms21041326
28. Suárez E, Bach D, Cadefau J, Palacin M, Zorzano A, Gumá A. A novel role of neuregulin in skeletal muscle. Neuregulin stimulates glucose uptake, glucose transporter translocation, and transporter expression in muscle cells. J Biol Chem. 2001;276(21):18257–18264.
29. Jackson RM, Griesel BA, Gurley JM, Szweda LI, Olson AL. Glucose availability controls adipogenesis in mouse 3T3-L1 adipocytes via up-regulation of nicotinamide metabolism. J Biol Chem. 2017;292(45):18556–18564. doi:10.1074/jbc.M117.791970
30. Leto D, Saltiel A. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13(6):383–396. doi:10.1038/nrm3351
31. Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409(6821):729–733. doi:10.1038/35055575
32. Moraes-Vieira PM, Saghatelian A, Kahn BB. GLUT4 expression in adipocytes regulates de novo lipogenesis and levels of a novel class of lipids with antidiabetic and anti-inflammatory effects. Diabetes. 2016;65(7):1808–1815. doi:10.2337/db16-0221
33. Nguyen TT, Ha TT, Nguyen TH, et al. Peptide fraction pOh2 exerts antiadipogenic activity through Inhibition of C/EBP-α and PPAR-γ expression in 3T3-L1 adipocytes. Biomed Res Int. 2017;2017.
34. Shimoda H, Tanaka J, Kikuchi M, et al. Effect of polyphenol rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J Agric Food Chem. 2009;57(5):1786–1792. doi:10.1021/jf803441c
35. Poulsen L, Siersbæk M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23(6):631–639. doi:10.1016/j.semcdb.2012.01.003
36. Lee JE, Ge K. Transcriptional and epigenetic regulation of PPARγ expression during adipogenesis. Cell Biosci. 2014;4:29. doi:10.1186/2045-3701-4-29
37. Tian J, Wu J, Chen X, et al. BHLHE40, a third transcription factor required for insulin induction of SREBP-1c mRNA in rodent liver. Elife. 2018;7:e36826. doi:10.7554/eLife.36826
38. Heo MG, Choung SY. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018;9(9):4906–4915. doi:10.1039/C8FO00986D
39. Chang YH, Chen YL, Huang WC, Liou CJ. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem Biophys Res Commun. 2018;495(1):197–203. doi:10.1016/j.bbrc.2017.11.022
40. Simão AL, Afonso MB, Rodrigues PM, et al. Skeletal muscle miR-34a/SIRT1: AMPK axis is activated in experimental and human non-alcoholic steatohepatitis. J Mol Med. 2019;97(8):1113–1126. doi:10.1007/s00109-019-01796-8
41. Gan CC, Ni TW, Yu Y, et al. Flavonoid derivative (Fla-CN) inhibited adipocyte differentiation via activating AMPK and up-regulating microRNA-27 in 3T3-L1 cells. Eur J Pharmacol. 2017;797:45–52. doi:10.1016/j.ejphar.2017.01.009
42. Du M, Yin J, Zhu MJ. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci. 2010;86(1):103–109. doi:10.1016/j.meatsci.2010.04.027
43. Lowe CE, O’Rahilly S, Rochford JJ. Adipogenesis at a glance. J Cell Sci. 2011;124(16):2681–2686. doi:10.1242/jcs.079699
44. Wolins NE, Quaynor BK, Skinne JR. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–460. doi:10.1194/jlr.D500037-JLR200
45. Nakae J, Kitamura T, Kitamura Y, Biggs III WH, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4(1):119–129. doi:10.1016/S1534-5807(02)00401-X
46. Moreno-Navarrete JM, Fernández-Real JM. Adipocyte differentiation. Adipose Tissue Biol. 2017;69–90.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.