Back to Journals » Clinical Interventions in Aging » Volume 15
The Frail’BESTest. An Adaptation of the “Balance Evaluation System Test” for Frail Older Adults. Description, Internal Consistency and Inter-Rater Reliability
Authors Kubicki A, Brika M, Coquisart L , Basile G , Laroche D , Mourey F
Received 26 January 2020
Accepted for publication 9 April 2020
Published 30 July 2020 Volume 2020:15 Pages 1249—1262
DOI https://doi.org/10.2147/CIA.S247332
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
A Kubicki,1,2 M Brika,2 L Coquisart,3 G Basile,3 D Laroche,1,4 F Mourey1
1INSERM UMR1093-CAPS, Université Bourgogne Franche-Comté, UFR Des Sciences Du Sport, Dijon F-21000, France; 2Institut De Formation Des Métiers De La Santé, Hôpital Nord Franche-Comté, 2 Rue Du Docteur Flamand, Montbéliard 25200, France; 3Centre Hospitalier Durécu-Lavoisier, Darnetal 76160, France; 4INSERM CIC 1432, Plateforme d’Investigation Technologique, University Hospital of Dijon, Dijon 21000, France
Correspondence: A Kubicki
INSERM UMR1093-CAPS, Université Bourgogne Franche-Comté, BP 27877, Campus Universitaire, Dijon 21078, France
Tel +33 684379141
Email [email protected]
Introduction: The Balance Evaluation System Test (BESTest) and the Mini-BESTest were developed to assess the complementary systems that contribute to balance function. These tests include functional tasks involving several high-level exercises to assess the balance function, which may be even more difficult in case of frailty. The Frail’BESTest has been developed to make it possible to include frail older adults in systemic assessment. In this first paper, the objective is to present the Frail’BESTest and to describe the usefulness and complementarity of each system and to test the inter-rater reliability of the score measurements in two health centers.
Methods: In the first center, 192 frail and non-frail older patients were enrolled to test I) the contribution of each system, II) internal consistency, and III) the threshold and ceiling effects. The scores of 32 patients from center 1 and 32 patients recruited in another center (center 2) were used to measure the inter-rater reliability of the measurements by means of Kendall’s tau coefficients.
Results: The internal consistency was moderate to good for five systems and limited for “biomechanical constraints”. The distribution of the Frail’BESTest was more centered than that of the Tinetti and Mini-Motor tests. The Kendall’s tau showed strong concordance in center 1 for all systems and only for 4 on 6 systems in center 2.
Discussion: Completing a systemic evaluation, the therapist may prioritize the patient’s needs identifying the most challenging systems. This paper presents the Frail’BESTest and confirms the psychometric properties at a first step level.
Keywords: frailty, geriatric assessment, systemic evaluation, motor evaluation, psychometric properties
Introduction
The BESTest (Balance Evaluation System Test) was validated in 2009 by Horak et al as an innovative diagnostic tool for therapists, using a systemic approach to patient balance. The BESTest helps to prioritize rehabilitation by identifying disabilities and classifying them into complementary systems called sections.1 Though the BESTest is a relevant test, the time to completion makes it difficult to implement in current care. The MiniBESTest was therefore developed and validated as a shorter version of the original.2 The BESTest and Mini-BESTest have been validated in various populations, including patients with Parkinson’s disease,3–5 multiple sclerosis,6,7 kidney disease,8 strokes,3,9-11 knee osteoarthritis12,13 and older adults.14–16
However, although these tests do not present important floor or ceiling effects,17 they include functional tasks involving several challenging exercises to assess balance, such as sit-to-stand or back-to-sit transfers without armchairs or rise-to-toes without support. These exercises are very hard to do for most frail older adults. Indeed, frailty18–21 entails significant fatigability and a considerable reduction in physical performance. The frailty state is associated with a decrease of physical and/or cognitive abilities, functional reserves and resistance to stressor events which can lead to increased physical inactivity, disability, biological disturbances, risk of fall, and hospitalizations.20,21 Frail older adults have various deficiencies and are unable to compensate adequately to maintain an optimal functional level.20 Typical deficiencies involve the sensorial system, the visual system,22 articular and associated proprioceptive impairments,23 and sarcopenia. Alteration of the proprioceptive system mainly contributes to slower execution of movements23 whereas sarcopenia is a loss of muscle mass contributing to a deficit in strength and in muscle power.24 Most of these deficiencies are associated with the functional level of frail patients.25 There is considerable evidence that deficits of balance and gait abilities can have consequences on functional activities of daily life associated with an increased risk of fall and risk of becoming dependent.26,27 In geriatric practice, balance is often assessed with functional tests28 to objectively quantify the patient's capacities. However, these functional tests do not give a lot of information about the balance deficits. A systemic approach like the BESTest could help, prioritizing the rehabilitation objectives by identifying the different deficiencies.
In furtherance of tests adapted for frail older adults, such as the Mini-Motor Test29 and the Backward Disequilibrium Scale,30 we have designed an adapted version of the BESTest that will make it practical for use in frail older adults: The Frail’BESTest. The modified test respects Horak’s systemic approach, which was to identify the disorders underlying balance control. Therapists can therefore directly manage therapeutic intervention for different types of balance deficiencies. Overall, six sub-systems have been addressed: A: anticipations, B: reactions, C: locomotion, D: sensorial orientation, E: biomechanical constraints and F: asymmetric gait. The organization of each system, the instructions and the evaluation criteria are described in the methods section.
The aim of this paper was to define the Frail’BESTest, to assess its psychometric properties, to describe the usefulness and complementarity of each system, to measure the distribution of scores in a sample of patients and to measure the inter-rater reliability of the measurements in two health centers. For the sake of clarity, a second paper will focus on the concurrent validity, the responsiveness, and the predictive capacities of the Frail’BESTest, as well as its ability to detect frailty.
Materials and Methods
Description of Frail’BESTest Items
The test is shown in Figure 1, and the instructions and evaluation criteria for all the assessed items are presented in Table 1.
 |
Table 1 English Version of the Frail’BESTest: Instructions and Evaluation |
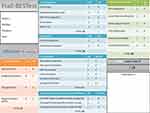 |
Figure 1 English version of the Frail’BESTest. |
Table 2 compares the items of the BESTest with the items of the Frail’BESTest. It clearly indicates when a test is maintained, adapted or removed, and each choice is justified. We limited the scoring scale, offering only a binary choice. This is in response to the needs of therapists who are in search of a tool that is (1) informative about the systems involved in the motor impairment and (2) easy and quick to complete.
 |
Table 2 Comparisons Between BESTest and Frail’BESTest Items |
The “anticipation” section is composed of five tests: two are focused on the diagnosis of anticipatory postural adjustments (APA) (ie Motor programming; Massion et al 1992, for a review)31 and three are focused on the diagnosis of potential deficiencies in motor planning processes.32,33 Both APA tests allow the therapist to detect potential impairments in postural anticipation by detecting the consequences of non-optimal movement preparation. APA testing is found in the BESTest,1 but the difficulty level has been adjusted for frail older adults. As explained in Table 1, the evaluator is asked to assess how well the patient prepares for the movement, not the patient’s ability to do the actual task. For example, in the “rise to toes” task, the patient takes the therapist’s hands and then has to raise his/her heels off the floor several times. If the patient initially tips back, pulling the therapist’s hands, an APA deficiency is probable, since the patient does not move his/her center of mass forward before contracting the soleus muscles in a concentric mode in order to rise.34 APA testing is overridden in a context of frailty: we know that most falls occur when frail older adults are coordinating their posture and movement.35 For the motor planning tests, the therapist assesses the sequence of movements: making sure that the different phases of the whole movement are respected for optimal execution (for example, tilting the trunk forward before pushing on the lower limbs to stand up).
The “locomotor tests” are relatively similar to the BESTest, and include the “sit on the floor and stand up” task, classified in the biomechanical constraints section in the BESTest.1
The “static postural control” section, similar to the “sensorial orientation” of the BESTest, is focused on three main compensations classically observed in geriatrics: visual-dependency (difficulty with eyes closed),36,37 polygonal dependency (difficulty reducing the support surface) and podal-dependency (difficulty on a foam surface).38,39
The “asymmetric gait” section targets a potential decreased step length and helps the therapist to find the subsequent causes: muscular deficiency, reduced range of motion or nociceptive inputs.
The “bio-mechanical constraints” section assesses various common types of movement limitations. The items are designed to test for a potential deficiency in muscle power,25,40 a limited range of motion in the ankle,41 a poor adaptation to effort,42 a reduction of the stability limits (adapted Functional Reach Test)43 and a directional preference in instability.30
Context, Methods and Participants
In the first center, 192 frail and non-frail older patients were enrolled to test I) the contribution of each system, II) internal consistency, and III) the threshold and ceiling effects. The scores of 32 patients from center 1 and 32 patients recruited in another center (center 2) were used to measure the inter-rater reliability of the measurements by means of Kendall’s tau coefficients.
Our enrolled patients were aged 67 to 95 years (mean ± standard deviation = 84.07 ± 5.17 years), and 65.7% female. The local ethics committee of the François Mitterrand hospital approved the experimental protocol, which was carried out in agreement with legal and international requirements (Declaration of Helsinki, 1964). Patients were informed about the research project and gave their written consent before the evaluation. The threshold of 0.65 m.s−1 in the gait speed test was used to detect the frailty state. The gait speed has been shown as a very good landmark for physical frailty and severe outcomes.44–46 However, patients were considered frail after a conscientious examination of their medical files, and the final diagnosis was made by geriatrician according to the clinical features of the syndrome. Patients were recruited from the first geriatric department (center 1) and a second geriatric department in another region of France (center 2). Data were collected between September 2014-April 2015 in center 1 and between January 2014 –September 2015 in center 2. Figure 2 displays the patient’s distribution in the two centers (Flow diagram).
 |
Figure 2 Patient distribution in center 1 and center 2. |
For clarity, the 192 patients from center 1 are presented in two subgroups: non-frail and frail patients. Please see patients’ characteristics in Table 3.
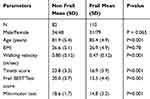 |
Table 3 Characteristics of the 192 Non-Frail and Frail Patients Enrolled in Center 1 |
Patient data were collected to calculate the following: I. system contribution, II. internal consistency, and III. distribution of the scores (see statistical analysis). Another double-blind evaluation was conducted with these patients in order to calculate the inter-rater reliability of the Frail’BESTtest.
In center 1, patients were recruited during their outpatient hospital consultation. The motor assessment was performed by an experienced physiotherapist before any of the other evaluations conducted in this center. This motor assessment included the Frail’BESTest, the Tinetti test (Performance Oriented Mobility Assessment)28 and the Mini-motor test.29 Tinetti and Mini-motor tests were used as comparators since they were being used by clinicians in the two centers at the time. The Tinetti test has proved its reliability in institutionalized aged adults with interrater reliability coefficients ranging from 0.80 to 0.95 and reported test–retest reliability of 0.72 to 0.86.47,48 The Tinetti test has also exhibited construct validity with gait speed in people with Parkinson's disease and with the Timed Up and Go in older adults.49,50 The Mini-motor test29 has very good inter-rater reliability (Pearson coefficients up to 0.95), with a limited redundancy between items (coefficients were far from 100%). The correlation between the Mini-motor test and the Katz index was significantly negative (p<0.05).
In center 2, the recruited patients were assessed during the first session of a rehabilitation program. The motor assessment included the Frail’BESTest and the Tinetti test, and was conducted at the beginning of the first rehabilitation session for all of the included participants. All of the patients were previously informed of the aim of the study and gave their written consent. The Frail’BESTest was the only supplementary test done specifically for the study.
In both centers, the exclusion criteria were the inability to stand up with help and the inability to understand basic instructions from the therapist.
Material
The motor assessments were done either in a large corridor (for gait analysis) or in the physiotherapist’s office (for the other evaluations). The Frail’BESTest requires only two specific devices: foam (mean density 55 kg/m3) for the “sensorial orientation” section and a 12-cm-high rehabilitation step for the “anticipations” section. Motor assessments were immediately scored by the physiotherapist and entered into a specific computer. For tests of inter-rater reliability, the two physiotherapists from each center scored the patient successively, suggesting a 10-min rest in the middle in order to avoid fatigue. The two physiotherapists alternated positions (first or second evaluator) for each patient.
Statistical Analysis
Our aim was to determine the internal consistency of the parameters, the construct validity and the reliability of the Frail’BESTest. Sub-scores were calculated for each of the six systems of the Frail’BESTest, and the total score was obtained by totaling these six sub-scores.
An a priori statistical power calculation was done to determine the expected number of subjects. It was based on a power of 0.90, an alpha of 0.05 and a Kendall’s Tau higher than 0.7. Based on this hypothesis 30 subjects are expected to achieve enough statistical power.
Internal Consistency
Internal consistency is defined by the COSMIN panel as the degree of inter-relatedness among the items of each sub-scores.51 As all sub-scores came from different dimensions, we tested the hypothesis that items from the same sub-score were closely linked and would evolve together (ie measure the same construct). To test this hypothesis, we evaluated the item-total correlation (correlation of each item to its sub-score). The Cronbach alpha coefficient and the Kendall’s tau were also used to test the consistency of sub-scores. A Cronbach Alpha and a Kendall’s tau higher than 0.5 were considered acceptable and higher than 0.7 as satisfactory.52,53
In a second point, we aim to test the correlation between each domain and the total score. We evaluated the correlation between each sub-score and the total patient score. The relationship between the sub-scores and the total scores was evaluated using both a Kendall’s Tau and the linearity of the ANOVA. To be part of the scale, we considered an item-total correlation (Kendall Tau) between 0.2 and 0.9. We then plotted the distribution of the Frail’BESTest, the Tinetti Test (considered the gold standard) and the Mini-motor test to seek a potential ceiling effect.
Distribution of the Scores
Distribution was assessed for all three scales (Frail’BEStest, Tinetti and Mini-Motor Test) and it was tested separately for frail and non-frail patients to visualize the homogeneity of the scores in the two samples. Skewness and kurtosis were computed to assess, respectively, the symmetry and the tailedness of the test distributions.
Inter-Rater Reliability
Kendall’s tau was computed to assess the inter-rater reliability of the measurement. A between day test–retest evaluation was performed with a 24-h interval. Evaluators in center 1 received more training for the Frail’BESTest than those in center 2. In both centers, the Kendall’s tau was calculated for total scores and sub-scores (scores for each section) of the Frail’BESTest. The Kendall’s tau was calculated to assess relative reliability using a two-way random effect with an average measure of absolute agreement. A Kendall’s tau higher than 0.6 was defined as strong and a Kendall’s tau higher than 0.8 was defined as very strong.54
Results
Overall, 192 patients were enrolled in center 1 and 32 patients in center 2 (see Figure 2 for flowcharts). Each patient completed all of the items in the scale. Among the 224 patients, 145 were diagnosed as frail according to their gait speed.
The results of the linearity of one-way ANOVAs showed that each system contributes to the total patient scores. Similarly, the linearity coefficient was significant for the relationships between each system and the total score of the Frail’BESTest. The ANOVAs indicated that there is significant linearity between each system and the total score of the Frail’BESTest (F>50; p<0.001).
Internal coherence, tested with the Cronbach alpha coefficient, was moderate to good for five systems (anticipations: 0.784; reactions: 0.636; locomotion: 0.789; sensorial preferences: 0.64; asymmetric gait: 0.698) and limited for the “biomechanical constraints” system (0.478). Item-total correlations were significant for all items and ranged from 0.3 to 0.75, excepted for one item, “System F-Gait symmetry” showed a correlation of 0.98 with its sub-score. Correlations between each sub-score and the total score were ranged between below 0.75 (anticipations: 0.66; reactions: 0.59; locomotion: 0.71; sensorial preferences: 0.56; asymmetric gait: 0.33; biomechanical constraints: 0.60).
The distribution of the scores obtained for each test and expressed as a percentage of the maximum score are provided in Figure 3 (frail patients in the upper portion and non-frail patients in the lower portion). The lower part of the figure (non-frail) and the significant negative skewness indicated that there is a ceiling effect for all scales: most of the patients obtained high-level scores. The medians, first quartiles and third quartiles show that the scores of Mini-motor tests are at the top of the distribution (median = 92.8%), followed by the Tinetti test (median = 77%) and the Frail’BESTest (median = 71.5%). Interestingly, only 13/73 non-frail patients had a score under 50% of the maximum score with the Tinetti test, whereas 21/73 non-frail patients had a score under 50% with the Frail’BESTest. The upper part of the figure (frail patients) reveals that there is no ceiling effect and a very homogeneous distribution of the scores for the Tinetti test (first quartile: 34.6% and third quartile: 61.5%) and the Frail’BESTest (first quartile: 32.1% and third quartile: 57.1%) showed by non-significant skewness and kurtosis. The distribution to the left side of the curve was more pronounced with the Frail’BESTest (median = 42.9%) compared with the Tinetti test (median = 46.2%).
As detailed in Figure 2, inter-rater reliability was studied at both care centers. The results for the Frail’BESTest are presented in graph form in Figure 4A for center 1 (expert physiotherapists and familiar with the Frail’BESTest) and in Figure 4B for center 2 (expert physiotherapists not familiar with the Frail’BESTest). As shown in Figure 4, the Kendall’s tau was calculated for these two pairs of evaluators. The Kendall’s tau for center 1 was higher than 0.6 indicated a strong concordance for all sub-scores of the Frail’BESTest score. The Kendall’s tau in center 2 was strong for 4 on 6 sub-scores of the Frail’BESTest score. Two scores (A and F systems) remain with moderate concordance (>0.41)(see Figure 4C).
 |
Figure 4 Presentation of the interrater reliability of the total Frail-BESTest Score in center 1 (A) and center 2 (B). Kendall’s tau coefficients for each system and each center (C). |
Discussion
The focus of the present study was to analyze a new Balance Evaluation System Test (BESTest),1 which is a systemic assessment of balance initially suggested by Horak et al. The modified version, named the Frail’BESTest, was designed for use in frail older adults. In clinical practice, the Frail’BESTest is feasible: an experienced physiotherapist needs eight to 10 min to complete the test with a mild-impaired patient. Completing the evaluation, the therapist may prioritize the patient’s needs identifying the most challenging systems. Prioritization is very important in a geriatric context: patient’s fatigability involves to choice the most efficient exercises in order to improve the rehabilitation outcomes. We presented the modified version of the scale, and we then conducted a validation analysis. In this paper, we wanted to verify the utility of each system, the complementarity of each system and the inter-rater reliability.
The contribution of each system was found to be good; each system contributed to total patient scores. The linearity F-score of the ANOVA and Kendall’s Tau were significant for the relationships between each system and the total score of the Frail’BESTest. Each system demonstrates ability in a specific domain and measures a specific aspect of balance. The mechanisms of age and frailty are complex,19 so it is important to target different potential affected systems in order to prioritize rehabilitation. The exhaustive exploration of deficiencies initially validated in the BESTest appears to be maintained in the Frail’BESTest but should be confirmed in further studies.
The internal consistency of the systems was satisfactory or acceptable, except for the “Biomechanical constraints” system. This issue was partly expected for a measurement of the constraints of both biological and mechanical capacities, and tests from different domains were included in this system. Muscle power, ankle range of motion, or the quality of the foot base of support were part of the mechanical testing, even though the effort has more of a biological aspect (cardio-respiratory limitations). In addition, directional preference could be influenced by a mechanical deficiency such as a peripheral vestibular lesion55 or a neurological failure (eg a hemiparetic “pusher”56 or backward disequilibrium30). Similarly, the anterior stability limit, measured by the validated “Functional Reach Test”,43 could be influenced by mechanical deficiencies such as limited ankle or hip range of motion, or by the psychological and neurological factors involved in Psycho-Motor Disadaptation Syndrome, which associates backward disequilibrium and fear of falling, especially falling forward.57 The internal coherence of the vast bio-mechanical system is low, but it does take into account the heterogeneous characteristic of frail older adults.
There was also an interesting distribution of the Frail’BESTest scores in the 192 patients studied. In non-frail patients, the three scores showed ceiling effects, though they were less pronounced than in the Tinetti test. Mini-motor test showed a considerable ceiling effect in the non-frail subgroup. However, this result can be considered coherent considering that the Mini-motor test was validated to measure the functional abilities of heavily impaired patients.29
In frail patients, the score distribution of the Frail’BESTest seemed to be more evenly distributed than the other scores, with a distribution centered on 42.9% of the total score, against 46.2% for the Tinetti test. Taken together, these results suggest that when compared with the Tinetti test, the Frail’BESTest under-evaluates patient capacities. The potential to progress should, therefore, be higher with this Frail’BESTest; an analysis of responsiveness will assess this assumption in part II of the companion paper.
We tested the inter-rater reliability of the Frail’BESTest in two different geriatric care centers with Kendall’s tau calculations. The first pair of physiotherapists (center 1) obtained a Kendall’s tau of 0.76 (total score) and the second pair (center 2) a Kendall’s tau of 0.77. There was no difference in reliability when considering the total score of the Frail’BESTest. However, Kendall’s tau coefficients were different when calculated within the systems in each center. As shown in Figure 4C, Kendall’s tau coefficients were “moderate” in center 2 for system A (Anticipations) and for system E (Biomechanical constraints) rather than “strong” or “very strong” in center 1. In both centers, the physiotherapists were geriatric rehabilitation experts with more than 3 years of experience. The difference could probably be explained by the exposure to the Frail’BESTest in the first center, where the scale was used daily for 3 months before the start of the study. In the second center, the physiotherapists were experienced in geriatric rehabilitation but not as familiar with the tool. Taken together, these Kendall’s tau coefficients show a very good overall inter-rater reliability for the Frail’BESTest. Considering the sub-scores obtained for each system, the results are satisfying in center 1, but the poor reliability observed in center 2 for two systems shows that a trainable period is necessary to use the Frail’BESTest with a strong reliability between operators.
One limitation of this study is the heterogeneity of the patients included. However, this heterogeneity gave us the opportunity to measure the performance of the Frail’BESTest in a large sample of patients with different motor and cognitive capacities. It could be interesting to compare the Frail’BESTest with other similar tools used in different geriatrics departments, long-term care facilities. Another limitation could be the frailty diagnosis used in the inclusion. Indeed, the gait speed was mainly used to diagnose frailty. As mentioned in the methods, gait speed is a very interesting test to spot the frailty state. However, other signs should complete the clinical examination in order to confirm the diagnosis, as the other Fried criteria.18
The use of a binary choice for scoring the Frail’BESTest could probably be considered another limitation for physical assessment. However, we decided on a pragmatic approach, making the duration of the test compatible with the everyday practices of therapists. This may have resulted in some inaccuracy in the motor evaluation.58 The motor evaluation section of the Frail’BESTest should, therefore, be assessed more thoroughly and compared with longer and previously validated tests. Moreover, we only tested the inter-rather reliability in this study. Another work should test the intra-rather reliability to complete the analysis. The next step consists on determine the concurrent validity, the responsiveness and the predictive capacities of the Frail’BESTest in terms of detection of frailty and the occurrence of falls.
Acknowledgments
The authors thank Suzanne Rankin and Frederic Desramault for their precious help.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Horak FB, Wrisley DM, Frank J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89(5):484–498. doi:10.2522/ptj.20080071
2. Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the balance evaluation systems test: the mini-BESTest. J Rehabil Med. 2010;42(4):323–331. doi:10.2340/16501977-0537
3. Goljar N, Giordano A, Schnurrer Luke Vrbanić T, et al. Rasch validation and comparison of Slovenian, Croatian, and Italian versions of the mini-BESTest in patients with subacute stroke. Int J Rehabil Res Int Z Rehabil Rev Int Rech Readaptation. 2017;40(3):232–239. doi:10.1097/MRR.0000000000000233
4. Benka Wallén M, Sorjonen K, Löfgren N, Franzén E. Structural validity of the mini-balance evaluation systems test (Mini-BESTest) in people with mild to moderate Parkinson disease. Phys Ther. 2016;96(11):1799–1806. doi:10.2522/ptj.20150334
5. Löfgren N, Benka Wallén M, Sorjonen K, Conradsson D, Franzén E. Investigating the Mini-BESTest’s construct validity in elderly with Parkinson’s disease. Acta Neurol Scand. 2017;135(6):614–621. doi:10.1111/ane.12640
6. Potter K, Anderberg L, Anderson D, et al. Reliability, validity, and responsiveness of the balance evaluation systems test (BESTest) in individuals with multiple sclerosis. Physiotherapy. 2018;104(1):142–148. doi:10.1016/j.physio.2017.06.001
7. Ross E, Purtill H, Uszynski M, et al. Cohort study comparing the berg balance scale and the mini-bestest in people who have multiple sclerosis and are ambulatory. Phys Ther. 2016;96(9):1448–1455. doi:10.2522/ptj.20150416
8. Jácome C, Flores I, Martins F, et al. Validity, reliability and minimal detectable change of the balance evaluation systems test (BESTest), mini-BESTest and brief-BESTest in patients with end-stage renal disease. Disabil Rehabil. 2018;40(26):3171–3176. doi:10.1080/09638288.2017.1375034
9. Madhavan S, Bishnoi A. Comparison of the mini-balance evaluations systems test with the berg balance Scale in relationship to walking speed and motor recovery post stroke. Top Stroke Rehabil. 2017;24(8):579–584. doi:10.1080/10749357.2017.1366097
10. Chinsongkram B, Chaikeeree N, Saengsirisuwan V, Horak FB, Boonsinsukh R. Responsiveness of the balance evaluation systems test (BESTest) in people with subacute stroke. Phys Ther. 2016;96(10):1638–1647. doi:10.2522/ptj.20150621
11. Chinsongkram B, Chaikeeree N, Saengsirisuwan V, Viriyatharakij N, Horak FB, Boonsinsukh R. Reliability and validity of the balance evaluation systems test (BESTest) in people with subacute stroke. Phys Ther. 2014;94(11):1632–1643. doi:10.2522/ptj.20130558
12. Tamura T, Otaka Y, Konno S, Sadashima K, Tomatsu T, Machida S. The impaired balance systems identified by the BESTest in older patients with knee osteoarthritis. PM R. 2016;8(9):869–875. doi:10.1016/j.pmrj.2016.02.002
13. Chan ACM, Pang MYC. Assessing balance function in patients with total knee arthroplasty. Phys Ther. 2015;95(10):1397–1407. doi:10.2522/ptj.20140486
14. Anson E, Thompson E, Ma L, Jeka J. Reliability and fall risk detection for the BESTest and Mini-BESTest in Older Adults. J Geriatr Phys Ther 2001. 2017. doi:10.1519/JPT.0000000000000123
15. Wang-Hsu E, Smith SS. Interrater and test-retest reliability and minimal detectable change of the balance evaluation systems test (BESTest) and subsystems with community-dwelling older adults. J Geriatr Phys Ther. 2001;41(3):173–179. doi:10.1519/JPT.0000000000000117
16. Yingyongyudha A, Saengsirisuwan V, Panichaporn W, Boonsinsukh R. The mini-balance evaluation systems test (Mini-BESTest) Demonstrates higher accuracy in identifying older adult participants with history of falls than do the bestest, berg balance scale, or timed up and go test. J Geriatr Phys Ther 2001. 2016;39(2):64–70. doi:10.1519/JPT.0000000000000050
17. King LA, Priest KC, Salarian A, Pierce D, Horak FB. Comparing the Mini-BESTest with the berg balance scale to evaluate balance disorders in Parkinson’s disease. Parkinson’s Dis. 2012;2012doi:10.1155/2012/375419
18. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.M146
19. Robinovitch SN, Normandin SC, Stotz P, Maurer JD. Time requirement for young and elderly women to move into a position for breaking a fall with outstretched hands. J Gerontol a Biol Sci Med Sci. 2005;60(12):1553–1557. doi:10.1093/gerona/60.12.1553
20. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet London Eng. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-9
21. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. doi:10.1016/j.cger.2010.08.008
22. Liljas AEM, Carvalho LA, Papachristou E, et al. Self-reported vision impairment and incident prefrailty and frailty in English community-dwelling older adults: findings from a 4-year follow-up study. J Epidemiol Community Health. 2017;71(11):1053–1058. doi:10.1136/jech-2017-209207
23. Castell MV, van der Pas S, Otero A, et al. Osteoarthritis and frailty in elderly individuals across six European countries: results from the European Project on OSteoArthritis (EPOSA). BMC Musculoskelet Disord. 2015;16(1):359. doi:10.1186/s12891-015-0807-8
24. Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, et al. The sit-to-stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Exp Gerontol. 2018;112:38–43. doi:10.1016/j.exger.2018.08.006
25. Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol a Biol Sci Med Sci. 2000;55(4):M192–M199. doi:10.1093/gerona/55.4.M192
26. Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16(2):105–114. doi:10.1089/rej.2012.1397
27. Eriksson S, Gustafson Y, Lundin-Olsson L. Risk factors for falls in people with and without a diagnose of dementia living in residential care facilities: a prospective study. Arch Gerontol Geriatr. 2008;46(3):293–306. doi:10.1016/j.archger.2007.05.002
28. Tinetti ME. Preventing Falls in Elderly Persons. N Engl J Med. 2003;8:42–49.
29. Mourey F, Camus A, d’Athis P, et al. Mini motor test: a clinical test for rehabilitation of patients showing psychomotor disadaptation syndrome (PDS). Arch Gerontol Geriatr. 2005;40(2):201–211. doi:10.1016/j.archger.2004.08.004
30. Manckoundia P, Mourey F, Pfitzenmeyer P, Van Hoecke J, Pérennou D. Is backward disequilibrium in the elderly caused by an abnormal perception of verticality? A pilot study. Clin Neurophysiol off J Int Fed Clin Neurophysiol. 2007;118(4):786–793. doi:10.1016/j.clinph.2006.11.274
31. Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38(1):35–56. doi:10.1016/0301-0082(92)90034-C
32. Allain P, Chaudet H, Nicoleau S, et al. [A study of action planning in patients with Alzheimer’s disease using the zoo map test]. Rev Neurol (Paris). 2007;163(2):222–230. French. doi:10.1016/S0035-3787(07)90393-8
33. Saimpont A, Mourey F, Manckoundia P, Pfitzenmeyer P, Pozzo T. Aging affects the mental simulation/planning of the “rising from the floor” sequence. Arch Gerontol Geriatr. 2010;51(3):e41–e45. doi:10.1016/j.archger.2009.11.010
34. Nardone A, Schieppati M. Shift of activity from slow to fast muscle during voluntary lengthening contractions of the triceps surae muscles in humans. J Physiol. 1988;395(1):363–381. doi:10.1113/jphysiol.1988.sp016924
35. Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet London Eng. 2013;381(9860):47–54. doi:10.1016/S0140-6736(12)61263-X
36. Teasdale N, Stelmach GE, Breunig A, Meeuwsen HJ. Age differences in visual sensory integration. Exp Brain Res. 1991;85(3):691–696. doi:10.1007/BF00231755
37. Woollacott MH. Systems contributing to balance disorders in older adults. J Gerontol a Biol Sci Med Sci. 2000;55(8):M424–M428. doi:10.1093/gerona/55.8.M424
38. Wiesmeier IK, Dalin D, Maurer C. Elderly use proprioception rather than visual and vestibular cues for postural motor control. Front Aging Neurosci. 2015;7:97. doi:10.3389/fnagi.2015.00097
39. Maitre J, Paillard TP. Influence of the Plantar Cutaneous Information in Postural Regulation Depending on the Age and the Physical Activity Status. Front Hum Neurosci. 2016;10:409. doi:10.3389/fnhum.2016.00409
40. Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12(6):403–409. doi:10.1016/j.jamda.2011.04.014
41. Deshpande N, Simonsick E, Metter EJ, Ko S, Ferrucci L, Studenski S. Ankle proprioceptive acuity is associated with objective as well as self-report measures of balance, mobility, and physical function. Age Dordr Neth. 2016;38(3):53. doi:10.1007/s11357-016-9918-x
42. Menezes AR, Lavie CJ, Milani RV, Arena RA, Church TS. Cardiac rehabilitation and exercise therapy in the elderly: should we invest in the aged? J Geriatr Cardiol JGC. 2012;9(1):68–75. doi:10.3724/SP.J.1263.2012.00068
43. Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45(6):M192–197. doi:10.1093/geronj/45.6.m192
44. van Kan GA, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med. 2010;26(2):275–286. doi:10.1016/j.cger.2010.02.002
45. Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease: FRAILTY IN HOSPITALIZED PATIENTS WITH CAD. J Am Geriatr Soc. 2006;54(11):1674–1681. doi:10.1111/j.1532-5415.2006.00914.x
46. Middleton A, Fritz SL, Lusardi M. Walking Speed: the Functional Vital Sign. J Aging Phys Act. 2015;23(2):314–322. doi:10.1123/japa.2013-0236
47. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. doi:10.1111/j.1532-5415.1986.tb05480.x
48. Canbek J, Fulk G, Nof L, Echternach J. Test-retest reliability and construct validity of the Tinetti performance-oriented mobility assessment in people with stroke. J Neurol Phys Ther JNPT. 2013;37(1):14–19. doi:10.1097/NPT.0b013e318283ffcc
49. Faber MJ, Bosscher RJ, van Wieringen PCW. Clinimetric properties of the performance-oriented mobility assessment. Phys Ther. 2006;86(7):944–954. doi:10.1093/ptj/86.7.944
50. Kegelmeyer DA, Kloos AD, Thomas KM, Kostyk SK. Reliability and validity of the Tinetti Mobility Test for individuals with Parkinson disease. Phys Ther. 2007;87(10):1369–1378. doi:10.2522/ptj.20070007
51. Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi:10.1016/j.jclinepi.2010.02.006
52. Cronbach LJ. Coefficient Alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. doi:10.1007/BF02310555
53. Nunnally JC. Psychometric Theory. New York: McGraw Hill; 1967.
54. Kendall M, Dickinson J,. Rank Correlation Methods (Charles Griffin Book Series) (9780195208375): books. https://www.amazon.com/Rank-Correlation-Methods-Charles-Griffin/dp/0195208374. Accessed November 5, 2019.
55. Arshad Q, Seemungal BM. Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front Neurol. 2016;7:231. doi:10.3389/fneur.2016.00231
56. Pérennou DA, Mazibrada G, Chauvineau V, et al. Lateropulsion, pushing and verticality perception in hemisphere stroke: a causal relationship? Brain J Neurol. 2008;131(Pt9):2401–2413. doi:10.1093/brain/awn170
57. Manckoundia P, Pérennou D, Pfitzenmeyer P, Mourey F. [Backward disequilibrium in the elderly: review of symptoms and proposition of a tool for quantitative assessment]. Rev Med Interne. 2007;28(4):242–249. French. doi:10.1016/j.revmed.2006.12.002
58. Kyriazos TA. Applied Psychometrics: the 3-Faced construct validation method, a routine for evaluating a factor structure. Psychology. 2018;09(08):2044–2072. doi:10.4236/psych.2018.98117
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

