Back to Journals » Drug Design, Development and Therapy » Volume 11
Synthesis, characterization, and antimicrobial evaluation of novel 5-benzoyl-N-substituted amino- and 5-benzoyl-N-sulfonylamino-4-alkylsulfanyl-2-pyridones
Authors Elgemeie G , Altalbawy F , Alfaidi M, Azab R, Hassan A
Received 21 August 2017
Accepted for publication 12 October 2017
Published 28 November 2017 Volume 2017:11 Pages 3389—3399
DOI https://doi.org/10.2147/DDDT.S149615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Lymperopoulos
Galal Elgemeie,1 Farag Altalbawy,2,3 Mohammed Alfaidi,3 Rania Azab,2,4 Atef Hassan5
1Department of Chemistry, Faculty of Science, Helwan University, Helwan, 2National Institute of Laser Enhanced Sciences (NILES), Cairo University, Giza, Egypt; 3Department of Biological Sciences, University College of Duba, Tabuk University, Tabuk, 4Department of Biological Sciences, Faculty of Science, Al-Baha University, Al-Baha, Saudi Arabia; 5Department of Mycology and Mycotoxins, Animal Health Research Institute, Agriculture Research Center, Giza, Egypt
Abstract: The present research describes the synthesis of novel 5-benzoyl-N-substituted-amino- and 5-benzoyl-N-sulfonylamino-4-alkylsulfanyl-2-pyridones 5a–c and 6a–c via the reaction of 2-benzoyl-3,3-bis(alkylthio)acrylonitriles 2a–c with N-cyanoacetohydrazide 3 and cyanoaceto-N-phenylsulfonylhydrazide 4, respectively. Also, the reactivity of the compounds 5a–c toward hydrazine hydrate to give product 1H-pyrazolo[4,3-c]pyridine derivative 7 was studied. In addition, the reactivity of the 2a–c toward 1-cyanoacetyl-4 arylidenesemicarbazides 8a–c afforded 3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile derivatives (12–14)a–c, which reacted with hydrazine hydrate to give 3H-pyrazolo[4,3-c][1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile derivatives 15a–c. The structures of the new products were characterized based on 1H nuclear magnetic resonance, 13C nuclear magnetic resonance, infrared, mass-spectroscopy, and elemental analyses. The products were screened in vitro for their antibacterial and antifungal activity properties.
Keywords: amino-2-pyridones, N-cyanoacetohydrazide, cyanoaceto-N-phenylsulfonylhydrazide, 2-benzoyl-3,3-bis(alkylthio)acrylonitriles, 5-benzoyl-N-sulfonylamino-4-alkylsulfanyl-2-pyridones, 5-benzoyl-N-substituted-amino-4-alkylsulfanyl-2 pyridones, antimicrobial activity
Introduction
Microorganisms that resist antimicrobial drugs are a complex problem affecting the health of people all over the world. More than 1 million people die from microbial infections every year, and the number of deaths is expected to increase as antimicrobial drug resistance increases.1 Innovation must be strengthened in research activities related to effective antimicrobial and antifungal drugs.2 N-substituted amino-2-pyridones are important heterocycles possessing a wide range of pharmaceutical applications.3 Many N-substituted amino-2-pyridones are known to possess antimicrobial and antifungal activities,4 antimalarials,5 and Alzheimer’s β-peptide aggregation inhibitors.6 In addition, sulfonyl heterocycles have a variety of pharmacological properties, such as apoptosis inhibition and ischemia treatment,7 metabolic liability,8 and glucokinase activation.9,10 We recently reported different innovative synthetic methods to prepare alkylsulfanyl, N-sulfonylamino and N-sulfonyl heterocycles, which found application and appear to constitute new classes of anticancer and antimicrobial agents.11–15 A series of one of our novel N-sulfonylpyrazoles were used by another group of research as an inhibitors of the enzyme cathepsin B.16 The studies demonstrated that N-sulfonylpyrazoles act as alternate substrates for cathepsin B, rather than as inhibitor metabolites. In another study, our N-sulfonylated pyrazoles were proven active as allosteric inhibitors of West Nile virus NS2B-NS3 proteinase.17 These promising results have motivated our research group to continue this work exploring novel molecular mechanisms of these synthetic compounds and their use as chemotherapeutic agents. In view of these findings and as a part of our program directed toward the preparation of potential antimetabolic agents,18–20 we recently reported different synthetic methods for preparation of azoloazines using cyanoketene dithioacetals.21–24 Derivatives of these ring systems are important as antimetabolic agents in biochemical reactions.25,26 In view of these reports and in continuation of our previous work in synthesis of bioactive heterocyclic compounds,27–34 the present research deals with a novel synthesis of 5-benzoyl-N-substituted amino- and 5-benzoyl-N-sulfonyl-amino-4-alkylsulfanyl-2-pyridones 5, 6 and 12–14 by the reaction of 2-benzoyl-3,3-bis(alkylthio)acrylonitriles 2a–c with substituted hydrazides.
Materials and methods
All melting points (MPs) were measured on an Electrothermal Gallenkamp apparatus (Weiss Technik, London, UK). Infrared (IR) spectra were recorded in potassium bromide disks on SP3300 (Pye Unicam, Cambridge, England) and 8101 PC (Shimadzu, Tokyo, Japan) IR spectrophotometers. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded on a Varian Mercury VXR-300 spectrometer (300 MHz). Mass spectra were recorded on Shimadzu GCMS-Q1000-EX and GCMS 5988-A HP spectrometers, and ionizing voltage was 70 eV. Elemental analyses were carried out at the Microanalytical Center of Cairo University, Giza, Egypt. Biological evaluation of the products was carried out in the Microbiology Division of the Microanalytical Center of Cairo University.
Synthetic procedures
1,6-diamino-5-benzoyl-2-oxo-4-(alkylthio)-1,2-dihydropyridine-3-carbonitrile derivatives 5a–c and N-[6-amino-5-benzoyl-3-cyano-4-(alkylthio)-2-oxopyridin-1(2H)-yl]benzene-sulfonamide derivatives 6a–c
A mixture of 2-benzoyl-3,3-bis(alkylthio)acrylonitrile (2a–c) (0.01 mol), 2-cyanoacetohydrazide (3) (0.99 g, 0.01 mol), and potassium hydroxide (0.67 g, 0.012 mol) in 1,4-dioxane (50 mL) was stirred at room temperature for 24 hours. The resultant product was acidified with hydrochloric acid. The precipitate formed was collected by filtration, washed with water, dried, and then crystallized from ethanol (EtOH)–dimethylformamide (DMF) to afford the corresponding compounds 5a–c. Repetition of the same procedure using the 2-benzoyl-3,3-bis(alkylthio)acrylonitrile 3a–c (0.01 mol) with 2-cyano-N′-(phenylsulfonyl)acetohydrazide (4) (2.39 g, 0.01 mol) yielded the respective products 6a–c.
1,6-diamino-5-benzoyl-4-(methylthio)-2-oxo-1,2-dihydropyridine-3-carbonitrile (5a)
Yield (85%), MP 197°C (from EtOH-DMF); IR (KBr) νmax 3,426 (NH2), 2,923 (aliphatic CH), 2,214 (CN), 1,659 (C=O), 1,623 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 2.89 (s, 3H, SCH3), 7.36–7.59 (m, 5H, ArH), 7.97 (s, 4H, 2NH2); 13C NMR (DMSO-d6) – δ 14.37 (SCH3), 84.86, 91.07, 115.13, 126.91, 129.39, 131.81, 140.06, 153.2, 155.8, 183.42, 188.66. MS m/z (%) 302 (M++2, 1.5), 301 (M++1, 1.1), 300 (M+, 10.5), 230 (30.71), 213 (20.16), 154 (21.47), 77 (100), 57 (55). Analysis calculated (Anal Calcd) for C14H12N4O2S (300.33): C, 55.99; H, 4.03; N, 18.65; S, 10.68. Found: C, 55.95; H, 4; N, 18.65; S, 10.66%.
1,6-diamino-5-benzoyl-4-(ethylthio)-2-oxo-1,2-dihydropyridine-3-carbonitrile (5b)
Yield (80%), MP 281°C (from EtOH-DMF); IR (KBr) νmax 3,423 (NH2), 2,923, 2,857 (aliphatic CH), 2,210 (CN), 1,664 (C=O), 1,550 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 1.21 (t, 3H, CH3), 2.89 (q, 2H, SCH2), 7.42–7.56 (m, 5H, ArH), 7.81 (s, 4H, 2 NH2). MS m/z (%): 316 (M++2, 2.81), 315 (M++1, 1.65), 314 (M+, 10.23), 291 (20.62), 275 (25.25), 211 (31.22), 106 (100), 75 (57.96), 56 (84.24). Anal Calcd for C15H14N4O2S (314.36): C, 57.31; H, 4.49; N, 17.82; S, 10.20. Found: C, 57.30; H, 4.43; N, 17.79; S, 10.14%.
1,6-diamino-5-benzoyl-2-oxo-4-(propylthio)-1,2-dihydropyridine-3-carbonitrile (5c)
Yield (85%), MP >300°C (from EtOH-DMF); IR (KBr) νmax 3,427 (NH2), 2,925 (aliphatic CH), 2,208 (CN), 1,655 (C=O), 1,555 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 0.85 (t, 3H, CH3), 1.5 (m, 2H, CH2), 2.78 (t, 2H, SCH2), 7.34–7.56 (m, 5H, ArH), 7.8 (s, 4H, 2NH2). MS m/z (%): 330 (M++2, 5.62), 329 (M++1, 3.21), 328 (M+, 8.62), 223 (38.55), 189 (40.23), 155 (22.42), 76 (100), 55 (85.87). Anal Calcd for C16H16N4O2S (328.39): C, 58.52; H, 4.91; N, 17.06; S, 9.76. Found: C, 58.48; H, 4.9; N, 17.05; S, 9.75%.
N-[6-amino-5-benzoyl-3-cyano-4-(mthylthio)-2-oxopyridin-1(2H)-yl]benzenesulfonamide (6a)
Yield (68%), MP 194°C (from EtOH-dioxane); IR (KBr) νmax – 3,421 (NH2), 2,935 (aliphatic CH), 2,219 (CN), 1,664 (C=O) cm−1; 1H NMR (DMSO-d6) – δ 2.78 (s, 3H, CH3), 7.35–7.68 (m, 10H, ArH), 8.14 (s, 2H, NH2), 9.22 (s, 1H, NH). MS m/z (%): 451 (M++1, 1.48), 454 (M+, 5.31), 440 (24.02), 409 (50.11), 330 (48.02), 175 (64.32), 77 (100), 55 (65.32). Anal Calcd for C20H16N4O4S2 (440.49): C, 54.53; H, 3.66; N, 12.72; S, 14.56. Found: C, 54.51; H, 3.63; N, 12.71; S, 14.53%.
N-[6-amino-5-benzoyl-3-cyano-4-(ethylthio)-2-oxopyridin-1(2H)-yl]benzenesulfonamide (6b)
Yield (82%), MP 225°C (from EtOH-dioxane); IR (KBr) νmax – 3,430 (NH2), 2,925 (aliphatic CH), 2,215 (CN), 1,660 (C=O), 1,549 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 1.38 (t, 3H, CH3), 2.99 (q, 2H, SCH2), 7.41–7.87 (m, 10H, ArH), 8.17 (s, 2H, NH2), 9.1 (s, 1H, NH). MS m/z (%): 455 (M++1, 1.05), 454 (M+, 2.11), 198 (54.22), 182 (45.11), 151 (60.52), 79 (100), 57 (65.32). Anal Calcd for C21H18N4O4S2 (454.52): C, 55.49; H, 3.99; N, 12.33; S, 14.11. Found: C, 55.42; H, 3.9; N, 12.31; S, 14.07%.
N-[6-amino-5-benzoyl-3-cyano-4-(propylthio)-2-oxopyridin-1(2H)-yl]benzenesulfonamide (6c)
Yield (54%), MP 212°C (from EtOH-dioxane); IR (KBr) νmax – 3,417 (NH2), 2,922 (aliphatic CH), 2,211 (CN), 1,668 (C=O), 1,558 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 0.96 (t, 3H, CH3), 1.49 (m, 2H, CH2), 2.86 (t, 2H, SCH2), 7.39–7.76 (m, 10H, ArH), 8.1 (s, 2H, NH2), 9.31 (s, 1H, NH). MS m/z (%): 469 (M++1, 0.65), 468 (M+, 1.81), 414 (24.02), 330 (42.11), 153 (67.5), 77 (100), 55 (65.54). Anal Calcd for C20H20N4O4S2 (468.55): C, 56.39; H, 4.3; N, 11.96; S, 13.69. Found: C, 56.32; H, 4.28; N, 11.91; S, 13.58%.
4,5-diamino-6-oxo-3-phenyl-5,6-dihydro-1H-pyrazolo[4,3-c]pyridine-7-carbonitrile (7)
To a solution of compound 1,6-diamino-5-benzoyl-4-(alkylthio)-2-oxo-1,2-dihydropyridine-3-carbonitrile (5a–c) (0.005 mol) in absolute EtOH, an equivalent amount of hydrazine hydrate was added, and then the reaction mixture was heated under reflux for 7 hours, cooled, and poured onto cold water. The solid that precipitated was filtered off, dried, and finally crystallized from mEtOH-water to give 7. Yield (95%), MP 138°C (from water-mEtOH); IR (KBr) νmax – 3,433, 3,141 (NH and NH2), 3,094 (ArH), 2,924 (aliphatic CH), 2,222 (CN), 1,629 (C=O), 1,562 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 7.49–7.8 (m, 5H, ArH), 8.31 (s, 4H, 2NH2), 14.31 (s, 1H, NH). MS m/z (%): 268 (M++2, 0.01), 267 (M++1, 0.02), 266 (M+, 0.02), 247 (1.53), 201 (2.17), 189 (3.29), 143 (2.84), 135 (2.39), 127 (3.23), 117 (12.87), 113 (17.49), 101 (14.82), 87 (21), 59 (100). Anal Calcd for C13H10N6O (266.25): C, 58.64; H, 3.79; N, 31.56%. Found: C, 58.61; H, 3.72; N, 31.5%.
8-benzoyl-2-(aryl)-7-(alkylthio)-5-oxo-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (12–14)a–c
A mixture of 2-benzoyl-3,3-bis(methylthio)acrylonitrile (2a) (0.01 mol), N′-[(aryl)-methylene]-2-cyanoacetohydrazide (8a–c) (0.01 mol) and potassium hydroxide (0.67 g, 0.012 mol) in 1,4-dioxane (50 mL) was stirred at room temperature for 24 hours. The resultant product was acidified with hydrochloric acid while stirring. The solid that precipitated was filtered off, dried, and crystallized from EtOH-dioxane to give the respective compounds 12a–c. The same procedure was repeated using each of 2-benzoyl-3,3-bis(ethylthio)acrylonitrile (2b) and 2-benzoyl-3,3-bis(propylthio)acrylonitrile (2c). N′-[(aryl)-methylene]-2-cyanoaceto-hydrazide (8a–c) yielded the 13a–c and 14a–c.
8-benzoyl-2-(4-methoxyphenyl)-7-(methylthio)-5-oxo-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (12a)
Yield (90%), MP >300°C (from dioxane); IR (KBr) νmax – 3,433 (NH), 3,059 (aromatic CH), 2,923 (aliphatic CH), 2,211 (CN), 1,656 (C=O), 1,610 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 3.65 (s, 3H, SCH3), 3.82 (s, 3H, OCH3), 6.85–8.15 (m, 9H, ArH), 12.05 (s, 1H, NH); 13C NMR (DMSO-d6) – δ 19.23 (SCH3), 55.63 (OCH3), 72.29, 81.5, 114.97, 117.05, 126.51, 128.37, 130.03, 130.83, 133.63, 140.57, 151.55, 152.22, 157.69, 161.53, 178.75, 186.65. MS m/z (%): 419 (M++3, 1.37), 418 (M++2, 1.36), 417 (M++1, 1.57), 416 (M+, 2.97), 415 (M+−1, 11.56), 414 (M+−2, 9.44), 133 (18.08), 105 (64.83), 77 (88.29), 63 (100), 57 (15.52). Anal Calcd for C22H16N4O3S (416.45): C, 63.45; H, 3.87; N, 13.45; S, 7.7. Found: C, 63.42; H, 3.85; N, 13.42; S, 7.68%.
8-benzoyl-7-(methylthio)-5-oxo-2-phenyl-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (12b)
Yield (88%), MP 220°C (from EtOH-dioxane); IR (KBr) νmax – 3,430 (NH), 3,059 (aromatic CH), 2,923 (aliphatic CH), 2,212 (CN), 1,658 (C=O), 1,549 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 3.55 (s, 3H, SCH3), 6.95–8.1 (m, 10H, ArH), 12 (s, 1H, NH); 13C NMR (DMSO-d6) – δ 16.9 (SCH3), 87.5, 92.5, 114.5, 117.55, 127.7, 128.04, 129.73, 130.16, 131.65, 134.27, 137.55, 147.5, 151.9, 161.96, 178.55, 185.5. MS m/z (%): 387 (M++1, 12.52), 386 (M+, 12.7), 385 (M+−1, 5.47), 206 (10.95), 131 (37.72), 105 (86.18), 77 (100), 63 (87), 57 (25.64). Anal Calcd for C21H14N4O2S (386.42): C, 65.27; H, 3.65; N, 14.5; S, 8.3. Found: C, 65.22; H, 3.55; N, 14.45; S, 8.25%.
8-benzoyl-2-(4-chlorophenyl)-7-(methylthio)-5-oxo-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (12c)
Yield (85%), MP >300°C (from EtOH-DMF); IR (KBr) νmax – 3,429 (NH), 3,065 (aromatic CH), 2,922 (aliphatic CH), 2,205 (CN), 1,648 (C=O), 1,605 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 3.5 (s, 3H, SCH3), 6.15–8.15 (m, 9H, ArH), 12.1 (s, 1H, NH); 13CNMR (DMSO-d6) – δ 19.22 (SCH3), 83.51, 111.36, 119.92, 128.18, 128.9, 129.13, 130.8, 133.67, 134.75, 138.64, 141.06, 152.37, 157.35, 157.53, 161.28, 192.72. MS m/z (%): 420 (M+, 1.59), 419 (M+−1, 1.72), 136 (100), 111 (11.15), 105 (55.52), 102 (46.9), 105 (86.18), 77 (48.59), 63 (75.48), 55 (13.07), 51 (28.75). Anal Calcd for C21H13ClN4O2S (420.87): C, 59.93; H, 3.11; N, 13.31; S, 7.62. Found: C, 59.9; H, 3.1; N, 13.3; S, 7.6%.
8-benzoyl-7-(ethylthio)-2-(4-methoxyphenyl)-5-oxo-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (13a)
Yield (85%), MP 235°C (from EtOH); IR (KBr) νmax – 3,440 (NH), 3,035 (aromatic CH), 2,931 (aliphatic CH), 2,212 (CN), 1,658 (C=O), 1,606 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 1.32 (t, 3H, CH3), 3.76 (q, 2H, SCH2), 3.88 (s, 3H, OCH3), 6.97–8.21 (m, 9H, ArH), 11.47 (s, 1H, NH); MS m/z (%): 432 (M++2, 0.01), 431 (M++1, 0.01), 430 (M+, 0.01), 405 (0.2), 379 (0.19), 329 (0.14), 305 (0.74), 271 (0.51), 259 (1.73), 201 (2.54), 189 (3.21), 143 (4.47), 117 (18.29), 101 (17.1), 59 (100). Anal Calcd for C23H18N4O3S (430.48): C, 64.17; H, 4.21; N, 13.01; S, 7.45. Found: C, 64.1; H, 4.2; N, 13; S, 7.4%.
8-benzoyl-7-(ethylthio)-5-oxo-2-phenyl-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (13b)
Yield (85%), MP 172°C (from EtOH); IR (KBr) νmax – 3,430 (NH), 3,045 (aromatic CH), 2,924 (aliphatic CH), 2,207 (CN), 1,656 (C=O), 1,595 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 1.23 (t, 3H, CH3), 3.31 (q, 2H, SCH2), 7.31–7.87 (m, 10H, ArH), 11.21 (s, 1H, NH). MS m/z (%): 402 (M++2, 0.01) 401 (M++1, 0.01), 400 (M+, 0.01), 379 (0.12), 317 (0.3), 289 (0.44), 260 (0.36), 201 (2.29), 189 (3.11), 131 (5.01), 117 (18.13), 87 (19.74), 59 (100). Anal Calcd for C22H16N4O2S (400.45): C, 65.98; H, 4.03; N, 13.99; S, 8.01. Found: C, 65.91; H, 4; N, 13.92; S, 8%.
8-benzoyl-2-(4-chlorophenyl)-7-(ethylthio)-5-oxo-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (13c)
Yield (85%), MP 161°C (from EtOH); IR (KBr) νmax – 3,439 (NH), 3,055 (aromatic CH), 2,924 (aliphatic CH), 2,219 (CN), 1,667 (C=O), 1,606 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 1.23 (t, 3H, CH3), 3.48 (q, 2H, SCH2), 7.35–7.63 (m, 9H, ArH), 11.21 (s, 1H, NH). MS m/z (%): 435 (M++1, 0.09), 434 (M+, 0.12), 307 (0.63), 280 (0.66), 248 (0.67), 201 (1.79), 143 (2.44), 131 (4.69), 113 (18.27), 87 (18.5), 59 (100). Anal Calcd for C22H15ClN4O2S (434.89): C, 60.76; H, 3.48; Cl, 8.15; N, 12.88; S, 7.37. Found: C, 60.7; H, 3.4; Cl, 8.1; N, 12.81; S, 7.3%.
8-benzoyl-2-(4-metoxyphenyl)-7-(propylthio)-5-oxo-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (14a)
Yield (85%), MP 240°C (from EtOH-dioxane); IR (KBr) νmax – 3,430 (NH), 3,061 (aromatic CH), 2,924 (aliphatic CH), 2,212 (CN), 1,626 (C=O) cm−1; 1H NMR (DMSO-d6) – δ 0.93 (t, 3H, CH3), 1.64 (m, 2H, CH2), 2.47 (t, 2H, SCH2), 3.34 (s, 3H, OCH3), 6.96–7.76 (m, 9H, ArH), 11.68 (s, 1H, NH). MS m/z (%): 446 (M++2, 0.05), 445 (M++1, 0.08), 444 (M+, 1.1), 369 (3.2), 292 (12.56), 184 (23.21), 177 (41.25), 105 (35.34), 77 (42.75), 57 (100). Anal Calcd for C24H20N4O3S (444.5): C, 64.85; H, 4.54; N, 12.6; S, 7.21. Found: C, 64.81; H, 4.52; N, 12.58; S, 7.2%.
8-benzoyl-5-oxo-2-phenyl-7-(propylthio)-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (14b)
Yield (85%), MP >300°C (from EtOH-DMF); IR (KBr) νmax – 3,438 (NH), 2,925 (aliphatic CH), 2,213 (CN), 1,658 (C=O), 1,612 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 0.96 (t, 3H, CH3), 1.46 (m, 2H, CH2), 2.28 (t, 2H, SCH2), 7.4–8.25 (m, 10H, ArH), 11.21 (s, 1H, NH). MS m/z (%): 416 (M++2, 1.12), 415 (M++1, 1.26), 414 (M+, 2.13), 338 (23.21), 261 (53.36), 215 (25.13), 105 (56.26), 77 (38.49), 57 (100). Anal Calcd for C23H18N4O2S (414.48): C, 66.65; H, 4.38; N, 13.52; S, 7.74. Found: C, 66.6; H, 4.31; N, 13.5; S, 7.7%.
8-benzoyl-2-(4-chlorophenyl)-5-oxo-7-(propylthio)-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (14c)
Yield (85%), MP 280°C (from EtOH-dioxane); IR (KBr) νmax – 3,428 (NH), 3,154 (aromatic CH), 2,923 (aliphatic CH), 2,214 (CN), 1,670 (C=O), 1,617 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 1.03 (t, 3H, CH3), 1.4 (m, 2H, CH2), 2.86 (t, 2H, SCH2), 7.13–8.23 (m, 9H, ArH), 11.36 (s, 1H, NH). Anal Calcd for C23H17ClN4O2S (448.92): C, 61.53; H, 3.82; Cl, 7.9; N, 12.48; S, 7.14. Found: C, 61.5; H, 3.8; Cl, 7.89; N, 12.4; S, 7.1%.
2-(aryl)-5-oxo-9-phenyl-5,7-dihydro-3H-pyrazolo[4,3-c][1,2,4]triazolo[1,5-a]pyridine-6-carbonitriles 15a–c
To a solution of each compound (12–14)a–c (0.005 mol) in dioxane, equivalent amounts of hydrazine hydrate were added, and then the reaction mixture was heated under reflux for 12 hours, cooled, and then poured onto cold water. The solid that precipitated was filtered off, dried, and finally crystallized from EtOH-dioxane to give the 15a–c at good yield.
2-(4-methoxyphenyl)-5-oxo-9-phenyl-5,7-dihydro-3H-pyrazolo[4,3-c][1,2,4]triazolo[1,5-a]-pyridine-6-carbonitrile (15a)
Yield (65%), MP 193°C (from EtOH-dioxane); IR (KBr) νmax – 3,445 (NH), 3,112 (aromatic CH), 2,945 (aliphatic CH), 2,218 (CN), 1,674 (C=O), 1,613 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 3.54 (s, 3H, OCH3), 6.96–8.01 (m, 9H, ArH), 11.31 (s, 1H, NH); 12.15 (s, 1H, NH). MS m/z (%): 384 (M++2, 1.01), 383 (M++1, 1.06), 382 (M+, 2.18), 368 (25.41), 294 (55.32), 267 (25.11), 105 (100), 77 (38.99), 57 (95.21). Anal Calcd for C21H14N6O2 (382.37): C, 65.96; H, 3.69; N, 21.98. Found: C, 65.91; H, 3.6; N, 12.4%.
5-oxo-2,9-diphenyl-5,7-dihydro-3H-pyrazolo[4,3-c][1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (15b)
Yield (73%), MP 202°C (from EtOH-dioxane); IR (KBr) νmax – 3,435 (NH), 3,120 (aromatic CH), 2,222 (CN), 1,670 (C=O), 1,618 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 7.02–7.98 (m, 10H, ArH), 11.22 (s, 1H, NH); 12.11 (s, 1H, NH). MS m/z (%): 354 (M++2, 0.06), 353 (M++1, 1), 352 (M+, 1.1), 326 (28.11), 274 (57.3), 206 (32.1), 105 (100), 77 (53.94), 57 (65.11). Anal Calcd for C20H12N6O (352.34): C, 68.17; H, 3.43; N, 23.85. Found: C, 68.11; H, 3.39; N, 23.68%.
2-(4-chlorophenyl)-5-oxo-9-phenyl-5,7-dihydro-3H-pyrazolo[4,3-c][1,2,4]triazolo[1,5-a]-pyridine-6-carbonitrile (15c)
Yield (73%), MP 231°C (from EtOH-dioxane); IR (KBr) νmax – 3,422 (NH), 3,110 (aromatic CH), 2,215 (CN), 1,665 (C=O), 1,610 (C=C) cm−1; 1H NMR (DMSO-d6) – δ 6.892–7.88 (m, 9H, ArH), 11.52 (s, 1H, NH); 12.31 (s, 1H, NH). MS m/z (%): 388 (M++2, 1.23), 387 (M++1, 1.42), 386 (M+, 3.14), 352 (35.32), 275 (47.12), 200 (52.14), 105 (100), 77 (51.66), 57 (45.18). Anal Calcd for C20H11ClN6O (386.79): C, 62.1; H, 2.87; Cl, 9.17; N, 21.73. Found: C, 61.97; H, 2.82; Cl, 9; N, 21.65%.
Antimicrobial evaluation
The yeast of Candida albicans and bacteria of Staphylococcus aureus and Escherichia coli were used in this study to evaluate the antifungal and antibacterial potential of the synthesized compounds. The selected isolates were recovered from diseased cases of humans and animals at the Laboratory of Microbiology, Health Research Institute, Cairo, Egypt.
Control antibacterial and antifungal
The control antifungal (fluconazole 20 μg) and antibacterial (levofloxacin 3.25 μg) were purchased from Sigma-Aldrich (St Louis, MO, USA) and used as comparable controls.
Evaluation of antimicrobial potential of synthesized compounds against C. albicans, S. aureus, and E. coli using well diffusion test
Spore suspension (1 mL) was added to adjust the inoculum of S. aureus and E. coli to 2.5×102 cells/mL and C. albicans to 5×104 cells/mL were incorporated with Sabouraud dextrose agar (SDA) medium plates (for yeast) or nutrient agar medium (for bacteria). Wells of 5 mm Φ were made on the medium surface of agar plates, and 100 μL of gradual concentrations (0, 1, 2, 3, 4 and 5 mg/mL) of the synthesized compounds was added to the wells. Then, plates were incubated at 35°C–37°C for 24–72 hours. After incubation, the plates were tested for growth-inhibitory concentration zones around wells in the SDA medium plates (for yeast) or nutrient agar medium plates (for bacteria). Similar methods were used for control antifungal and antibacterial.35–37
Evaluation of antimicrobial potential of synthesized compounds against C. albicans, S. aureus, and E. coli using broth-dilution antifungal susceptibility testing of filamentous fungi
The minimum inhibitory concentration (MIC) of synthesized compounds for the tested isolates was determined by a broth-microdilution method based on the National Committee for Clinical Laboratory Standards. In sterile 12×75 mm plastic test tubes, 900 μL of RPMI 1640 broth medium or SD broth medium (for fungi) or nutrient broth (for bacteria) was inoculated separately, then 100 μL spore suspension added to adjust the inocula of S. aureus, E. coli (2.5×103 cells/mL), and Candida albicans to 5×104 cells/mL, and 100 μL of tested synthesized compound concentrations (1, 2, 3, 4, 5 mg/mL) for bacteria and fungi were added. The traditional antifungal agent fluconazole (20 μg) and antibacterial agent levofloxacin (3.25 μg) were included in separate assays as positive controls.38
The experiment was repeated twice. The MIC for fungi and bacteria was defined as the lowest synthesized compound concentration that showed no visible fungal or bacterial growth after incubation. After incubation, 5 μL of tested broth was inoculated on the sterile nutrient agar plates for bacteria and SDA plate for fungi and incubated at 37°C for 24 hours to 2 weeks. The MIC was determined as the lowest concentration of synthesized compounds inhibiting the visual growth of the test cultures on the agar plate. The turbidity of the growth in tubes was observed every 24 hours. Growth was assayed by measurement of optical density and transmittance of the contents of each tube at 405 nm using spectrophotometry.
Scanning electron microscopy of treated microbial cells
Morphological changes in C. albicans, S. aureus, and E. coli treated by the synthesized compound were observed with scanning electron microscopy (SEM). All tube contents were centrifuged and the sediments of each dehydrated separately through a graded series of EtOH (30%, 50%, 60%, 70%, 80%, 90%, 95%, and 100%), each level was applied twice for 15 minutes each time, and then EtOH:isoamyl acetate (3:1, 1:1, 1:3) and 100% isoamyl acetate were applied twice for 30 minutes. Solutions in wells were dried with a critical-point drier using liquid CO2 and coated with gold for 5 minutes. Coated samples were observed under SEM (JSM-5600LV) with accelerating voltage of 10 kVj.39
Results
Chemistry
It was found that 2-benzoyl-3,3-bis(alkylthio)acrylonitriles 2a–c prepared by the reaction of benzoyl acetonitrile 1, carbon disulfide, and alkyl iodide in the presence of sodium hydride reacted with cyanoaceto-N-phenylsulfonylhydrazide 4 at room temperature for 24 hours in the presence of KOH-dioxane to give the corresponding 5-benzoyl-N-[4-(alkylthio)-2-oxopyridin-1(2H)-yl]benzenesulfonamide 6a–c. Structures of 6a–c were established on the basis of spectroscopic data and elemental analysis (13C NMR, 1H NMR, IR, MS; Scheme 1). Compound 6 can also be prepared by reaction of the corresponding 1,6-diamino-5-benzoyl-2-oxo-4-(alkylthio)-1,2-dihydropyridine-3-carbonitriles 5a–c with benzene sulfonyl chloride in KOH-dioxane. When 2-benzoyl-3,3-bis(alkylthio)acrylonitriles 2a–c was treated with cyanoacetohydrazide 3 at room temperature for 24 hours in the presence of KOH-dioxane, 5a–c was obtained at excellent yield. Compound 5 reacted with hydrazine in refluxing EtOH to give the pyrazolopyridine 7. The structure of compound 7 was established on the basis of elemental analysis and spectral data, as outlined in Scheme 1.
In order to explore the reactivity of cyanoketene dithioacetals 2 with other classes of substituted cyanoacetohydrazide, we investigated the reaction of 2a–c with N′-[(aryl)-methylene]-2-cyanoacetohydrazides 8a–c. As such, we treated 8a–c with one equivalent of compound 2a–c in KOH-dioxane at room temperature for 24 hours and obtained the corresponding 7-(alkylthio)-3,5-dihydro[1,2,4]triazolo[1,5-a]pyridines 12–14 at good yield. The structure of 12–14 was established on the basis of its IR, MS, 1H NMR, spectroscopic, and elemental analysis. The formation of 12–14 from 2 and 8 was assumed to proceed via intermediate Michael adducts 9–11, which cyclized to yield the novel triazolopyridine derivatives 12–14. Compounds 12–14 reacted with hydrazine in refluxing dioxane to give the corresponding pyrazolo[4,3-c][1,2,4]triazolo[1,5-a]pyridines 15a–c. The structure of compound 15 was established on the basis of elemental analysis and spectral data, as outlined in Scheme 2.
Antimicrobial evaluation
In the present work, the antifungal and antibacterial potentials of synthesized compounds were evaluated against C. albicans, S. aureus, and E. coli using well diffusion tests. Inhibition-zone diameters for C. albicans were larger than for bacteria of S. aureus and E. coli strains (Tables 1 and 2). The MIC of synthesized compounds 6a–c and 15a–c was 4 mg/mL. Compound 6c had more antibacterial potential against S. aureus and E. coli, and showed zones of growth inhibition of 12 and 18 mm, respectively, at a concentration of 5 mg/mL. Other synthesized compounds – 5a–c, 7, 15a–b, and 12a–c – showed no antimicrobial potential up to a concentration of 5 mg/mL. On the other hand, the antifungal potential of the chemicals used showed more pronounced effects at comparatively lower concentrations, where the MIC against C. albicans was 1 mg/mL. The MIC of antifungal potential of synthesized compounds 6a–c and 15a–c was 1 mg/mL, while for chemicals of 15b the MIC was 4 mg/mL. The zone of fungal inhibition of chemical number 15c was 20 mm at a concentration of 1 mg/mL, and reached 35 mm at a concentration of 5 mg/mL. Other tested compounds showed no antimicrobial potential till concentrations had reached 5 mg/mL.
In the present study, the scanning of treated fungal and bacterial cells by SEM analysis showed interactions between synthesized compounds and the membrane structure of bacterial or fungal cells through significant changes to their membranes, recognized by the formation of “pits” on their surfaces, and finally resulted in the formation of pores and cell death and hence increasing the antimicrobial function of these compounds (Figures 1–3).
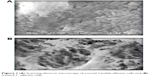  | Figure 1 (A) Scanning electron microscopy of normal Candida albicans cells and (B) treated C. albicans cells. |
  | Figure 2 (A) Scanning electron microscopy of normal Staphylococcus aureus cells and (B) treated S. aureus cells. |
  | Figure 3 (A) Scanning electron microscopy of normal Escherichia coli cells and (B) treated E. coli cells. |
Conclusion
In summary, we achieved a region-specific synthesis of novel 5-benzoyl-N-substituted amino- and 5-benzoyl-N-sulfonylamino-4-alkylsulfanyl-2-pyridones and their corresponding pyrazolopyridines and pyrazolotriazolopyridones by the reaction of 2-benzoyl-3,3-bis(alkylthio)acrylonitriles 2a–c with cyanohydrazide 3 and its derivatives 4 and 8. The antibacterial potential of the synthesized compounds was evaluated in order to assess their antimicrobial potential.
Acknowledgment
Support from the National Institute of Laser Enhanced Sciences (NILES), Cairo University is gratefully acknowledged.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Li Q, Mitscher LA, Shen LL. The 2-pyridone antibacterial agents: bacterial topoisomerase inhibitors. Med Res Rev. 2000;20:231–293. | ||
Fassihi A, Abedi D, Saghaie L, et al. Synthesis, antimicrobial evaluation and QSAR study of some 3-hydroxy-pyridine-4-one and 3-hydroxypyran-4-one derivatives. Eur J Med Chem. 2009;44:2145–2157. | ||
Desai NC, Rajpara KM, Joshi VV. Synthesis of pyrazole encompassing 2-pyridone derivatives as antibacterial agents. Bioorg Med Chem Lett. 2013;23:2714–2717. | ||
Manna F, Chimenti F, Bolasco A, Filippelli A, Lampa E. Antiinflammatory, analgesic and antipyretic 4,6-disubstituted 3-cyanopyridine-2-ones and 3-cyano-2-aminopyridines. Pharm Res. 1992;26:267–277. | ||
Yeates CL, Batchelor JF, Capon EC, et al. Synthesis and structure-activity relationships of 4-pyridones as potential antimalarials. J Med Chem. 2008;51:2845–2852. | ||
Norman AV, Chorell F, Westermark E, Olofsson A, Eriksson AS, Almqvist FA. Microwave-assisted decarboxylation of bicyclic 2-pyridone scaffolds and identification of Aβ-peptide aggregation inhibitors. Org Biomol Chem. 2005;3:2817–2823. | ||
Li L, Jiang X, Huang S, et al. Discovery of highly potent 2-sulfonyl-pyrimidinyl derivatives for apoptosis inhibition and ischemia treatment. Med Chem Lett. 2017;8:407–412. | ||
Pfefferkorn JA, Lou J, Minich ML, et al. Pyridones as glucokinase activators: identification of a unique metabolic liability of the 4-sulfonyl-2-pyridone heterocycle. Bioorg Med Chem Lett. 2009;19:3247–3252. | ||
Litchfield J, Sharma R, Atkinson K, et al. Intrinsic electrophilicity of the 4-methylsulfonyl-2-pyridone scaffold in glucokinase activators: role of glutathione-S-transferases and in vivo quantitation of a glutathione conjugate in rats. Bioorg Med Chem Lett. 2010;20:6262–6267. | ||
Azzam RA, Elgemeie GH, Elsayed RE, Jones PG. Crystal structure of N′-[2-(benzo[d]thiazol-2-yl)acetyl]-4-methylbenzenesulfonohydrazide. Acta Crystallogr E Crystallogr Commun. 2017;73:1041–1043. | ||
Elgemeie GH, Hanfy N, Hopf H, Jones PG. 5-Amino-1-phenylsulfonyl-4-pyrazolin-3-one. Acta Crystallogr C. 1998;54:136–137. | ||
Elgemeie GH, Hanfy N. Novel synthesis of 5-amino-1-arylsulfonyl-4-pyrazolin-3-one as a new class of N-sulfonylated pyrazoles. J Chem Res. 1999:385–386. | ||
Elgemeie GH, Sayed SH, Jones PG. True symmetry or pseudosymmetry: 5-amino-1-(4-methylphenylsulfonyl)-4-pyrazolin-3-one and a comparison with its 1-phenylsulfonyl analogue. Acta Crystallogr C. 2013;69:90–92. | ||
Elgemeie GH, Jones PG. Crystal structure of 1-amino-2-oxo-2,5,6,7,8,9-hexahydro-1H-cyclohepta[b]pyridine-3-carbonitrile. Acta Crystallogr E Crystallogr Commun. 2016;72:1239–1241. | ||
Elgemeie GH, Salah AM, Abbas NS, Hussein HA, Mohamed RA. Pyrimidine non-nucleoside analogs: a direct synthesis of a novel class of N-substituted amino and N-sulfonamide derivatives of pyrimidines. Nucleosides Nucleotides Nucleic Acids. 2017;36:213–223. | ||
Michael CM, Andrew DN, Nuzhat M, et al. Identification and characterization of 3-substituted pyrazolyl esters as alternate substrates for cathepsin B: the confounding effects of DTT and cysteine in biological assays. Bioorg Med Chem Lett. 2007;17:4761–4766. | ||
Sidique S, Shiryaev SA, Ratnikov BI, et al. Structure-activity relationship and improved hydrolytic stability of pyrazole derivatives that are allosteric inhibitors of West Nile virus NS2B-NS3 proteinase. Bioorg Med Chem Lett. 2009;19:5773–5777. | ||
Elgemeie GH, Abu-Zaied MA. Heterocyclic thioglycosides in carbohydrate research: synthesis of thiophene thioglycosides. Nucleosides & Nucleotides. 2017;36:511–519. | ||
Elgemeie GH, Abu-Zaied MA, Loutfy SA. 4-Aminoantipyrine in carbohydrate research: design, synthesis and anticancer activity of a novel class of derivatives of 4-aminoantipyrine thioglycosides and their corresponding pyrazolopyrimidine and pyrazolopyridine thioglycosides. Tetrahedron. 2017;73:5853–5861. | ||
Elgemeie GH, Fathy NM, Farag AB, Al-Kursani SA. Antimetabolites: design, synthesis, and cytotoxic evaluation of novel dihydropyridine thioglycosides and pyridine thioglycosides. Nucleosides Nucleotides Nucleic Acids. 2017;36:355–377. | ||
Elgemeie GH, Salah AM, Abbas NS, Hussein HA, Mohamed RA. Nucleic acid components and their analogs: design and synthesis of novel cytosine thioglycoside analogs. Nucleosides Nucleotides Nucleic Acids. 2017;36:139–150. | ||
Elgemeie GH, Mohamed RA, Hussein HA, Jones PG. Crystal structure of N-(2-amino-5-cyano-4-methylsulfanyl-6-oxo-1,6-dihydropyrimidin-1-yl)-4-bromobenzenesulfonamide dimethylformamide monosolvate. Acta Crystallogr E Crystallogr Commun. 2015;71:1322–1324. | ||
Elgemeie GH, Abouzeid M, Jones PG. Crystal structure of 4-{[(cyanoimino)(methylsulfanyl)methyl]amino}-1,5-dimethyl-2-phenyl-2,3-dihydro-1H-pyrazol-3-one. Acta Crystallogr E Crystallogr Commun. 2015;71:104–105. | ||
Elgemeie GH, Mohamed RA. Application of dimethyl N-cyanodithioiminocarbonate in synthesis of fused heterocycles and in biological chemistry. Heterocycl Comm. 2014;20:313–331. | ||
Elgemeie GH, El-Ezbawy SR, El-Aziz HA. The design and synthesis of structurally related mercaptopurine analogues: reaction of dimethyl N-cyano-dithioiminocarbonate with 5-aminopyrazoles. Synth Commun. 2001;31:3453–3458. | ||
Elgemeie GH, Elzanate AM, Elghandor AH, Ahamed SA. Novel intramolecular cyclization of pyrazolone ketene S, N-acetals for the construction of methylsulfanylpyrazolo[4,3-b]pyridines. Synth Commun. 2002;32:3509–3517. | ||
Elgemeie GH, Jones PG. 6-Amino-4-(methylsulfanyl)-2-oxo-1-tolyl-1,2-dihydropyridine-3,5-dicarbonitrile. Acta Crystallogr E Crystallogr Commun. 2004;60:O2107–O2109. | ||
Elgemeie GH, Elghandor AH, Abd-Elaziz GW. Potassium 2-cyanoethylene-1-thiolate: a new preparative route to 2-cyanoketene S, N-acetals and pyrazole derivatives. Synth Commun. 2004;34:3281–3291. | ||
Altalbawy FM, Mohamed GG, Mohamed MI. Synthesis, characterization and biological activity of some transition metals complexes with Schiff base derived from 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol and p-methoxysalicyldehyde. Asian J Chem. 2010;22:7291–7307. | ||
Altalbawy FM. Synthesis, in vitro antimicrobial, anticancer evaluation of some new pyridazines and polyfunctionally substituted heterocyclic compounds. Asian J Chem. 2015;27:4361–4368. | ||
Altalbawy FM. Synthesis and antimicrobial evaluation of some novel bis-α,β-unsaturated ketones, nicotinonitrile, 1,2-dihydropyridine-3-carbonitrile, fused thieno[2,3-b]pyridine and pyrazolo-[3,4-b] pyridine derivatives. Int J Mol Sci. 2013;14:2967–2979. | ||
Altalbawy FM, Darwish SS. Synthesis and antimicrobial activity of 1,2,4-triazolo[4,3-b][1,2,4,5]tetrazines. Asian J Chem. 2011;23:2951–2955. | ||
Altalbawy FM, Mohamed GG, Sayed MA, Mohamed MI. Synthesis, characterization, and biological activity of some transition metal complexes with Schiff base ligands derived from 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol and salicaldehyde. Monatsh Chem. 2012;143:79–89. | ||
Elgemeie GH, Hanfy N, Hopf H, Jones PG. 2-Dicyanomethylene-5,6-dimethyl-1,2-dihydropyridine-3-carbonitrile. Acta Crystallogr C. 1998;54:820–821. | ||
Amichai B, Grunwald MH. Adverse drug reactions of the new oral antifungal agents: terbinafine, fluconazole, and itraconazole. Int J Dermatol. 1998;37:410–415. | ||
Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149:296–305. | ||
Hassan AA, Mahmoud HK, Hesham T, El-Ahl RM, Mahmoud HH. Herbal biosynthesis of zinc nanoparticles and evaluation of their antifungal and antibacterial effect for buffaloes skin affections. Int J Curr Res. 2015;7:24338–24349. | ||
Rex JH, Ghannoum MA. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 2nd ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2008. | ||
Gong P, Li HM, He XX, et al. Preparation and antibacterial activity of Fe3O4 @Ag nanoparticles. Nanotechnology. 2007;18:285604. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




