Back to Journals » Drug Design, Development and Therapy » Volume 11
Synthesis and antimicrobial activity of chiral quaternary N-spiro ammonium bromides with 3',4'-dihydro-1'H-spiro[isoindoline-2,2'-isoquinoline] skeleton
Authors Bielawski K , Leszczyńska K, Kałuża Z, Bielawska A, Michalak O, Daniluk T, Staszewska-Krajewska O, Czajkowska A, Pawłowska N, Gornowicz A
Received 25 January 2017
Accepted for publication 8 May 2017
Published 5 July 2017 Volume 2017:11 Pages 2015—2028
DOI https://doi.org/10.2147/DDDT.S133250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Krzysztof Bielawski,1 Katarzyna Leszczyńska,2 Zbigniew Kałuża,3 Anna Bielawska,4 Olga Michalak,3 Tamara Daniluk,2 Olga Staszewska-Krajewska,3 Anna Czajkowska,4 Natalia Pawłowska,1 Agnieszka Gornowicz4
1Department of Synthesis and Technology of Drugs, 2Department of Microbiology, Medical University of Bialystok, Bialystok, 3Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, 4Department of Biotechnology, Medical University of Bialystok, Bialystok, Poland
Abstract: A new class of highly functionalized tetrahydroisoquinolines with a quaternary carbon stereocenter was synthesized starting from an easily accessible l-tartaric acid. Nine strains of bacteria (Staphylococcus aureus, Streptococcus pyogenes, Streptococcus mutans, Streptococcus salivarius, Bacillus subtilis, Enterococcus faecalis, Moraxella catarrhalis, Escherichia coli, Campylobacter jejuni) were used for the determination of minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of synthesized compounds. The influence of analyzed compounds on viability and induction of apoptosis in human skin fibroblasts was determined. A majority of the synthesized compounds showed the strongest antibacterial properties toward some gram-negative bacteria (M. catarrhalis and C. jejuni) with a high level of selectivity. High antibacterial compounds have bactericidal activity ratio MBC/MIC =4. Our studies also proved that the novel compounds do not possess cytotoxic and proapoptotic potential in normal cells.
Keywords: quaternary ammonium compounds, tetrahydroisoquinolines, antimicrobial activity, antibiotic resistance
Introduction
The growing numbers of bacterial strains resistant to currently used drugs constitute a real threat to the health of global community. Multidrug-resistant (MDR), extensively drug-resistant (XDR) and even pandrug-resistant (PDR) bacteria that are resistant to all available antibiotics are considered to be responsible for serious hospital infections, particularly in immunocompromised individuals. The use of antibiotics is the single most important factor leading to antibiotic resistance.1,2 Antibiotics are among the most commonly prescribed drugs used in human medicine. However, up to 50% of all antibiotics prescribed for people are not needed or are ineffective. Antibiotics are also commonly used in feeding animals to prevent, control and treat disease and to promote the growth of food-producing animals.3 Unfortunately, this dramatic increase of antibiotic-resistant bacteria in recent years has no reflection in the number of new antibacterial agents introduced into the market, thus limiting therapeutic options for patients infected by such bacteria. Therefore, the development of new methods of treatment for infections is one of the most urgent necessities in medicine today.4
Quaternary ammonium compounds (QACs) that belong to organic ionic chemical agents are one model for the design of novel antibacterial compounds. In particular, they are an essential component of infection control practices and are useful in the prevention of nosocomial infections.5 Despite these applications, our knowledge of the molecular mechanism of action of QACs is very limited. In general, QACs have a more comprehensive spectrum of activity than antibiotics, and while antibiotics tend to have specific intracellular targets these compounds may have multiple targets.6 A further exploration of QACs, which are more selective for bacterial cells than eukaryotic cells, is needed. Several natural compounds having QAC moiety have shown antimicrobial activity, including the ability to inhibit the biofilm formation.7 These include chelerythrine, sanguinarine and berberine from the group of alkaloids which contain isoquinoline scaffold and quaternary ammonium moiety (Scheme 1). Sanguinarine interacts with DNA, RNA and caps telomerases that leads to the rapid induction of apoptosis.8,9 Its antiplaque efficacy in humans was also proved, and sanguinarine has been used as a component of mouthwashes and toothpastes in the UK and USA.10 Other studies based on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) demonstrated that berberine could destroy cellular proteins and lead to damage in bacterial cells.11
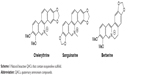  | Scheme 1 Natural bioactive QACs that contain isoquinoline scaffold. |
Collier et al12 found that decamethylene bisisoquinolinium bromide had a significant antimicrobial activity. Until now, a number of isoquinolinium derivatives have been synthesized and found to have a superior activity against gram-positive and gram-negative bacteria.13 These compounds have been shown to be antibacterial through membrane disruption14 as well as through binding to the cytosolic components, mainly nucleic acids.15 We have elaborated the procedure for the preparation of enantiopure hexahydropyrroloisoquinolines from L-tartaric acid,16–18 and their further transformation into highly functionalized tetrahydroisoquinolines with a quaternary carbon stereocenter. The main task of this study was to carry out the synthesis and assess the bacteriostatic and bactericidal activity of chiral quaternary N-spiro ammonium bromides with 3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinoline] skeleton. The effect of tested compounds on the viability of human skin fibroblasts was also determined.
Materials and methods
Nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 at room temperature (except where indicated otherwise) using a Varian VNMR500 spectrometer or Bruker Avance 500 (Bruker Corporation, Billerica, MA, USA). Chemical shifts are quoted in parts per million relative to tetramethylsilane (TMS) for 1H NMR and CDCl3 for 13C NMR. Coupling constants J are reported in Hertz. Infrared (IR) spectra were obtained using a Fourier transform infrared (FTIR) Jasco 6200 or FTIR Spectrum 2000 (PerkinElmer Inc., Waltham, MA, USA) and are reported in reciprocal centimeters (cm−1). Mass spectra were recorded using an AMD-604 Intectra GmbH or a Mariner Perseptive Biosystem mass spectrometer. X-ray analysis was performed on Bruker AXS, APEX diffractometer (Bruker Corporation). Optical rotations were measured at 23°C with a Jasco P2000 digital polarimeter. Thin-layer chromatography was performed using precoated silica gel plates (Merck Kieselgel 60 F254 [Merck Millipore, Billerica, MA, USA], 0.2 mm layer thickness). Visualization of the developed chromatogram was performed by ultraviolet (UV) absorbance and cerium molybdate water solution. Flash chromatography was carried out using Merck Kieselgel (230–400 mesh). All air and moisture sensitive reactions were performed under an argon atmosphere in flame-dried glassware using anhydrous solvents. Most reagents were obtained from commercial suppliers and were used without further purification, unless noted otherwise. Tetrahydrofuran (THF) was distilled from Na and benzophenone, and dichloromethane (DCM) and toluene were distilled from CaH2. The compounds 1, 4 and 5 were prepared according to the literature methods.17,18 The same procedure was applied for the synthesis of compounds 2 and 3.
General procedure for the preparation of amino alcohols 6–9
Step I: Into a solution of diacetate 1–5 (5 mmol) in anhydrous methanol (50 mL), MeONa (5 mmol) was added at room temperature. Stirring was continued until disappearance of the substrate (~0.5 h), and solid CO2 was added and concentrated in vacuum. Crude product was dissolved in CH2Cl2, the precipitate was filtered off and the filtrate was concentrated.
Step II: Crude diol was dissolved in CH3CN (25 mL), and a solution of NaIO4 (4.28 g, 20 mmol) in 38 mL H2O was added. Stirring was continued until disappearance of the substrate (~4–6 days; thin layer chromatography control). Precipitate was filtered off and washed with ethyl acetate. The filtrate was concentrated in vacuo, approximately to 1/2 initial volume and extracted with ethyl acetate (4×50 mL). Collected extracts were dried with MgSO4, filtrated and concentrated in vacuo. Crude product was used in the next step.
Step III: The obtained product in step II was dissolved in THF (30 mL), and during intensive stirring NaOH (1.20 g, 30 mmol) dissolved in water (30 mL) was added. Stirring was continued until it was judged that the reaction was over (thin layer chromatography control, 0.5–2 h). The solution was diluted with water (50 mL) and extracted with DCM (3×50 mL). Collected extracts were dried (MgSO4) and concentrated in vacuo. Crude product was purified on silica gel or via crystallization. Overall, the yield of amino alcohols was in the range of ~40%–60%.
(S)-(6,7-dimethoxy-1-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl)methanol (6)
Yield 42%, amorphous solid. Purified on silica gel using DCM:MeOH:NH3aq = (95:5:0.5 → 90:10:0.5) as an eluent. [α]D22 =−42.4 (c =1, MeOH). 1H NMR (CDCl3): 1.28 (s, 3H), 2.04 (s, 3H), 2.78 (m, 1H), 2.80 (m, 1H), 3.25 (m, 1H), 3.30 (m, 1H), 3.80 (d, 1H, J =11.8 Hz), 3.85 (s, 3H), 3.86 (s, 3H), 4.09 (d, 1H, J =11.8 Hz), 6.58 (s, 1H), 6.63 (s, 1H), 6.88 (bs, 2H). 13C NMR (CDCl3): 26.2, 29.20, 38.80, 58.36, 58.88, 66.64, 68.78, 111.12, 114.14, 129.99, 150.43, 150.66, 180.20. MS (EI, HR) m/z: (M+H+) Calcd. for C13H20NO3: 238.2055. Found: 238.2061.
(S)-(6,7-dimethoxy-1-(4-methoxyphenyl)-1,2,3,4-tetrahydroisoquinolin-1-yl)methanol (7)
Yield 57%, amorphous solid. Purified on silica gel using DCM:MeOH:NH3aq = (95:5:0.5 → 90:10:0.5) as an eluent. [α]D22 =−26.7 (c =1, MeOH). 1H NMR (CDCl3): 2.72 (m, 2H), 2.88 (m, 1H), 2.96 (m, 1H), 3.05 (m, 1H), 3.73 (s, 3H), 3.78 (s, 3H), 3.89 (s, 3H), 3.96 (d, 1H, J =11.2 Hz), 4.17 (d, 1H, J =11.2 Hz), 6.46 (s, 1H), 6.64 (s, 1H), 6.82 (d, 1H, J =8.8 Hz), 7.25 (d, 1H, J =8.8 Hz). 13C NMR (CDCl3): 29.13, 38.61, 55.22, 55.80, 56.04, 63.24, 67.47, 110.31, 111.80, 113.62, 128.54, 129.10, 129.52, 136.92, 147.46, 148.01, 158.74. MS (EI, HR) m/z: (M+) Calcd. for C19H24NO4: 329.1695. Found: 329.1693.
(S)-(1-(4-bromophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl)metanol (8)
Yield 45%, amorphous solid. Purified on silica gel using DCM:MeOH:NH3aq (95:5:0.5 → 90:10:0.5) as an eluent. [α]DD21 =+33.6 (c =1, MeOH). IR (film): 3,322, 3,057, 2,999, 2,934, 2,833, 1,609, 1,515, 1,464 cm−1. 1H NMR (CDCl3): 2.55 (bm, 2H), 2.72 (dt, 1H, J =16.6, 5.1 Hz), 2.85 (m, 1H), 2.94 (m, 1H), 3.04 (m, 1H), 3.74 (s, 3H), 3.88 (s, 3H), 3.95 (d, 1H, J =11.2 Hz), 4.11 (d, 1H, J =11.2 Hz), 6.41 (s, 1H), 6.65 (s, 1H), 7.23 (d, 2H, 8.6 Hz), 7.41 (d, 2H, 8.6 Hz). 13C NMR (CDCl3): 29.19, 38.71, 55.81, 63.16, 67.25, 110.16, 111.97, 121.44, 128.69, 128.97, 129.79, 131.40, 144.21, 147.54, 148.18. MS (EI, HR) m/z: (M+) Calcd. for C18H21NO3Br: 378.0705. Found: 378.0696.
(S)-(1-cyclohexyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl)methanol (9)
Yield 46%, amorphous solid. Purified on silica gel using DCM:MeOH:NH3aq (95:5:0.5 → 90:10:0.5). [α]DD23 =−62.9 (c =1, MeOH). 1H NMR (CDCl3): 0.9–1.94 (m, 11H), 2.01 (s, 3H), 2.71 (m, 1H), 2.84 (m, 1H), 3.23 (m, 1H), 3.32 (m, 1H), 3.80 (d, 1H, J =11.8 Hz), 3.85 (s, 3H), 3.86 (s, 3H), 4.08 (d, 1H, J =11.8 Hz), 6.58 (s, 1H), 6.63 (s, 1H), 6.82 (bs, 2H). 13C NMR (CDCl3): 25.51, 28.86, 29.28, 29.50, 29.91, 30.23, 30.78, 42.40, 49.31, 58.46, 58.83, 66.66, 68.76, 111.15, 114.13, 129.98, 150.47, 150.68, 180.24. MS (EI, HR) m/z: (M+H+) Calcd. for C18H28NO3: 306.2065. Found: 306.2069.
General procedure for the preparation of quaternary ammonium salts 11a–14c
Into a solution of amino alcohol (0.4 mmol) in MeCN or DCM (2 mL), a proper dibromide (0.5 mmol) was added followed by the addition of N, N-diisopropylethylamine (DIPEA; 0.5 mmol). The reaction was run for 24 h at room temperature, except for low-reactive cyclohexyl amino alcohol 9, which required elevated temperature (60°C). The reaction mixture was concentrated in vacuo, and crude product was purified via crystallization or chromatography on silica gel. Due to considerable broadening of some NMR signals of ammonium salts with a bulky substituent at C-1′ (aryl, cyclohexyl), only selected data are given.
(S)-1′-(hydroxymethyl)-6′,7′-dimethoxy-1′-methyl-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (11a)
[α]D20 =+15.8 (c =1, MeOH). IR (film): 3,230, 3,010, 2,936, 1,611, 1,523, 1,493, 1,408, 1,362 cm−1. 1H NMR (CD3OD): 1.85 (s, 3H), 3.25 (m, 1H), 3.41 (m, 1H), 3.83 (s, 3H), 3.84 (s, 3H), 3.89 (m, 1H), 3.98 (d, 1H, J =13.7 Hz), 4.21 (d, 1H, J =13.7 Hz), 4.39 (m, 1H), 4.82 (d, 1H, J =15.5 Hz), 5.00 (d, 1H, J =15.5 Hz), 5.87 (d, 1H, J =15.6 Hz), 5.87 (s, 1H), 5.92 (s, 1H), 7.34 (d, 1H, J =7.1 Hz), 7.41 (m, 3H). 13C NMR (CD3OD): 18.13, 23.48, 55.07, 55.43, 55.78, 63.76, 65.98, 67.09, 74.91, 109.82, 111.48, 122.48, 122.62, 123.61, 125.41, 128.75, 128.85, 132.11, 133.33, 148.51, 149.84. MS (EI, HR) m/z: (M+) Calcd. for C21H26NO3: 340.1913. Found: 340.1910.
(S)-1′-(hydroxymethyl)-5,6,6′,7′-tetramethoxy-1′-methyl-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (11b)
[α]D22 =+14.5 (c =1, MeOH). IR (film): 2,989, 2,978, 2,039, 2,898, 2,771, 2,664, 1,608, 1,512, 1,467, 1,445 cm−1. 1H NMR (CD3OD): 1.84 (s, 3H), 3.41 (m, 1H), 3.80 (s, 3H), 3.84 (s, 9H), 3.91 (m, 1H), 3.95 (d, 1H, J =13.7 Hz), 4.19 (d, 1H, J =13.7 Hz), 4.39 (m, 1H), 4.72 (dd, 2H, J =7.9, 15.2 Hz), 4.89 (d, 1H, J =14.4 Hz), 5.78 (d, 1H, J =15.4), 6.86 (s, 1H), 6.90 (s, 1H), 6.91 (s, 1H), 7.0 (s, 1H). 13C NMR (CD3OD): 17.90, 23.51, 42.42, 54.46, 55.29, 55.31, 55.98, 64.07, 66.33, 67.12, 74.83, 105.62, 105.74, 109.81, 111.48, 123.68, 123.70, 124.96, 125.49, 148.49, 149.84, 150.39, 150.49. MS (EI, HR) m/z: (M+) Calcd. for C23H30NO5: 400.2124. Found: 400.2123.
(S)-1′-(hydroxymethyl)-6′,7′-dimethoxy-1′-methyl-3′,4′,5,7-tetrahydro-1′H-spiro[[1,3]dioxolo[4,5-f]isoindole-6,2′-isoquinolin]-2′-ium bromide (11c)
[α]DD21 =+14.2 (c =1, MeOH). IR (film): 3,227, 2,963, 2,939, 2,834, 1,611, 1,520, 1,481 cm−1. 1H NMR (CD3OD): 1.85 (s, 3H), 3.28 (m, 1H), 3.41 (m, 1H), 3.85 (s, 6H), 3.92 (m, 2H), 3.96 (d, 1H, J =13.6 Hz), 4.19 (d, 1H, J =13.6 Hz), 4.38 (m, 1H), 4.68 (d, 2H, J =15.1 Hz), 5.73 (d, 1H, J =13.3 Hz), 6.01 (dd, 2H, J =0.9, 3.5 Hz), 6.77 (s, 1H), 6.86 (s, 1H), 6.87 (s, 1H), 6.92 (s, 1H). 13C NMR (CD3OD): 17.96, 23.83, 55.07, 55.42, 55.93, 63.32, 63.90, 67.07, 74.97, 101.97, 103.74, 102.83, 109.83, 111.48, 123.65, 124.71, 125.38, 125.99, 148.51, 148.98, 149.07, 149.86. MS (EI, HR) m/z: (M+) Calcd. for C22H26NO5: 384.1811. Found: 384.1811.
(S)-1′-(hydroxymethyl)-6′,7′-dimethoxy-1′-(4-methoxyphenyl)-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (12a)
[α]DD21 =−0.85 (c =0.7, MeOH). IR (film): 3,227, 2,963, 2,939, 2,834, 1,611, 1,520, 1,481 cm−1. 1H NMR (CD3OD): 3.33–3.38 (m, 2H), 3.67 (s, 3H), 3.71–3.75 (m, 1H), 3.80 (s, 3H), 3.90 (s, 3H), 4.04–4.09 (m, 1H), 4.38 (d, 14.4 Hz, 1H), 4.58 (d, 13.6 Hz, 1H), 4.90 (d, 15.0 Hz, 1H), 5.14 (d, 13.6 Hz, 1H), 5.52 (d, 14.4 Hz, 1H), 5.70 (d, 15.0 Hz, 1H), 6.58 (s, 1H), 6.94–6.97 (m, 3H), 7.25–7.28 (m, 1H), 7.32–7.36 (m, 3H), 7.40–7.57 (m, 2H). 13C NMR (CD3OD) 23.9, 54.5, 54.7, 55.1, 55.2, 63.0, 65.3, 65.6, 78.9, 111.0, 111.4, 113.6, 148.4, 149.8, 161.0. MS (EI, HR) m/z: (M+) Calcd. for C27H30NO4: 432.2175. Found: 432.2173.
(S)-1′-(hydroxymethyl)-5,6,6′,7′-tetramethoxy-1′-(4-methoxyphenyl)-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (12b)
[α]DD21 =−2.33 (c =1, MeOH/DCM 8/2). IR (film): 3,402, 3,244, 2,979, 2,942, 2,689, 1,725, 1,607, 1,515, 1,466 cm−1. 1H NMR (CDCl3): 3.11–3.18 (m, 1H), 3.29–3.35 (br, 2H), 3.69 (s, 3H), 3.70–3.78 (m, 1H), 3.82 (s, 3H), 3.83 (s, 3H), 3.84 (s, 3H), 4.18–4.27 (br, 1H), 4.65 (d, 13.6 Hz, 1H), 4.72–4.88 (br, 1H), 4.94 (d, 13.9 Hz, 1H), 5.19–5.34 (br, 1H), 5.42–5.51 (br, 1H), 6.22 (br, 1H), 6.37 (br, 1H), 6.70 (s, 1H), 6.73 (s, 1H), 6.84 (s, 1H), 6.86–6.70 (br, 1H). 13C NMR (CDCl3): 24.5, 53.7, 54.1, 55.4, 56.1, 56.2, 56.3, 56.3, 64.9, 67.9, 72.8, 106.0, 106.7, 110.9, 111.6, 114.1, 148.1, 149.7, 150.0, 150.3, 160.8. MS (EI, HR) m/z: (M+) Calcd. for C29H34NO6: 492.2386. Found: 492.2386.
(S)-1′-(hydroxymethyl)-6′,7′-dimethoxy-1′-(4-methoxyphenyl)-3′,4′,5,7-tetrahydro-1′H-spiro[[1,3]dioxolo[4,5-f]isoindole-6,2′-isoquinolin]-2′-ium bromide (12c)
[α]D22 =−0.31 (c =1, MeOH/DCM 9/1). IR (film): 3,402, 3,244, 2,979, 2,942, 2,689, 1,725, 1,607, 1,515, 1,482 cm−1. 1H NMR (CDCl3): 3.24–3.41 (m, 1H), 3.69 (s, 3H), 3.83 (s, 3H), 3.91 (s, 3H), 4.14 (br, 1H), 4.67 (br, 1H), 4.92 (br, 1H), 5.17 (br, 1H), 5.47 (br, 1H), 5.93–6.00 (m, 2H), 6.38 (s, 1H), 6.61 (s, 1H), 6.71–6.76 (m, 2H), 6.84–7.01 (br, 2H). 13C NMR (CDCl3): 24.4, 53.8, 55.4, 56.1, 56.2, 67.8, 101.9, 103.5, 104.4, 110.9, 160.8. MS (EI, HR) m/z: (M+) Calcd. for C29H30NO6: 476.2073. Found: 476.2078.
(S)-1′-(4-bromophenyl)-1′-(hydroxymethyl)-6′,7′-dimethoxy-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (13a)
[α]D22 =−1.44 (c =1, MeOH/DCM 9:1). IR (film): 3,213, 2,925, 2,854, 2,685, 2,517, 2,490, 1,736, 1,612, 1,586, 1,520, 1,489, 1,465 cm−1. 1H NMR (CD3OD): 3.33 (br, 1H), 3.38–3.43 (m, 1H), 3.67 (s, 3H), 3.89 (s, 3H), 4.02 (br, 1H), 4.40 (br, 1H), 4.60 (d, 13.5 Hz, 1H), 4.93 (d, 15.1 Hz, 1H), 5.14 (d, 13.5 Hz, 1H), 5.63 (br, 1H), 5.73 (d, 15.1 Hz, 1H), 6.56 (s, 1H), 6.96 (s, 1H). 13C NMR (CD3OD): 23.8, 55.1, 63.1, 65.4, 66.1, 78.1, 111.0, 111.1, 148.6, 150.0. MS (EI, HR) m/z: (M+) Calcd. for C26H27NO3Br: 480.1174. Found: 480.1174.
(S)-1′-(4-bromophenyl)-1′-(hydroxymethyl)-5,6,6′,7′-tetramethoxy-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (13b)
[α]D22 =+1.41 (c =1, MeOH/DCM 9:1). IR (film): 3,202, 3,004, 2,962, 2,939, 2,836, 1,722, 1,612, 1,586, 1,512, 1,466 cm−1. 1H NMR (CD3OD): 3.34–3.39 (m, 1H), 3.67 (s, 3H), 3.71–3.75 (m, 1H), 3.78 (s, 3H), 3.80 (s, 3H), 3.89 (s, 3H), 4.12 (br, 1H), 4.34 (br, 1H), 4.81 (d, 14.8 Hz, 1H), 5.07 (d, 13.4 Hz, 1H), 5.48 (br, 1H), 5.64 (d, 14.8 Hz, 1H), 6.56 (s, 1H), 6.80 (s, 1H), 6.56 (s, 1H), 6.88 6.56 (s, 1H), 6.95 6.56 (s, 1H). 13C NMR (CD3OD): 24.0, 55.7, 63.8, 65.7, 66.3, 78.9, 106.7, 106.7, 111.5, 111.8, 148.7, 150.3, 150.5, 150.5. MS (EI, HR) m/z: (M+) Calcd for C28H31NO5Br: 540.1386. Found: 540.1385.
(S)-1′-(4-bromophenyl)-1′-(hydroxymethyl)-6′,7′-dimethoxy-3′,4′,5,7-tetrahydro-1′H-spiro[[1,3]dioxolo[4,5-f]isoindole-6,2′-isoquinolin]-2′-ium bromide (13c)
[α]D22 =+1.57 (c =1, MeOH). IR (film): 3,218, 2,972, 2,942, 2,684, 2,518, 2,492, 1,612, 1,520, 1,482, 1,396, 1,353, 1,298 cm−1. 1H NMR (CDCl3): 3.36 (br, 1H), 3.70 (s, 3H), 3.77 (br, 1H), 3.91 (s, 3H), 4.23 (br, 1H), 4.71 (br, 1H), 4.88 (br, 1H), 5.07 (br, 1H), 5.57 (br, 1H). 13C NMR (CDCl3): 24.5, 49.4, 54.1, 54.4, 56.1, 56.3, 64.6, 67.5, 72.8, 101.9, 103.6, 104.3. MS (EI, HR) m/z: (M+) Calcd. for C27H27NO5Br: 524.1073. Found: 524.1074.
(S)-1′-cyclohexyl-1′-(hydroxymethyl)-6′,7′-dimethoxy-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (14a)
[α]DD24 =−13.9 (c =0.8, MeOH). IR (film): 3,547, 3,464, 3,408, 3,186, 3,009, 2,934, 2,855, 1,609, 1,520, 1,453 cm−1. 1H NMR (CDCl3): 0.99–1.23 (m, 4H), 1.29–1.41 (m, 1H), 1.85–1.94 (m, 1H), 2.05–2.14 (m, 1H), 2.20–2.27 (m, 1H), 3.21–3.40 (m, 2H), 3.73–3.82 (m, 1H), 3.89 (s, 3H), 3.97 (s, 3H), 4.20 (d, 14.7 Hz, 1H), 4.75 (m, 1H), 4.83 (d, 14.1 Hz, 1H), 5.86–5.93 (m, 2H), 6.70 (s, 1H), 7.03 (s, 1H). 13C NMR (CDCl3): 24.3, 25.7, 27.0, 27.4, 29.5, 31.2, 45.9, 55.0, 56.0, 57.0, 58.9, 67.3, 68.9, 80.6, 111.3, 111.6, 147.9, 149.4. MS (EI, HR) m/z: (M+) Calcd. for C26H34NO3 408.2539. Found: 408.2529.
(S)-1′-cyclohexyl-1′-(hydroxymethyl)-5,6,6′,7′-tetramethoxy-3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinolin]-2-ium bromide (14b)
[α]DD24 =−13.9 (c =0.8, MeOH). IR (film): 3,230, 2,928, 2,854, 1,737, 1,612, 1,514, 1,465, 1,454, 1,350, 1,363, 1,226 cm−1. 1H NMR (CDCl3): 0.85–0.92 (m, 1H), 1.02–1.15 (m, 3H), 1.56–1.71 (m, 4H), 1.84–1.96 (m, 3H), 3.18–3.43 (m, 3H), 3.80 (s, 3H), 3.84 (s, 3H), 3.86 (s, 3H), 3.89 (s, 3H), 4.15 (d, 14.3 Hz, 1H), 4.67–4.84 (m, 3H), 5.83 (d, 14.3 Hz, 1H), 5.91 (d, 13.6 Hz, 1H), 6.63 (s, 1H), 6.70 (s, 1H), 6.96 (s, 1H), 7.04 (s, 1H). 13C NMR (CDCl3): 24.4, 25.3, 25.7, 27.5, 29.7, 46.0, 55.2, 55.9, 56.2, 56.3, 56.9, 65.6, 67.7, 69.1, 80.6, 105.5, 106.3, 111.4, 111.6, 148.0, 149.5, 150.2, 150.3. MS (EI, HR) m/z: (M+) Calcd. for C28H38NO5: 468.2750. Found: 468.2740.
(S)-1′-cyclohexyl-1′-(hydroxymethyl)-6′,7′-dimethoxy-3′,4′,5,7-tetrahydro-1′H-spiro[[1,3]dioxolo[4,5-f]isoindole-6,2′-isoquinolin]-2′-ium bromide (14c)
[α]DD23 =−1.90 (c =1, MeOH). IR (film): 3,243, 2,935, 2,855, 2,691, 1,715, 1,611, 1,521, 1,483, 1,365 cm−1. 1H NMR (CD3OD): 1.03 (br, 1H), 1.11–1.23 (br, 3H), 1.41–1.69 (br, 4H), 1.88 (br, 1H), 2.15–2.26 (m, 2H), 3.32–3.44 (m, 2H), 3.85 (s, 3H), 3.86 (s, 3H), 4.03–4.11 (br, 1H), 4.34 (d, 14.6 Hz, 1H), 4.50–4.70 (m, 3H), 5.51 (d, 14.5 Hz, 1H), 5.66 (d, 14.5 Hz, 1H), 5.95–6.02 (m, 2H), 6.67 (s, 1H), 6.87 (s, 1H), 6.91 (s, 1H), 6.94 (s, 1H). 13C NMR (CD3OD): 23.5, 25.6, 26.4, 27.0, 28.9, 31.3, 42.5, 45.3, 54.5, 56.5, 66.8, 67.8, 80.3, 111.6, 112.1, 147.4, 148.7. MS (EI, HR) m/z: (M+) Calcd. for C27H34NO5: 452.2437. Found: 452.2429.
Biological assays
Antibiotics and bacterial strains
Norfloxacin (Nor) was obtained from Merck & Co., Inc. (Whitehouse Station, NJ, USA). Bacterial reference strains with well-characterized properties from American Type Culture Collection (ATCC, Manassas, VA, USA) were used in the study, as well as strains isolated from invasive infections among in- and outpatients, from the collection of the Department of Microbiology, Medical University of Bialystok. All strains were stored according to the international standards at −80°C in cryobank tubes (Mast Diagnostica, Reinfeld, Germany), containing microporous beads suspended in a special hypertonic preserving solution, ensuring long-term storage and preservation of microorganisms, including fastidious ones. Briefly, all bacterial strains were retrieved from fresh bacterial agar cultures, suspended in the preserving solution, mixed with the beads and, after removing excess of the solution, stored in a low-temperature freezer at −80°C. Prior to experiments, the bacterial strains under the study were revitalized in Trypticase Soy Broth medium (BD, Franklin Lakes, NJ, USA), a universal medium to culture various microorganisms, including aerobic and facultative anaerobic bacteria as well as fungi. Subsequently, bacterial suspensions were prepared from fresh, 24-hour cultures, to obtain inoculum 108 colony-forming unit (CFU)/mL. Antibacterial activity of the chemical agents was tested on the following bacterial species: bacterial ATCC reference strains, gram-positive coccus Staphylococcus aureus ATCC 25923; Streptococcus pyogenes ATCC 19615; Streptococcus mutans ATCC 35668; Streptococcus salivarius ATCC 13419; Enterococcus faecalis ATCC 29212; gram-negative coccus Moraxella catarrhalis ATCC 25238 and gram-negative rod Escherichia coli ATCC 25922 (facultative anaerobe); Campylobacter jejuni ATCC33560 (microaerophile). Tests were performed using clinical bacterial isolates, including MDR strains: S. aureus (isolates from skin and soft tissue infections); S. pyogenes (isolates from pharyngitis cases and skin infections); S. mutans and S. salivarius (isolates from periodontal pockets and oral mucous membranes); E. faecalis (isolates from urinary tract infections); M. catarrhalis (isolates from respiratory tract infections); E. coli (isolates from urinary tract infections and skin and wound infections); C. jejuni (isolates from feces of patients with diarrhea). Bacillus subtilis strains were isolated from food.
Assessment of bacterial susceptibility to the chemical agents
A quantitative method, microdilution in liquid (broth) medium performed on microtiter plates, was used to determine the antibacterial activity of the chemical agents under study. Briefly, the method allowed estimating in vitro minimal inhibitory concentrations (MICs, mg/L) and minimal bactericidal concentrations (MBCs, mg/L) of the tested agents. To that end, broth media containing serial dilutions of the chemical agents were inoculated with tested bacterial strains, and after 24-hour incubation the turbidity of the medium was recorded.19
Assessment of bacteriostatic activity
Susceptibility of non-fastidious bacteria and fastidious bacteria to the chemical agents under study was estimated on microtiter plates according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or Clinical and Laboratory Standard Institute (CLSI) guidelines. Briefly, Mueller-Hinton broth (MHB; Emapol, Gdansk, Poland) and MHB supplemented with 5% horse blood and 20 mg/L beta-nicotinamide adenine dinucleotide were used to culture non-fastidious bacteria and fastidious bacteria, respectively. The chemical agents were diluted with ethanol to obtain the stock solutions of concentration 400 mg/L. Subsequently, serial dilutions from 200 to 0.195 mg/L were prepared in the appropriate MHB and inoculated with bacterial suspensions to obtain the final inoculum 105 CFU/mL. The MIC values were recorded after 24-hour incubation at 35°C. The highest dilution that inhibits bacterial growth was assumed as MIC. All experiments were performed in triplicate.20–22
Assessment of bactericidal activity
MBCs that kill 99.9% of bacteria in the initial inoculum under standardized in vitro conditions were used to determine the bactericidal activity of the chemical agents. To that end, after the estimation of MICs, bacteria from the dilutions were subcultured on agar media (Columbia Agar and sheep blood; Oxoid Limited, Basingstoke, UK), and MBC values were recorded after 24-hour incubation at 35°C. The lowest concentration that killed at least 99.9% of bacteria was assumed as MBC. All experiments were performed in triplicate.23
Controls
The following internal quality controls were included in the experiments: control A – to determine the quality of bacterial growth in broth media without the chemical agents under study; control B – as the verification of the sterility of microbiological media; control C – to determine in vitro bacteriostatic activity (MIC) of Nor antibiotic for the bacterial strains under study as the reference control, which were performed simultaneously with the planned experiments with the chemical agents.
Red blood cell lysis
The hemolytic activity of compounds 11a–c, 12a–c, 13a–c and 14a–c (0–200 mg/L), against human red blood cells (Rockland Immunochemicals Inc., Limerick, PA, USA), was tested. Cells were suspended in phosphate-buffered saline (PBS) and incubated for 1 h at 37°C after the addition of test molecules. The hemoglobin concentration in supernatants after centrifugation at 2,000× g was monitored by measuring the absorbance at 540 nm. Then, 100% hemolysis (positive control) was taken from samples in which 2% Triton X-100 was added.24 The research protocol was approved by the Committee for Ethics and Supervision on Human and Animal Research, Medical University of Bialystok (Nr N/ST/ZB/15/001/2209 [153-09-535F]).
Cell culture fibroblasts
Human skin fibroblasts (CCD 1112Sk) were obtained from ATCC. Cells were maintained in Dulbecco’s Minimal Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 50 U/mL penicillin and 50 mg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Cells were cultured in Costar flasks and grown in 5% CO2 at 37°C in high humid atmosphere to subconfluence (90%–95%). Subconfluent cells were treated with 0.05% trypsin and 0.02% EDTA in calcium-free PBS, counted in a hemocytometer and seeded at 5×105 cells/well in six-well plates (Nunc) in 2 mL of growth medium. The cells were grown in a monolayer (80% confluence) before being used for further analysis.
Cell viability assay
The growth inhibitory effects of the test compounds against human skin fibroblast cells were measured by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Compounds were dissolved in dimethyl sulfoxide (DMSO) and screened at a range of concentrations against normal cells. The cytotoxic activity was determined after 24 h. All experiments were repeated at least three times.
Fluorescent microscopy assay
The cell viability was estimated 24 h after the addition of the study compounds to assess apoptosis. The cell suspension (250 μL) was stained with 10 μL of the dye mixture (10 μM acridine orange and 10 μM ethidium bromide), which was prepared in PBS. Acridine orange (fluorescent DNA-binding dye) intercalated into DNA, making it appear green, and bound to RNA, staining it red/orange. Ethidium bromide was only taken up by nonviable cells; its fluorescence predominated that of the acridine orange, making the chromatin of necrotic cells appear orange. Cells cultured in a drug-free medium were used as controls. Analysis was performed using Nikon Eclipse Ti inverted microscope, and the results were analyzed with NIS-Elements software (both from Nikon Instruments, Melville, NY, USA).
Statistical analysis
All numerical data are presented as mean ± SD from at least three independent experiments. Statistical analysis was conducted using the Origin 7.5 software (OriginLab Corporation, Northampton, MA, USA). Statistical differences in multiple groups were determined by one-way ANOVA followed by Tukey’s test. P<0.05 was considered statistically significant.
Results
Chemistry
We envisioned that a new type of spiro-QACs can be obtained via double alkylation of easily available, by our methodology, enantiopure amino alcohols with 1,2-bis(bromomethyl)benzene. To verify this idea, several 10b(S) configurated hexahydropyrroloisoquinolines (1–4) were obtained from L-tartaric acid using our procedure16,17 (Scheme 2). Cyclohexyl derivative 5 was prepared via Pd-catalyzed hydrogen reduction of a 10β-phenyl substituted compound 4. Therefore, obtained pyrroloisoquinolines were submitted to base hydrolysis of acetate esters. Isolated corresponding diols were oxidized with sodium periodate to give an epimeric mixture of cyclic hemiaminals type A.18
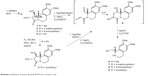  | Scheme 2 Synthesis of amino alcohols 6–9 from L-tartaric acid. |
The masked dialdehydes A treated with a strong base (NaOH) underwent an intramolecular, highly chemoselective Cannizzaro reaction, to give corresponding β-amino alcohols 6–9. Finally, the title quaternary N-spiro ammonium bromides 11a–14c were obtained by double alkylation reaction of enantiopure amino alcohols 6–9 with 1,2-bis(bromomethyl)benzene derivatives 10a–c (Scheme 3) in the presence of DIPEA.
  | Scheme 3 Synthesis of compounds 11a–14c. |
Antimicrobial activity
The antimicrobial activity of compounds 11a–14c was tested against gram-positive cocci of the genus Staphylococcus, Enterococcus and Streptococcus and gram-positive bacilli of the genus Bacillus. We also tested the antimicrobial activity of these compounds against gram-negative bacilli E. coli of the family Enterobacteriaceae and C. jejuni, as well as against gram-negative M. catarrhalis. Nor was used for the control to analyse tested strains for their susceptibility/resistance. The new quaternary ammonium salts inhibited the growth of more than 95% of gram-positive and gram-negative bacteria tested in the concentration range from 12 to 200 mg/L (Tables 1 and 2). The results of antibacterial activity showed that gram-negative bacteria were more susceptible toward the tested compounds than gram-positive bacteria. The compounds 11a–c showed MIC above 200 mg/L against S. aureus and B. subtilis. In contrast, the compound 12a at a concentration of 25 mg/L inhibited the growth of cocci S. aureus and S. mutans. Five of the compounds 12c, 13a, 13b, 14a, 14c showed inhibition of the growth of bacilli in the range of 50–200 mg/L.
  | Table 1 MIC of compounds 11a–14c on the studied bacterial strains (mg/L) |
  | Table 2 MBC of compounds 11a–14c on the studied bacterial strains (mg/L) |
We observed more differences in the MIC and MBC values of the tested compounds obtained from gram-negative bacteria. Most of the tested compounds inhibited the growth of E. coli at a concentration range of 100–200 mg/L. It was found that only 13c inhibited the growth of a strain of E. coli by the MIC =50 mg/L. The compounds 12a, 13a, 13b, 14a, 14b inhibited the growth of M. catarrhalis at the lowest concentration (MIC value between 15 and 50 mg/L). The compounds 11c, 12b, 12c, 13c, 14c inhibited the growth of C. jejuni at the lowest concentration (MIC in the range of 12–25 mg/L).
The structure–antimicrobial activity relationship of the synthesized compounds revealed that the presence of phenyl substituent at the quaternary carbon stereocenter in the compounds 12a–c and 13a–c leads to higher activity than those having cyclohexyl and methyl substituents. Among compounds 12 and 13, those having methoxyphenyl moieties showed greater activity than the compounds with bromophenyl units. Substitution of the phenyl ring in positions 3 and 4 at isoindoline moiety does not influence the antimicrobial activity significantly.
The selective antibacterial activity of the compounds is often a desirable feature. The on-target therapies are recommended antibiotic/chemotherapeutic agents with a narrow spectrum of activity directed against specific pathogens, without adversely affecting the physiologic flora saline environment. Because the MBC is usually a multiple of the MIC values, the ratio MBC/MIC is an additional indicator of antimicrobial activity. High antibacterial compounds have bactericidal activity ratio MBC/MIC ≤4. Compounds 11a–14c have MBC/MIC ratio of 1–4 for 100% of the strains (Table 3).
The very promising compound from the group of the tested compounds was 12a. It possessed stronger antimicrobial potential than Nor against S. mutans and B. subtilis thus might be an alternative to use in lower doses to receive a bactericidal effect. The compounds 11c, 12b, 12c, 14c were more active against C. jejuni in comparison with the control.
Evaluation of cell toxicity
Nonspecific insertion of antibacterial QACs into host cell membranes can cause toxicity. Host cell membrane permeabilization can be measured by the release of proteins such as hemoglobin from the cytosol to the extracellular space. We showed no significant membrane permeabilization in the range at which the compounds 11a–14c have bactericidal activity by evaluating hemoglobin release (Figure 1). However, an increase in hemoglobin release was observed with increasing concentration. The compound 11c was the strongest hemolytic agent, but even for 11c, bactericidal concentrations against C. jejuni were below its minimal hemolytic concentration.
Viability of human skin fibroblasts
The viability of human skin fibroblasts was determined after 24 hours of incubation with tested compounds. Our studies revealed that all compounds were not cytotoxic for normal cells. The viability in all cases was similar to control (untreated cells). We detected more than 95% of viable cells after 24 hours of incubation with compounds 11a–14c used in three concentrations: 50, 100 and 200 μM (Figure 2). To check the influence of tested compounds on apoptosis, we carried out an assessment of the dual acridine orange/ethidium bromide fluorescent staining, and visualized it under a fluorescent microscope (Figure 3). We can observe that live cells with normal nuclei appeared uniformly green in the control as well as after treatment with compounds 11a–14c using the high concentration (200 μM). Dual acridine orange/ethidium bromide fluorescent staining confirmed the results obtained by cell viability assay. We proved that all compounds had no effect on induction of apoptosis or necrosis in normal cells.
Discussion
Our novel chiral quaternary N-spiro ammonium bromides with 3′,4′-dihydro-1′H-spiroisoindoline-2,2′-isoquinoline skeleton possess antimicrobial activity against gram-positive and gram-negative bacteria. We observed that the inhibition of the growth of selected bacteria depends on the concentration of tested compounds and there were differences between sensitivity of selected bacteria species.
A majority of the synthesized compounds show the strongest antibacterial properties toward some gram-negative bacteria (M. catarrhalis and C. jejuni) with a high level of selectivity. It has been shown for many years that QACs are either membrane-active agents or the plasma membrane agents in yeasts.25 On the model proposed by Salton, microorganisms exposed to these cationic agents follow the specific sequence of events. The agent is adsorbed and penetrated into the cell wall, then the reaction with cytoplasmic membrane occurs followed by membrane disorganization. The consequence of these molecular events is the degradation of proteins and nucleic acids and wall lysis caused by autolytic enzymes.26,27
The composition of cell membrane layers depends on the organism type and may act as a permeability barrier, in which there may be a reduced uptake.28–32 Teichoic acid and peptidoglycan are major components of staphylococci cell wall.6,33–38 None of these compounds are an effective barrier to the entry of antiseptics and disinfectants. The high molecular weight substances can easily pass through the cell wall of staphylococci and vegetative Bacillus spp., and it could explain the high sensitivity of these strains to QAC agents.30,39 Studies based on staphylococci and gram-negative bacteria as well as mutants of E. coli and S. typhimurium proved that the outer membrane of gram-negative bacteria acts as a barrier that limits the entry of different types of antibacterial agents.40–43 The cell membrane, lipopolysaccharides, in wild-type gram-negative bacteria act as a barrier which limits the access of hydrophobic molecules to phospholipid and thence to the cell interior. Apart from the hydrophilic and hydrophobic input paths, the third way was proposed to be by cationic agents such as QAC and biguanides. It is argued that they damage the outer membrane, thus fostering their own uptake.6,44 Our in vitro data confirmed that all tested compounds do not possess cytotoxic and proapoptotic potency in normal cells such as human skin fibroblasts. Our study needs further examination to explain in detail the molecular mechanisms of action of chiral quaternary N-spiro ammonium bromides with 3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinoline] skeleton. In further studies, we will try to check whether their mechanism of action appears to be associated with damage to the cell membrane or whether also to inhibition of DNA replication or binding to other components of the cell.
Conclusion
Our findings proved that all novel tested chiral quaternary N-spiro ammonium bromides with 3′,4′-dihydro-1′H-spiro[isoindoline-2,2′-isoquinoline] skeleton inhibited the growth of more than 95% of gram-positive and gram-negative bacteria tested in the concentration range from 12 to 200 mg/L. A majority of the synthesized compounds show the strongest antibacterial properties toward some gram-negative bacteria (M. catarrhalis and C. jejuni) with a high level of selectivity. High antibacterial compounds have bactericidal activity ratio MBC/MIC ≤2. Our studies revealed that all tested compounds are active only toward bacteria, but do not possess cytotoxic and proapoptotic activity in normal cells such as human skin fibroblasts.
Acknowledgments
We thank Dr Paweł Czerwiński for the help in the synthesis of selected compounds. The authors are thankful to the National Science Centre (Grant DEC-2012/07/B/NZ7/04382) for financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119(6 suppl 1):S3–S10. | ||
Lee CR, Cho IH, Jeong BC, Lee SH. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health. 2013;10(9):4274–4304. | ||
Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283. | ||
Hawkey PM. The changing epidemiology of resistance. Antimicrob Chemother. 2009;64(suppl 1):3–10. | ||
McBain AJ, Ledder RG, Moore LE, Catrenich CE, Gilbert P. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl Environ Microbiol. 2004;70(6):3449–3456. | ||
Maillard JY. Bacterial target sites for biocide action. J Appl Microbiol. 2002;92(suppl):16S–27S. | ||
Jennings MC, Minbiole KP, Wuest WM. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect Dis. 2015;1(7):288–303. | ||
Croaker A, King GJ, Pyne JH, Anoopkumar-Dukie S, Liu L. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17(9):1414. | ||
Matkar SS, Wrischnik LA, Hellmann-Blumberg U. Production of hydrogen peroxide and redox cycling can explain how sanguinarine and chelerythrine induce rapid apoptosis. Arch Biochem Biophys. 2008;477(1):43–52. | ||
Southard GL, Boulware RT, Walborn DR, Groznik WJ, Thorne EE, Yankell SL. Sanguinarine, a new antiplaque agent: retention and plaque specificity. J Am Dent Assoc. 1984;108(3):338–341. | ||
Peng L, Kang S, Yin Z, et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int J Clin Exp Pathol. 2015;8(5):5217–5223. | ||
Collier HO, Potter MD, Taylor EP, et al. Antibacterial activities of some bisisoquinolinium salts. Br J Pharmacol Chemother. 1953;8(1):34–37. | ||
Babbs M, Collier HO, Austin WC, et al. Salts of decamethylene- bis-4-aminoquinaldinium (“Dequadin”), a new antimicrobial agent. J Pharm Pharmacol. 1956;8(2):110–119. | ||
D’Auria FD, Simonetti G, Strippoli V. Antimicrobial characteristics of a tincture of dequalinium chloride. Ann Ig. 1989;1(5):1227–1241. | ||
Hugo WG, Frier M. Mode of action of the antibacterial compounds dequalinium acetate. Appl Microbiol. 1969;17(1):118–127. | ||
Mostowicz D, Wójcik R, Dołęga G, et al. Diastereoselective synthesis of 10b-substituted hexahydropyrroloisoquinolines from L-tartaric acid. Creation of a quaternary carbon stereocentre via N-acyliminium ion cyclization. Tetrahedron Lett. 2004;45:6011–6015. | ||
Kałuża Z, Mostowicz D, Dołęga G, et al. 10b-Substituted hexahydropyrroloisoquinolines: studies on diastereoselective formation of a quaternary carbon stereocenter via N-Acyliminium ion cyclization. Tetrahedron. 2006;62:943–953. | ||
Kałuża Z, Mostowicz D, Dołęga G, et al. A new route to optically pure highly functionalized tetrahydroisoquinolines with a quaternary carbon stereocenter. Tetrahedron. 2008;64:2321–2328. | ||
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. | ||
European Committee on Antimicrobial Susceptibility Testing [homepage on the Internet]. Breakpoints Tables for Interpretation of MICs and Zones Diameters. Version 5.0. 2015. Available from: http://www.eucast.org. Accessed June 14, 2017. | ||
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard. 9th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. CLSI document M07-A9. | ||
Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. | ||
Jorgensen JH, Turnidge JD. Susceptibility test methods: dilution and disk diffusion methods. In: Jorgensen J, Pfaller M, Carroll K, Funke G, Landry M, Richter S, Warnock D, editors. Manual of Clinical Microbiology. Eleventh ed. Washington, DC: ASM Press; 2015:1253–1273. | ||
Evans BC, Nelson CE, Yu SS, et al. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J Vis Exp. 2013;9(73):e50166. | ||
Gerba CP. Quaternary ammonium biocides: efficacy in application. Appl Environ Microbiol. 2015;81(2):464–469. | ||
Salton MR. Lytic agents, cell permeability, and monolayer penetrability. J Gen Physiol. 1968;52(1):227–252. | ||
Lambert PA. Mechanisms of action of biocides. In Fraise AP, Lambert PA, Maillard JY, editors. Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization. 4th ed. Oxford: Blackwell Publishing Ltd; 2004:139–153. | ||
Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J Appl Microbiol. 2002;92:55S–64S. | ||
Cloete TE. Resistance mechanisms of bacteria to antimicrobial compounds. Int Biodeterior Biodegradation. 2003;51:277–282. | ||
Russell AD. Similarities and differences in the responses of microorganisms to biocides. J Antimicrob Chemother. 2003;52(5):750–763. | ||
Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67(4):593–656. | ||
Gilbert P, McBain AJ. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin Microbiol Rev. 2003;16(2):189–208. | ||
Borges-Walmsley MI, Walmsley AR. The structure and function of drug pumps. Trends Microbiol. 2001;9(2):71–79. | ||
De Oliveira FA, Brandelli A, Tondo EC. Antimicrobial resistance in Salmonella enteritidis from foods involved in human salmonellosis outbreaks in southern Brazil. New Microbiol. 2006;29(1):49–54. | ||
Denyer SP, Maillard JY. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J Appl Microbiol. 2002;92:35S–45S. | ||
Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. | ||
Davin-Regli A, Pagès JM. Cross-resistance between biocides and antimicrobials: an emerging question. Rev Sci Tech. 2012;31(1):89–104. | ||
Gilbert P, McBain DGA. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J Appl Microbiol. 2002;92:98–110. | ||
Aiello AE, Larson E. Antibacterial cleaning and hygiene products as an emerging risk factor for antibiotic resistance in the community. Lancet Infect Dis. 2003;3(8):501–506. | ||
Walsh SE, Maillard JY, Russell AD, Catrenich CE, Charbonneau DL, Bartolo RG. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J Appl Microbiol. 2003;94(2):240–247. | ||
Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria. Drugs. 2004;64(2):159–204. | ||
Singla R, Goel H, Ganguli A. Novel synergistic approach to exploit the bactericidal efficacy of commercial disinfectants on the biofilms of Salmonella enterica serovar Typhimurium. J Biosci Bioeng. 2014;118:34–40. | ||
Svetlíková Z, Skovierová H, Niederweis M, Gaillard JL, McDonnell G, Jackson M. Role of porins in the susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to aldehyde-based disinfectants and drugs. Antimicrob Agents Chemother. 2009;53(9):4015–4018. | ||
Tattawasart U, Hann AC, Maillard JY, Furr JR, Russell AD. Cytological changes in chlorhexidine-resistant isolates of Pseudomonas stutzeri. J Antimicrob Chemother. 2000;45:145–152. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.