Back to Journals » Drug Design, Development and Therapy » Volume 9
Synergistic antitumor effect of adenovirus armed with Drosophila melanogaster deoxyribonucleoside kinase and nucleoside analogs for human breast carcinoma in vitro and in vivo
Authors Tang M, Zu C, He A, Wang W, Chen B, Zheng X
Received 28 January 2015
Accepted for publication 27 February 2015
Published 14 July 2015 Volume 2015:9 Pages 3301—3312
DOI https://doi.org/10.2147/DDDT.S81717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Shu-Feng Zhou
Miao Tang,1 Cong Zu,2 Anning He,2 Wenqian Wang,1 Bo Chen,1 Xinyu Zheng1,2
1Department of Breast Surgery, The First Hospital of China Medical University, 2Laboratory 1, Cancer Institute, China Medical University, Shenyang, People’s Republic of China
Background: Suicide gene therapy in cancer can selectively kill tumors without damaging normal tissues. Drosophila melanogaster multisubstrate deoxyribonucleoside kinase (Dm-dNK), an original suicide kinase, makes use of the carcinomatous suicide gene therapy for broader substrate specificity and a higher catalytic rate.
Methods: To enhance the anti-tumor efficacy of Dm-dNK and maintain its substrate specificity and safety control in the meantime, the conditionally replicative gene–viral system, ZD55–dNK (which contains the selective replication adenovirus, ZD55, encoded with Dm-dNK), was investigated in pushing a deeper development of this strategy. Selective replication, cell killing efficacy, and cytotoxicity, in combination with chemotherapy, were applied to two breast cell lines (MDA231 and MCF7 cells), two normal cell lines (WI38 and MRC5 cells), and the MCF7 xenograft model in vivo.
Results: The preclinical study showed that ZD55–dNK, combined with 2',2'-difluorodeoxycytidine (DFDC), synergistically inhibited adenovirus replication in vitro but maintained specifically cancer cell killing efficacy. ZD55–dNK also greatly improved the antineoplastic effect in vitro and in breast cancer xenograft in vivo.
Conclusion: The concomitant use of ZD55–dNK and DFDC is possibly a novel and promising approach to breast cancer treatment, and further investigation on the safe control of excessive virus replication and the efficacy of this approach in humans is warranted.
Keywords: Dm-dNK, oncolytic adenovirus, cancer suicide gene therapy, nucleoside analogs, safety control
Introduction
Breast carcinoma is the principal cause of cancer deaths in women and the most frequently diagnosed cancer globally.1 A significant number of new anticancer agents, including chemotherapy, radiation treatment, and hormonal therapy for hormone-receptor-positive cancers, bisphosphonates, and adjuvant chemotherapy (trastuzumab) for human epidermal growth factor receptor 2-overexpressing patients, have been developed and shown to be beneficial to the treatment of breast cancer. However, side effects are commonly observed and the strategies of these treatments are not effective in all patients. Moreover, treatments are limited by the life expectancy in patients with metastatic disease. The curative rate of advanced or recurrent breast cancer is less than 5%.2 Therefore, novel gene therapy has promise as an alternative choice of treatments in breast cancer.
Suicide gene therapies for cancer have been thoroughly developed and became increasingly important during the past 20 years since incipiency as a type of gene therapy.3 The suicide gene system of killing neoplastic cells aims to deliver an enzyme containing a gene to the cancer locations, followed by systemic management of a prodrug that is transformed locally to a cytotoxic form through the duplication of the gene. Therefore, the cells expressing the enzyme and the neighboring “bystander” cells are killed.4 The most commonly accepted suicide gene system is herpes simplex virus thymidine kinase/ganciclovir (HSVtK/GCV). GCV, a kind of antiviral drug, can be phosphorylated by HSVtK to its monophosphate form, which is quickly shifted to diphosphate and triphosphate forms by cellular kinases, the latter of which is virulent to cells. The GCV triphosphate is merged into DNA during cell division, causing single-strand DNA breaks and inhibition of DNA polymerase, which results in programmed cell death.5 Nonetheless, the potency of suicide gene therapy for treatment of carcinoma in humans is still low because of its limited efficacy and safety.6
The multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster (Dm-dNK), purified from D. melanogaster, has been used as a classic suicide gene and valued for its efficiency in multiple cancer cells.7,8 Dm-dNK has an advanced catalytic rate and extensive substrate specificity. In previous studies, Dm-dNK was considered as a suicide gene in human gastric and breast cancer cell lines by means of a replication-deficient retroviral vector.9,10 In view of the prior findings, we developed a conditional replicative adenoviruses vector (pZD55)11 armed with Dm-dNK. This recombinant adenovirus, named pZD55–dNK, is a brand-new E1B 55 kDa-deficient adenovirus that resembles ONYX-015 and can only replicate in p53-mutant cells. Because E1B 55 k mainly inhibits p53-dependent pathways, E1B 55 k can make adenovirus conditionally duplicate in a large number of carcinoma cells and gives rise to oncolysis effects similar to that of ONYX-015. As pZD55–dNK virus replicates, the level of expression of Dm-dNK can accelerate.12 Additionally, the cytomegalovirus (CMV) core promoter, which was inserted into the Dm-dNK expression cassette, increased the expression in our study.
Dm-dNK was evaluated as a suicide gene using a replication-deficient retroviral vector by Zheng et al8 who detected that human cells can express Dm-dNK and that its enzyme maintains the enzymatic activity. The cells expressing Dm-dNK displayed an increasing susceptibility to some cytotoxic nucleoside analogs, among which (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU) and 2′,2′-difluorodeoxycytidine (DFDC)13,14 were the most efficient prodrug candidates. Furthermore, nucleoside analogs, such as BVDU, the triphosphate form of which is BVDU-TP, can compete with the substrate deoxynucleotide for the viral DNA polymerase. BVDU-TP can restrain the incorporation of deoxynucleotide into the viral DNA, though incorporating itself as a rotational substrate, thus inducing deficient viral DNA on the structure and function.15 As a result, the conditional replicative adenovirus, ZD55–dNK, combined with cytotoxic nucleoside analogs, such as BVDU or DFDC, will possibly induce a synergistic inhibition impact on adenovirus replication, and then keep specific killing activity to reduce the side effects and enhance the therapeutic index of the virus as much as possible at the same time. In addition, the therapeutic potential of combining conditional enzymatic activity of the suicide gene product, Dm-dNK, induced by prodrugs with their feasible synergic impact is reasonable.
Materials and methods
Cell lines and cell culture
Adenovirus packing the human embryonic kidney HEK293 cell line was obtained from Microbix Biosystems, Ltd. (Toronto, Canada). Human breast cell lines (MDA231, estrogen receptor negative, ERα+/ERβ+ and MCF7, estrogen receptor positive, ERα+/ERβ+) were purchased from Shanghai Cell Collection (Shanghai, People’s Republic of China). The normal human embryonic lung fibroblast cells (MRC5 and WI38) were purchased from the American Type Culture Collection (Manassas, VA, USA).
MDA231 cells were cultured in Leibovitz’s L-15 Medium (Invitrogen, Carlsbad, CA, USA). MCF7, WI 38, MRC5, and HEK293 cells were cultured in high glucose Dulbecco’s Modified Eagle’s Medium containing 4 mmol/L l-glutamine, 100 units/mL of penicillin, and 100 μg/mL of streptomycin, and 10% heat-inactivated fetal bovine serum at 37°C with a gas phase of 5% CO2.
Virus construction and production
The following plasmids were used in our experiments, including pXC1 (the wild type adenovirus plasmid) from Microbix Biosystems, Ltd. (Toronto, Canada), pZD55 (blank virus vector), pZD55-EGFP (E1B 55-kD gene was deleted in the plasmid as ONYX-015), PENTR12, PENTR13, and PPE3-RC. All of the above plasmids were purchased from Sino-gene Ltd. (Shanghai, People’s Republic of China).
The upstream primer, 5′-CCG GAA TTC (EcoRI) ACC ATG GCG GAG GCA-3′, and the downstream primer, 5′-CGC GGA TCC (BamHI) TCA TTA TCT GGC GAC-3′, was utilized for amplification of cDNA sequence of the Dm-dNK gene by polymerase chain reaction (PCR) from pLXSN-dNK. The synthetic DNA sequence was released by endonucleases (EcoRI and BamHI; New England Biolabs, Beverley, MA, USA) and then ligated into pENTER12 (Sinogene Scientific Co., Ltd., Beijing, People’s Republic of China) containing the sequence of the CMV promoter to generate pENTER12-dNK. PPE3-RC and pENTER12-dNK were recombined with LR enzyme (Invitrogen, Carlsbad, CA, USA). PPE3-CMV-dNK was acquired by inserting the dNK gene into the PPE3-RC in an orthograde direction. pZD55 and PPE3-CMV-dNK were co-transfected into HEK293 cells through Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA). After 9–14 days of transfection, viral plaques emerged and were sublimated three times. After collecting the virus by the QIAamp DNA blood kit (Qiagen, Valencia, CA, USA), recombinant adenoviruses were verified by PCR assay and then entitled ZD55–dNK (virus vector with the Dm-dNK gene). The replicative adenovirus (WtAd) and replicative deficiency adenovirus (AdGFP) were applied as the control.
Reverse-transcription polymerase chain reaction assay
To estimate Dm-dNK expression, MDA231, MCF7 (breast cancer cell line), MRC5, and WI38 (normal human embryonic lung fibroblast cell line) were infected with ZD55–dNK and ZD55 at a multiplicity of infection (MOI) of 1.0 pfu/cell for 48 hours in the 24-well plates (5×104 cells/well). The cells were harvested and washed in 1× phosphate-buffered saline (PBS). Total RNA was extracted from infected cells by TRIZOL (Invitrogen). Then, 1 μg of total RNA and the oligo (dt) primer was applied for reverse-transcription polymerase chain reaction (RT-PCR) assay utilizing Two Step RT-PCR kit (TakaRa, Otsu, Japan), and the procedure was done according to the manufacturer’s instructions. Dm-dNK gene was amplified by PCR in the following process: 94°C for 4 minutes, 35 cycles at 94°C for 1 minute each, 60°C for 1 minute, and 1.5 minute at 72°C. The GAPDH primers were: sense, 5′-ACC ACA GTC CAT GCC ATC AC-3′; and antisense, 5′-TCC ACC ACC CTG TTG CTG TA-3′. The Dm-dNK primers were: sense, 5′ CCG GAA TTC ACC ATG GCG GAG GCA 3′; and antisense, 5′ CGC GGA TCC TCA TTA TCT GGC GAC 3′. Finally, 1.5% agarose gel electrophoresis was done for visualing the amplification products.
Infection efficiency analysis
To estimate the infection efficacy, MDA231, MCF7, MRC5, and WI38 cells were infected with AdGFP at a multiplicity of infection of 10 pfu/cell for 3 days in the 24-well plates (5×104 cells/well). The green fluorescence was observed by the fluorescence microscope.
Enzyme assays
Normal cell lines (MRC5, WI38) and breast cancer cell lines (MDA231, MCF7) were cultured in six-well plates at a density of 5×105 cells/well. After 48 hours of infection with ZD55–dNK and ZD55 at a MOI of 1 pfu/cell, cell proteins were extracted as previously described.16 The experiments were carried out in a mixture consisting of 50 mM Tris-HCl (pH 7.6), 5 mM MgCl2, 5 mM adenosine triphosphate, 2 mM dithiothreitol, 15 mM NaF, 100 mM KCl, 0.5 mg/mL of bovine serum albumin, 2.5 μL cold ThD, and 0.6 mg protein extracts in a total volume of 35 μL. Methyl-3HdThd (2.5 mM; Moravek Biochem, Brea, CA, USA) was induced and blended with the same amount of unlabeled substrates. After 10 minute, 20 minute, and 30 minute incubations at 37°C, the reaction mixture was dropped on Whatman DE-81 filters. The Whatman filters were rinsed three times by 5 mM ammonium formate for 15 minutes and the radioactivity was detected using a scintillation counter.
Cytotoxic effect of ZD55–dNK plus prodrug
Exponentially growing cells (MDA231, MCF7, WI38, and MRC5) were seeded at a density of 1×104 cells/well in 96-well plate (Corning, New York, USA). The cells were infected 24 hours later with pZD55–dNK, pZD55, DL1520, and WtAd in 200 μL of serum-free culture medium per well at serial MOIs (0.01–100). After 7 days of incubation, cell viability was examined by methylthiazol tetrazolium assay (MTT) to decide the oncolytic effectiveness of the viral infection. ZD55–dNK, ZD55, DL1520, and WtAd at a MOI of 10 were added to each well. The viral inocula were discarded after 2 hours. The cells were rinsed twice with PBS and incubated at 37°C for 3 days, and nucleoside prodrugs (BVDU or DFDC) were added at concentrations of 0 μM, 0.01 μM, 0.1 μM, 1 μM, and 10 μM; after an additional 4 days (7 days total). The cell viability was assessed by MTT to conclude the combined cytotoxic effects. The absorbance was examined by an enzyme immunoassay instrument at 570 nm to determine cell viability. The cytopathological effects on cells were captured using an inverted microscope (Olympus, Tokyo, Japan).
In vitro apoptosis assays
Induction of apoptosis was analyzed by flow cytometer with an Annexin V-FITC kit (Genmed, Shanghai, People’s Republic of China). Briefly, MDA231, MCF7, MRC5, and WI38 cells were seeded in six-well plates at a density of 5×105 cells/well and cultured for 24 hours, followed by transduction with ZD55–dNK at a MOI of 1 pfu/cell. After 3 days of transduction, DFDC were treated for 4 days, then trypsinized, pelleted, and rinsed by PBS. Cells were resuspended in 1× binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, and 2.4 mM MgCl2) and stained with 5 μL Annexin V-FITC (20 μg/mL) and 10 μL propidium iodide (PI, 50 μg/mL) for 10 minutes at room temperature away from the light. The ratio of apoptosis was assessed by a FACScan flow cytometer equipped with CELLQUEST and ModFITLT for Mac (V1.01 software; Becton-Dickinson, San Jose, CA, USA).
Viral duplication assay
MBA231, MCF7, MRC5, and WI38 cells were seeded in six-well plates (105 cells/well) 1 day before viral transduction and then infected with pZD55–dNK, pZD55, DL1520, or WtAd (MOI =1). Virus inocula were gotten rid of after 2 hours. The cells were rinsed twice with PBS and incubated at 37°C for 3 days, and treated with nucleoside prodrugs (BVDU or DFDC) at a 1 μM for an additional 4 days (7 days of therapy). The cells and media were collected after 3 days and 7 days, with three freeze–thaw cycles. Successive dilutions of the lysates were titered on human embryonic kidney 293 cells using a tissue culture infectious dose (TCID)50 assay,17 and normalized with the beginning of infection, and reported as multiples.
Antineoplasm efficacy of Dm-dNK/nucleoside analog system in vivo
BALB/C nude mice, 6–8 weeks old, were obtained from Vital River Laboratory Animal Technology Co., Ltd (Beijing, People’s Republic of China). All animals were housed in a specific pathogen-free environment and were maintained on standard mouse chow at an environmental temperature of 24°C ±1°C and a 12/12 hour light/dark cycle with water ad libitum. All animal experiments were approved by the Animal Care and Use Committee of China Medical University, which complies with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The breast carcinoma cell line, MCF7 (1×107 cells suspended in 100 μL of PBS), were subcutaneously injected into the right flank of each of 24 mice. Mice were randomly divided into three groups (eight mice per group), including PBS control (group A), ZD55–dNK (group B) and DFDC with ZD55–dNK (group C). When neoplasms achieved 100–150 mm3, 109 pfu of adenovirus were injected intratumorally three times at 48-hour intervals, and DFDC was injected (10 mg/kg intraperitoneally twice on day 2 and day 8) after virus injection. The neoplasm size was monitored by calipers every 5 days, for 50 days. The neoplasm volume was calculated by the following formula: V (mm3) = ½ length (mm) × width (mm)2. Survival analysis expressed as time to progression was performed according to the method of Kaplan–Meier (log rank test for statistical significance).
Statistical analysis
All data were analyzed with SPSS statistical software version 19.0. All data are expressed as means ± SD. Tumor growth curves were compared using one-way analysis of variance for significance. A P-value of less than 0.05 was considered statistically significant.
Results
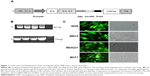
Construction and infection efficacy of recombinant adenovirus
An expression cassette encoding Dm-dNK gene controlled by human CMV promoter was plugged into plasmid ZD55 to produce plasmid ZD55–dNK, a kind of wild-type 5 adenovirus, where the viral E1B 55-kDa gene was removed (Figure 1A). Adenovirus ZD55–dNK was affirmed by DNA using PCR. Electrophoresis indicated the extent of the CMV promoter was 521 bp, and the Dm-dNK sequence was 779 bp (Figure 1B). The infection efficacy of the cell lines was decided through the wild-type 5 adenovirus with the expression of green fluorescence (Ad5GFP) (Figure 1C). The neoplastic cell lines (MDA231 and MCF7) and the nonneoplastic cell lines (WI38 and MRC5) revealed similar infection efficacy. The fluorescence-activated cell sorting (FACS) analysis results also indicated that there were no significant differences in the infection efficiency among the MDA231, MCF7, WI38, and MRC5 cells (P>0.05, Figure S1).
Expression and enzymatic activity of Dm-dNK after transduction
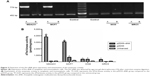
ZD55–dNK expressing the Dm-dNK gene had a distinctly increasing efficiency in neoplastic cells (MDA231 and MCF7) in comparison with the nonneoplastic cells (WI38 and MRC5). RT-PCR results showed the alternative expression of ZD55–dNK at a MOI =1 after 2 days of infection (Figure 2A). In the experiment of the enzyme assay, enzyme activity was decided by the phosphorylation of dThd, and the cells infected by ZD55 and mock cells indicated negligible levels of dThd phosphorylation, while cells infected by ZD55–dNK raised enzymatic activity approximately 30-fold compared with the parent cell line of MCF7. Expression of Dm-dNK revealed dThd kinase activity that was 40-fold higher than the 231 parent cells as well. In the normal cell lines (WI38 and MRC5), the activity of cells infected by ZD55–dNK decreased 10-fold relative to cancer cells; the cause might be alternative expression of ZD55–dNK (P<0.05, Figure 2B). This study demonstrated that human breast carcinoma cells transduced with ZD55–dNK showed enzymatically-active Dm-dNK and reserved enzymatic activity. Eventually, the expression led to a rise in nucleoside and nucleoside analog phosphorylation.
Alternative duplication of ZD55–dNK virus in breast carcinoma cells
The MTT and TCID50 titer assays were performed to determine the selective cytotoxicity of ZD55–dNK. ZD55–dNK infected breast carcinoma cell lines (MDA231 and MCF7) and human normal cell lines (WI38 and MRC5) at all kinds of viral MOI for 5 days. The results shown in Figure 3A demonstrate that ZD55–dNK not only showed more apparent cytolysis against MDA231 and MCF7 in comparison with DL1520 and WtAd infection (P<0.05), but also showed less cytotoxicity than WtAd against WI38 and MRC5. All kinds of cell lines were transduced with ZD55–dNK, ZD55, DL1520, and WtAd at a MOI of 1 for 48 hours as well. The data from the TCID50 titer assay indicated that the duplication potency of ZD55–dNK in the neoplastic cell lines was from 25-fold to 30-fold higher than the nonneoplastic cell lines (P<0.05, Figure 3B).
Alternative cell cytotoxic activity of ZD55–dNK in combination with prodrugs
The synergistic impact on cell toxicity by combining ZD55–dNK with the chemotherapy medicine, BVDU, and DFDC relative to ZD55, DL1520, or WtAd with medicine was investigated in the breast neoplastic cell line (MCF7) and the human non-neoplastic cell line (MRC-5). The experiment of MTT assay showed that ZD55–dNK in the presence of prodrugs had an ability to destroy breast carcinoma cells more effectively than additional three viruses, on the contrary in the non-carcinoma MRC-5 cells, ZD55–dNK with drugs decreased cytotoxic activity compared with the additional viruses (P<0.05, Figure 4A). DFDC was more sensitive to ZD55–dNK than BVDU. The derivatives DFDC-TP phosphorylated by ZD55–dNK restrained duplication of the adenovirus. The optical patterns of the cytopathological effect verify these data (Figure 4B). The FACS assay sustains the finding of MTT as well (Figure 4C). The ZD55–dNK (MOI =1) with 10 μM DFDC led to the greatest amount of cell apoptosis (86%±10%, right upper and lower quadrants) in the MCF7 carcinoma cells, which was 50% greater than ZD55 and DL1520 with DFDC. In the MRC5 cell line with 10 μM DFDC, apoptosis of 10%±3% was mediated by ZD55–dNK, which was obviously lower than ZD55, DL1520, and WtAd.
ZD55–dNK combined with DFDC restrained duplication of adenovirus
The duplication of ZD55–dNK with medicine was evaluated with the TCID50 assay in MCF7 cancer cells and normal MRC5 cells. The titers of the ZD55–dNK virus with BVDU and DFDC were extremely lower (approximately 1,000-fold) than the standard of viruses (ZD55, DL1520, and WtAd) without or with prodrugs in MCF7 cells. Moreover, the inhibition of DFDC was apparently greater in ZD55–dNK than BVDU (Figures 5A and B).
Antineoplastic efficacy of ZD55–dNK with DFDC in xenograft mice
The anticancer efficacy of combination treatment was assessed in the MCF7 xenograft model in vivo with subcutaneous neoplasms in treatment with ZD55–dNK with or without DFDC (Figure 6A). Statistically significant antitumoral effect with respect to neoplasm size was examined the ZD55–dNK in the DFDC-treated groups (1295.03±326.63 mm3) compared with ZD55–dNK virus only (2670.78±159.43 mm3) or the mock control group (3101.29±336.61 mm3, P<0.05). Furthermore, the mean survival time of group PBS, ZD55–dNK, and DNK + DFDC were 22 days, 30.5 days, and 52.5 days, respectively. Moreover, group C significantly prolonged the survival time compared to the group B (P=0.0146, Figure 6B). These data demonstrate that combination therapy of MCF7 xenografts with ZD55–dNK/DFDC elicited a significant antitumor effect.
Discussion
Over the last two decades, the morbidity and death rate from breast cancer in the People’s Republic of China has markedly risen.18 Although, certain current improvements in chemotherapy and endocrine treatment have produced moderate enhancements in survival, particularly in patients with terminal disease, with remarkable side effects from advanced treatment.19 As a result, novel therapeutic methods should be investigated. Gene-directed enzyme/drug treatment furnishes a novel approach for therapy of malignant neoplasms. In our study, oncolytic adenoviral vector combined with the suicide gene, Dm-dNK, was applied to establish ZD55–dNK. The efficiency of ZD55–dNK in terms of replication capacity, gene expression, and anticancerous cytotoxic effects in breast cancer cell lines, was examined in vitro. The antineoplastic impact on the combination of ZD55–dNK and chemotherapeutic agents, such as DFDC and BVDU, was inspected as well.
In our present study, the duplication productivity of ZD55–dNK can definitely duplicate and lead to prominent cytopathological effect in mammary carcinoma cells, with minimal influence in noncancerous cells. Moreover, ZD55–dNK revealed apparently powerful enzyme activity in breast cancer cells, but marginal enzyme activity in noncancerous cells, indicating that this oncolytic adenovirus could target malignant cells specifically. The MTT assay results showed that the nucleoside analogs exhibited lower toxicity to nontransduced cells. However, the cells conveying Dm-dNK had higher sensitivity to the compound, in particular for DFDC. Additionally, FACS data identified that apoptotic events primarily led to the cytotoxic impact on ZD55–dNK in combination with DFDC.
It is well recognized that the dose of chemotherapy is limited by the associated side effects. In our study, we combined ZD55–dNK and BVDU or DFDC together. However, as a therapeutic drug, the extreme dose of prodrugs that was used must preserve the normal cells. In this study, ZD55–dNK was utilized in combination with DFDC and had an ability to kill breast cancer cells. In following studies, we would explore the optimal lowest dosage of DFDC, without severe toxicity in normal cells. We speculate that this therapeutic strategy would have an up-and-coming synergistic impact on killing cancer cells, but protecting normal cells.
Our data displayed that ZD55–dNK plus DFDC caused cancerous cell destruction more dramatically than ZD55, DL1520, or WtAd with or without combining with the prodrugs. Interestingly, the adenovirus titers were obviously decreased after supplying the prodrugs in vitro (Figure 5). We viewed that the DFDC conversion phosphorylated by Dm-dNK probably caused apoptosis to the adenovirus as well. The replication of adenovirus could be administered through several kinds of Dm-dNK that contain nucleoside analogs to preserve the normal cells. Several reports have showed that the antitumor effect of HSVtK expressing through oncolytic adenovirus was augmented by GCV therapy.20,21 However, some other research pointed out that GCV did not promote more oncolytic capabilities of duplicating adenovirus.22,23 The controversy over the use of GCV is possibly because GCV-TP not only killed the cells, but also repressed the virus vector expression of HSVtK successively by leading to the apoptosis of virus, which is identical to our findings.
Recently, there has been a focus on exploiting a delivery system to maximize the efficiency of producing toxic metabolites.24 The probability of applying specific promoters to tumors will facilitate this treatment, for instance ribonuclease reductase 2 promoters25 for selective gene expression in breast carcinoma, carcinoembryonic antigen promoter for gene expression in colon cancer,26 and human telomerase reverse-transcriptase promoter could be applied to administrate selective transgene expression in a large majority of cancerous cells.27 It might be possible to improve the specificity of treatment in breast carcinoma by means of incorporation with promoter afterward. In addition, dual suicide gene systems can be applied to enhance the efficacy of killing of cancer cells.28 Perhaps some other strategies could be combined with gene-directed enzyme or drug systems, such as immunologic instruments.29,30 The next step of development in cancer therapeutics is to determine the optimal way to maximize the toxicity for cancer cells, but to minimize the side effects on normal cells.
Conclusion
We showed the effects of conditional replicative adenovirus vector (pZD55) armed with the Dm-dNK suicide gene in combination with nucleoside prodrugs. Specifically, DFDC induced high toxicities against MCF7 breast cancer cells in vitro and in vivo, while suppressing the replication of the adenovirus in vitro and protecting the normal MRC5 cells. Although the mechanisms of synergetic effects and safety strategies remain unclear and need further study, implementation of conditional replicative adenoviruses plus the Dm-dNK/nucleoside analog system in patients with breast cancer is prospectively highly efficient without inducing systemic toxicity.
Acknowledgments
The present work was granted Grants-in-Aid for the Hi-Tech Research Development Program of China (863 Program, 2006AA02Z493) and the China National Natural Science Foundation (No 81071900, No 81172199, and No 81272920).
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Rustogi A, Budrukkar A, Dinshaw K, Jalali R. Management of locally advanced breast cancer: evolution and current practice. J Cancer Res Ther. 2005;1(1):21–30. | ||
Portsmouth D, Hlavaty J, Renner M. Suicide genes for cancer therapy. Mol Aspects Med. 2007;28(1):4–41. | ||
van Dillen IJ, Mulder NH, Vaalburg W, de Vries EF, Hospers GA. Influence of the bystander effect on HSV-tk/GCV gene therapy. A review. Curr Gene Ther. 2002;2(3):307–322. | ||
Beltinger C, Fulda S, Kammertoens T, Uckert W, Debatin KM. Mitochondrial amplification of death signals determines thymidine kinase/ganciclovir-triggered activation of apoptosis. Cancer Res. 2000;60(12):3212–3217. | ||
Cai X, Zhou J, Chang Y, Sun X, Li P, Lin J. Targeting gene therapy for hepatocarcinoma cells with the E. coli purine nucleoside phosphorylase suicide gene system directed by a chimeric alpha-fetoprotein promoter. Cancer Lett. 2008;264(1):71–82. | ||
Chao DC, Hu KQ. Update on rescue therapies in patients with lamivudine-resistant chronic hepatitis B. Drug Des Devel Ther. 2013;7:777–788. | ||
Zheng X, Johansson M, Karlsson A. Bystander effects of cancer cell lines transduced with the multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster and synergistic enhancement by hydroxyurea. Mol Pharmacol. 2001;60(2):262–266. | ||
Zhu Z, Zhao L, He A, et al. Retrovirus-mediated Drosophila melanogaster multisubstrate deoxyribonucleoside kinase gene therapy of gastric cancer cells in vitro and in vivo. Anticancer Res. 2010;30(7):2641–2649. | ||
Ma S, Zhao L, Zhu Z, et al. The multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster as a therapeutic suicide gene of breast cancer cells. J Gene Med. 2011;13(6):305–311. | ||
Zheng JN, Pei DS, Mao LJ, et al. Inhibition of renal cancer cell growth in vitro and in vivo with oncolytic adenovirus armed short hairpin RNA targeting Ki-67 encoding mRNA. Cancer Gene Ther. 2009;16(1):20–32. | ||
Zhang ZL, Zou WG, Luo CX, et al. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res. 2003;13(6):481–489. | ||
Zheng X, Johansson M, Karlsson A. Nucleoside analog cytotoxicity and bystander cell killing of cancer cells expressing Drosophila melanogaster deoxyribonucleoside kinase in the nucleus or cytosol. Biochem Biophys Res Commun. 2001;289(1):229–233. | ||
Knecht W, Mikkelsen NE, Clausen AR, et al. Drosophila melanogaster deoxyribonucleoside kinase activates gemcitabine. Biochem Biophys Res Commun. 2009;382(2):430–433. | ||
De Clercq E. Antiviral drug discovery and development: where chemistry meets with biomedicine. Antiviral Res. 2005;67(2):56–75. | ||
Arnér ES, Spasokoukotskaja T, Eriksson S. Selective assays for thymidine kinase 1 and 2 and deoxycytidine kinase and their activities in extracts from human cells and tissues. Biochem Biophys Res Commun. 1992;188(2):712–718. | ||
Usuba O, Schulman JL, Deatly AM, Bona CA, Moran TM. New method for titration of virus infectivity by immunostaining. Viral Immunol. 1990;3(3):237–241. | ||
Yu KD, Di GH, Wu J, et al. Development and trends of surgical modalities for breast cancer in China: a review of 16-year data. Ann Surg Oncol. 2007;14(9):2502–2509. | ||
Monnier A. Clinical management of adverse events in adjuvant therapy for hormone-responsive early breast cancer. Ann Oncol. 2007;18(Suppl 8):viii36–viii44. | ||
Wildner O, Morris JC, Vahanian NN, Ford H, Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999;6(1):57–62. | ||
Wildner O, Morris JC. Therapy of peritoneal carcinomatosis from colon cancer with oncolytic adenoviruses. J Gene Med. 2000;2(5): 353–360. | ||
Lambright ES, Amin K, Wiewrodt R, et al. Inclusion of the herpes simplex thymidine kinase gene in a replicating adenovirus does not augment antitumor efficacy. Gene Ther. 2001;8(12):946–953. | ||
Raki M, Hakkarainen T, Bauerschmitz GJ, et al. Utility of TK/GCV in the context of highly effective oncolysis mediated by a serotype 3 receptor targeted oncolytic adenovirus. Gene Ther. 2007;14(19):1380–1388. | ||
Wierdl M, Potter PM. Update on gene therapy approaches for cancer. Curr Hematol Rep. 2005;4(4):294–299. | ||
Yun HJ, Cho YH, Moon Y, et al. Transcriptional targeting of gene expression in breast cancer by the promoters of protein regulator of cytokinesis 1 and ribonuclease reductase 2. Exp Mol Med. 2008;40(3):345–353. | ||
Zhang G, Liu T, Chen YH, et al. Tissue specific cytotoxicity of colon cancer cells mediated by nanoparticle-delivered suicide gene in vitro and in vivo. Clin Cancer Res. 2009;15(1):201–207. | ||
Fujiwara T, Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Uirusu. 2008;58(1):11–18. | ||
Zhang P, Zeng H, Wei Q, et al. Improved effects of a double suicide gene system on prostate cancer cells by targeted regulation of prostate-specific membrane antigen promoter and enhancer. Int J Urol. 2008;15(5):442–448. | ||
Zhu Y, Cheng M, Yang Z, et al. Mesenchymal stem cell-based NK4 gene therapy in nude mice bearing gastric cancer xenografts. Drug Des Devel Ther. 2014;8:2449–2462. | ||
Ahn YH, Yi H, Shin JY, et al. STAT3 silencing enhances the efficacy of the HSV.tk suicide gene in gastrointestinal cancer therapy. Clin Exp Metastasis. 2012;29(4):359–369. |
Supplementary material
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.