Back to Journals » International Journal of Nanomedicine » Volume 13
Surface engineered polyanhydride-based oral Salmonella subunit nanovaccine for poultry
Authors Renu S , Markazi AD, Dhakal S , Lakshmanappa YS, Gourapura SR, Shanmugasundaram R, Senapati S , Narasimhan B , Selvaraj RK, Renukaradhya GJ
Received 28 August 2018
Accepted for publication 31 October 2018
Published 30 November 2018 Volume 2018:13 Pages 8195—8215
DOI https://doi.org/10.2147/IJN.S185588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Sankar Renu,1,2,* Ashley D Markazi,3,* Santosh Dhakal,1,2 Yashavanth S Lakshmanappa,1,2 Suren R Gourapura,1,2 Revathi Shanmugasundaram,3 Sujata Senapati,4 Balaji Narasimhan,4 Ramesh K Selvaraj,5 Gourapura J Renukaradhya1,2
1Food Animal Health Research Program, Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, OH 44691, USA; 2Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210, USA; 3Department of Animal Sciences, Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, OH 44691, USA; 4Department of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA; 5Department of Poultry Science, University of Georgia, Athens, GA 30602, USA
*These authors contributed equally to this work
Purpose: Salmonellosis is a severe economic threat in poultry and a public health concern. Currently available vaccines are ineffective, and thus, developing effective oral Salmonella vaccine is warranted. Especially, a potent oral vaccine such as the mucoadhesive polyanhydride nanoparticle (PNP) protects the vaccine cargo and delivers to intestinal immune sites to elicit robust mucosal immunity and mitigate Salmonella colonization and shedding.
Materials and methods: We designed a Salmonella subunit vaccine using PNP containing immunogenic Salmonella outer membrane proteins (OMPs) and flagellar (F) protein-entrapped and surface F-protein-coated PNPs (OMPs-F-PNPs) using a solvent displacement method. Using high-throughput techniques, we characterized the OMPs-F-PNPs physicochemical properties and analyzed its efficacy in layer birds vaccinated orally.
Results: The candidate vaccine was resistant in acidic microenvironment and had ideal physicochemical properties for oral delivery in terms of particle size, charge, morphology, biocompatibility, and pH stability. In vitro, in vivo, and ex vivo studies showed that F-protein surface-anchored nanoparticles were better targeted to chicken immune cells in peripheral blood and splenocytes and intestinal Peyer’s patch sites. In layer chickens inoculated orally with OMPs-F-PNPs, substantially higher OMPs-specific IgG response and secretion of Th1 cytokine IFN-γ in the serum, enhanced CD8+/CD4+ cell ratio in spleen, and increased OMPs-specific lymphocyte proliferation were observed. OMPs-F-PNPs vaccination also upregulated the expression of toll-like receptor (TLR)-2 and -4, TGF-β, and IL-4 cytokines’ genes in chicken cecal tonsils (lymphoid tissues). Importantly, OMPs-F-PNPs vaccine cleared Salmonella cecal colonization in 33% of vaccinated birds.
Conclusion: This pilot in vivo study demonstrated the targeted delivery of OMPs-F-PNPs to ileum mucosal immune sites of chickens and induced specific immune response to mitigate Salmonella colonization in intestines.
Keywords: Salmonella antigens, polyanhydride nanoparticles, oral delivery, ileum, chickens
Introduction
Salmonella enterica causes foodborne disease in humans worldwide.1 Salmonella enterica serovar enteritidis is the most common infectious agent for animal and human Salmonellosis.2 Salmonella enteritidis reduces egg production in chickens and is responsible for economic losses to the poultry industry.3 Importantly, S. enteritidis-infected poultry meat and contaminated eggs are primary sources of human Salmonellosis.4 Salmonellosis has been reported worldwide. Millions of people are affected with S. enteritidis infection resulting in three million deaths annually.5 It is strongly believed that controlling Salmonellosis in poultry would reduce human Salmonellosis.6
Numerous approaches have been tried to control Salmonella shedding in poultry including vaccination.6 It is expected that Salmonella vaccines decrease intestinal colonization, bacterial shedding in feces, and environmental contamination, thereby reducing the public health risk.7 Currently, live-attenuated and killed/subunit vaccines are used to control S. enteritidis.8 Though live attenuated vaccines are effective, the possibility of reversal to virulence limits its application.9 Killed Salmonella vaccines, though safe, are often contaminated with the endotoxin lipopolysaccharide10 and generally provide limited protection owing to their inability to induce cell-mediated immunity.11 Potent subunit vaccines have the potential to induce high levels of antibody responses and long-lasting immune memory and can be an alternate to killed vaccines currently used.5,12 Salmonella outer membrane proteins (OMPs) are immunogenic and contain porins.13 Porins induce antibody and cell-mediated immune response against Salmonella.14,15 Partially purified OMPs have better immunogenicity than both killed and live Salmonella vaccines.15,16 Salmonella OMPs delivered orally induced mucosal immune response in chickens.16 Immunogenicity of subunit vaccines could be augmented when delivered with adjuvants.5 OMPs activate antigen-presenting cells (APCs) and induce the protective immune response in mice.17 Flagellin interacts with a toll-like receptor (TLR)–5 that subsequently activates the production of inflammatory cytokines and augments innate18 and adaptive immune responses.19
Oral application of polyanhydride nanoparticles (PNPs) vaccines in poultry is highly desirable because it delivers antigens to the gut-associated lymphoid tissues (GALT).20 Orally delivered potent PNPs-based vaccines induce levels of intestinal mucosal antibody response higher than any other routes of vaccination in mice.20 One of the major limitations of oral vaccination is the degradation of antigens in the gastric environment.21,22 Mucoadhesive nanoparticle-based delivery system protects the vaccine antigens from degradation in the acidic pH conditions and delivers antigens to the small intestine in rodent models.20,23,24
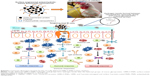
Polyanhydride is a natural mucoadhesive polymer.25 It is cleaved in the gut exposing the carboxylic acid groups, which form hydrogen bonds with hydroxyl groups of glycoproteins in the gut mucus and thus possess the mucoadhesive properties.26 PNPs activate TLRs-2 and −4, innate immunity, complement system, and APCs and induce long-lasting immunity.27,28 The PNPs provide a sustained release of vaccine antigens via surface erosion.29 Vaccine antigens entrapped in PNPs induce high levels of Th1 and Th2 immune responses.20,24,30 Furthermore, surface-modified PNPs enhance the bioadhesive nature of the particles and have been shown to specifically target the ileal Peyer’s patches (PP) in rats.23,24 In this study, we designed PNPs containing Salmonella OMPs and flagellar (F)-protein-entrapped and surface F-protein-coated PNPs (OMPs-F-PNPs) and subjected them to extensive physical and biological characterizations for oral delivery in birds. Furthermore, the ability of OMPs-F-PNPs delivered orally was analyzed for targeting to intestinal immune sites and the induction of OMPs-specific immune response in chickens (Scheme 1).
Materials and methods
Isolation of OMPs
OMPs from S. enteritidis (Phage type 13A from NVSL) were isolated using the sequential detergent extraction method as described previously31 with some modifications. Briefly, bacterial cultures were washed with PBS (pH 7.4) and lysed using the French press (Tissue Lyser LT; Qiagen NV, Venlo, the Netherlands). The bacterial inner membrane was solubilized by treating with 1% Sarkosyl (Sigma-Aldrich Co., St Louis, MO, USA) for 30 minutes and centrifuged (Optima L-100 XP ultracentrifuge: Beckman Coulter, IN, USA) at 20,000× g for 30 minutes. The pellet was suspended in 10% sodium dodecyl sulfate in 0.5 M Tris-HCl (pH 6.8) for 30 minutes and centrifuged at 20,000× g for 30 minutes. The supernatant containing soluble OMPs-enriched extract was dialyzed using the Milli-Q water and freeze-dried with 5% sucrose as a cryoprotectant. The protein concentration was estimated using the Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) as per the manufacturer’s instruction.
Isolation of F-Protein
S. enteritidis (Phage type 13A from NVSL) was grown initially in Trypticase soy agar plate and followed by inoculation into brain heart infusion broth and incubated at 37°C without shaking for 48 hours. Bacteria were washed three times in PBS and centrifuged at 7,000× g for 30 minutes. The bacterial pellet was treated with 3 M potassium thiocyanate (Sigma-Aldrich Co.) in PBS solution for 2 hours under magnetic stirring. Supernatant containing F-protein-enriched extract was collected after centrifugation at 35,000× g for 30 minutes. Extracted F-protein was dialyzed against PBS (pH 7.4) followed by using Milli-Q water and freeze-dried using 5% sucrose. The protein concentration was estimated using the Micro BCA™ Protein Assay Kit as per the manufacturer’s instructions.
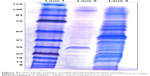
Analyses of OMPs and F-protein by SDS-PAGE
The isolated OMPs and F-protein extracts were mixed with a gel loading dye containing β-mercaptoethanol, denatured at 95°C for 5 minutes before loading onto the gel. Separation of the protein was achieved using SDS-PAGE analyses using 5% (v/v) stacking and 10% (v/v) separation gels, followed by staining with Coomassie brilliant blue R-250 for 2 hours. The stained gel was destained, and the protein molecular weight was determined using the standard protein molecular weight size marker ladder (235 to −9 kDa) (Figure S1).
Preparation of OMPs-F-PNPs and surface F-protein-coated PNPs
The OMPs-F-PNPs were formulated by a solvent displacement method as described previously20 with some modifications. Briefly, 100 mg of polyanhydride (MW ~216,000; Sigma-Aldrich Co.) polymer was dissolved in 2 mL of acetone by sonication. Both OMPs and F-protein (2.5 mg each) were dispersed in 3 mL acetone and mixed with 100 mg polyanhydride solution under magnetic stirring. A total of 50 μL of Span® 80 (Sigma-Aldrich Co.) was added to the mixture and the solution was magnetically stirred for 1 hour at room temperature. Polymer was desolvated by adding 7 mL of absolute ethanol. The nanoparticles were surface-coated with F-protein by adding 2.5 mg of F-protein in 3 mL of deionized water under magnetic stirring for 1 hour to evaporate the organic solvents. The formulated nanoparticles’ suspension was cross-linked by incubation with 1,3-diaminopropane (100 μg) for 5 minutes. OMPs-F-PNPs were collected by centrifugation at 27,000× g for 20 minutes and freeze-dried with 5% sucrose as a cryoprotectant. Empty PNPs and PNPs coated with F-protein (PNPs-F) were similarly prepared without OMPs for targeted in vitro, in vivo, and ex vivo studies.
Analysis of nanoparticle shape, size, charge, and surface labeling with F-protein
Hitachi S-4700 Field emission scanning electron microscope (FESEM) was applied to determine the morphological characteristics of OMPs-F-PNPs. Freeze-dried nanoparticles were placed on carbon taped stubs and coated with platinum prior to FESEM analysis. The average particle size distribution and charge of OMPs-F-PNPs were determined using a Zetasizer Nano ZS90 (Malvern Panalytical, MA, USA). The UV–visible spectrum of F-protein, PNPs, and PNPs-F was obtained using the Beckman Coulter DU-800 UV–vis Spectrophotometer in the range between 200 and 800 nm.
Encapsulation efficiency
The OMPs-F-PNPs formulation was digested in 0.1 M NaOH for 4 hours at 37°C, sonicated, and dispersed in PBS as described previously.30 The amount of protein released into the supernatant was measured using the Micro BCA™ Protein Assay Kit. The protein loading efficiency in PNPs was estimated by calculating difference between initial amount of protein added to formulate nanoparticles and the amount of protein released after digesting the nanoparticles. The surface loading efficiency of F-protein in nanoparticle vaccine was estimated by calculating difference between initial amount of F-protein added to preformed empty nanoparticles and the amount of protein released after digesting the particles.
Hemolysis assay
Two milliliters of fresh chicken blood was collected in EDTA. Red blood cells (RBCs) were harvested by centrifugation at 1,000× g for 10 minutes. RBCs were washed three times in sterile PBS and suspended in 3 mL of PBS. A total of 100 μL of RBCs’ suspension was treated with positive control (Triton X-100), negative control (PBS), or 250, 500, 750, and 1,000 μg of PNPs for 1 hour at 37°C and centrifuged at 12,000× g for 10 minutes. The absorbance of released hemoglobin in the supernatant was read at 575 nm in the ELISA plate reader (SpectraMax Plus 384; Molecular Devices LLC, Sunnyvale, CA, USA). The percentage of lysis was calculated as described previously32 by using the following formula: ([treatment sample absorbance − negative control]/[positive control − negative control]) ×100. The erythrocyte-treated nanoparticles were examined in a light microscope under 4× objectives (EVOS™ FL Cell Imaging System; Thermo Fisher Scientific).
pH stability analysis
Six milligrams of PNPs were incubated in 4 mL of 3.5, 4, 4.5, 5.5, 6.5, and 7.4 pH solutions at room temperature (22°C). At various time points of incubation, 100 μL of aliquots were collected and analyzed for turbidity reduction in duplicates in a spectrophotometer at 405 nm as described previously.30 The pH stability was expressed as the percentage of turbidity reduction and calculated as (initial absorbance − time point absorbance)/initial absorbance ×100.
Isolation of cells and in vitro immune cell uptake study
The PNPs and PNPs-F were tagged with fluorescent dye by incubating with 1.25 mg of rhodamine B isothiocyanate (RITC) (Sigma-Aldrich Co.) for 5 minutes. The fluorchrome-labeled nanoparticles were obtained by centrifugation at 10,500× g for 10 minutes and freeze-dried with 5% sucrose.
Ten days after Salmonella challenge, blood samples were collected in sterile EDTA tubes and spleen in 2 mL RPMI medium (GE Healthcare Bio-Sciences Corp., Piscataway, NJ), the latter containing 10% FBS (Sigma-Aldrich Co.), antibiotic–antimycotic (Thermo Fisher Scientific), sodium pyruvate, 1 M HEPES, Minimum Essential Medium Non-Essential Amino Acid Solution, and 2-mercaptoethanol, hereafter named as E-RPMI. Peripheral blood mononuclear cells (PBMCs) were isolated by using the Ficoll-Paque™ PLUS (GE Healthcare Bio-Sciences Corp.) as per the manufacturer’s protocol with slight modifications. Briefly, blood was diluted in PBS (1:1 ratio), an equal volume of Ficoll-Paque™ PLUS solution was added and centrifuged at 450× g for 25 minutes at 20°C. Cells’ interface were collected, washed 2× in PBS, and suspended in E-RPMI. Splenocytes were isolated by teasing spleen cells through a cell strainer using PBS. An equal volume of Ficoll-Paque™ PLUS solution was added, and the suspension was centrifuged at 450× g for 30 minutes at 4°C. Splenocytes at the interface were collected and washed two times with PBS, and cells were suspended in E-RPMI. The isolated cells were used in in vitro and the mixed lymphocyte proliferation assay.
For in vitro nanoparticle uptake study, PBMCs or splenocytes harvested from unvaccinated mock chickens were seeded at 5 million cells/well into a 12-well cell culture plate and incubated overnight at 39°C in 5% CO2. The adhered cells were treated with 1 mL of E-RPMI or RITC dye-tagged PNPs (150 μg/mL) or RITC dye-tagged PNPs-F for 4 hours at 39°C. Cells were washed 2× times with PBS, and RPMI without phenol red was added. Cells were examined under the red channel under a fluorescent microscope (IX70; Olympus Corporation, Tokyo, Japan), and images were taken at 20× magnification.
An immunofluorescence assay was performed to detect the cellular uptake of PNPs-F using a flagellin-specific antibody. For this, PBMCs (1 million cells/well) in a 96-well cell culture plate were seeded and incubated overnight at 39°C in 5% CO2. Adherent cells (macrophages) were treated with E-RPMI or F-protein (10 μg) either in soluble form or coated on PNPs (PNPs-F). After 4 hours of incubation, the cells were fixed with 80% acetone and stained with rabbit anti-flagellin antibody (Abcam, Cambridge, MA, USA) followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Thermo Fisher Scientific).33 The immunostained cells were observed under the green channel in a fluorescent microscopy (IX70), and images were taken at 20× magnification.
In vivo and ex vivo mucoadhesive study
To analyze the mucoadhesive nature of PNPs-F in chicken intestine, 15-week-old layer chickens were inoculated orally with control PBS or 0.6 mg of RITC dye either in soluble form or tagged to PNPs or PNPs-F suspended in 1 mL PBS. After 4 hours of inoculation, the birds were euthanized and approximately 1 cm ileum was harvested, washed in PBS, and fixed in Tissue-Tek® OCT compound (Sakura Finetek, Torrance, CA, USA), and frozen at −80°C. The frozen samples were sectioned (5 μm thick) in cryostat microtome (CM1510S; Leica Microsystems, Wetzlar, German), mounted on poly-L-lysine precoated slides, stained with DAPI, and examined in a cell imaging microscope (EVOS™ FL Cell Imaging System) under 2× objective. For ex vivo mucoadhesive analyses, approximately 10 cm of ileum was harvested from healthy layer chickens, washed thoroughly in PBS, and incubated with PBS or 0.6 mg RITC dye either in the soluble form or tagged to PNPs or PNPs-F suspended in 1 mL of PBS for 4 hours at 37°C. The tissues were fixed, sectioned, and visualized under the cell imaging microscope as described earlier.
OMPs-F-PNPs’ vaccination trial in layer chickens
One-day-old Austra White laying chicks (n=40) were raised at the OARDC animal house facility under standard animal husbandry practices. Birds were provided with ad libitum water and feed and housed in layer cages. All experimental procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University (Protocol number: 2016A00000060). Animal experiment was performed as per the recommendations by the Public Health Service Policy, USDA Regulations, National Research Council’s Guide for the Care and Use of Laboratory Animals and the Federation of Animal Science Societies’ Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. At the age of 6 weeks, hens were randomly separated into four experimental groups (n=10 per group) and inoculated orally with PBS (mock and mock + challenge groups) or soluble OMPs (50 μg) + F-protein (50 μg) in PBS (soluble antigen group) or equivalent amount of proteins-loaded OMPs-F-PNPs suspension in 1 mL of sterile PBS. The same vaccine dose and oral delivery was repeated twice more at the age of 9 and 12 weeks.
Nalidixic acid-resistant S. enteritidis pure culture (Phage type 13A) was grown in 10 mL of tryptic soy broth (TSB) at 37°C. At 8 hours of incubation, 100 μL of the bacterial suspension was transferred into 10 mL fresh TSB and incubated overnight at 37°C. One milliliter of the bacterial suspension was transferred into 100 mL of fresh TSB and incubated at 37°C until the culture absorbance reached 1.1. The bacteria were washed in PBS 3×, serially diluted, and plated on xylose lactose tergitol-4 (XLT4) agar plates to determine colony-forming units (CFUs). At the age of 15 weeks, birds were fasted for 8 hours and except mock group other experimental groups were challenged with S. enteritidis (1×109 CFU in 1 mL PBS) by oral gavage. Birds were placed as pairs in cages in a randomized design and necropsied at 10 days after Salmonella challenge infection for sample collection.
Antibody response in OMPs-F-PNPs-vaccinated birds
Chicken anti-OMPs IgG and IgA antibody titers were analyzed by ELISA in serum, cloacal swab, bile, small intestinal wash, and tracheal wash samples both at pre- and post-Salmonella challenge infections. Blood samples were collected at the age of 6, 9, 12, and 15 weeks.
Cloacal swab samples were collected in PBS, vortexed, and centrifuged at 3,000× g for 10 minutes. Aliquots of serum and cloacal supernatants were stored at −80°C until use. Small intestine and trachea samples were collected in 2 mL PBS at termination (10 days after challenge), cut into tiny pieces, vortexed, and centrifuged at 3,000× g for 10 minutes. Aliquots of the supernatant were stored at −80°C until use. Bile samples were collected from the gallbladder using an insulin syringe on the day of necropsy, and aliquots were stored at −80°C until use.
Flat bottom, high binding 96-well plates (Greiner Bio-One, Monroe, NC, USA) were coated with pretitrated amount of OMPs (2 μg/mL for IgG and 7.5 μg/mL for IgA ELISA) in 0.05 M sodium carbonate–bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Plates were washed three times and blocked with 5% skim milk powder in PBS Tween-20 (0.05%) (PBST) for 1 hour at room temperature. Plates were washed in PBST 3×. Serum and bile samples were diluted in 2.5% skim milk, and 50 μL of each sample was added to duplicate wells. Undiluted cloacal swab, small intestinal wash, and tracheal wash samples in 50 μL volume were added to duplicate wells. Plates were incubated for 2 hours at 4°C and washed 3×, and 50 μL of goat anti-chicken IgG-conjugated HRP (Southern Biotech, Birmingham, AL, USA) (1:10,000 in 2.5% skim milk powder in PBST) or goat anti-chicken IgA-conjugated HRP (Gallus Immunotech, Cary, NC, USA) (1:3,000 in 2.5% skim milk powder in PBST) secondary antibodies were added. Plates were incubated for 2 hours at room temperature and washed 3×, and 50 μL/well of TMB peroxidase substrate (1:1 mixture of TMB peroxidase substrate and TMB peroxidase substrate solution B) (KPL, Gaithersburg, MD, USA) was added to each well. The reaction was stopped after 10–20 minutes by adding 1 M phosphoric acid. The OD was measured at 450 nm using an ELISA plate reader. The corrected OD was obtained by subtracting the treatment group OD from blank control OD.
CD8+/CD4+ cell ratio analysis by flow cytometry
Splenocytes (1×106 cells/well) were seeded in 96-well plate and incubated with pretitrated 1:200 dilution of fluorescein isothiocyanate-conjugated mouse anti-chicken CD4 (Southern Biotech, Birmingham, AL, USA), pretitrated 1:400 dilution of phycoerythrin-conjugated mouse anti-chicken CD8α (Southern Biotech), or 1:100 diluted mouse IgG specific isotype control antibody for 20 minutes at 4°C. Cells were washed 3× by centrifugation at 750× g for 3 minutes at 4°C. The percentage of CD4+ and CD8+ cells was analyzed by flow cytometry (Guava Easy-Cyte; Merck Millipore, Billerica, MA, USA) as described previously.36 The result was expressed as CD8+/CD4+ cell ratio.
Mixed lymphocyte proliferation response in OMPs-F-PNPs-vaccinated birds
For the lymphocytes’ proliferation assay, PBMCs and splenocytes were suspended in E-RPMI in triplicate wells and were seeded in 96-well flat bottom tissue culture plates (Greiner Bio-One) at 1×106 cells/well in 100 μL volume. Cells were re-stimulated with OMPs (5 μg/mL) in 100 μL E-RPMI and incubated for 72 hours at 39°C in a 5% CO2 incubator. After incubation, 20 μL of MTS + PMS solution was added into cells and incubated for 4 hours at 37°C in a 5% CO2 incubator. The OD at 490 nm was recorded by an ELISA plate reader. Stimulation index (SI) was calculated by dividing the OD of stimulated cells by the OD of unstimulated control cells of the same chicken.
TLRs and cytokines gene expression in the cecal tonsillar lymphoid cells
Cecal tonsils were collected at 10 days post-Salmonella challenge. Total RNA was extracted by using TRIzol reagent (Thermo Fisher Scientific) and dissolved in Tris-EDTA (pH 7.5) buffer, and the concentration of RNA was determined by using NanoDrop™ 2000c Spectrophotometer (Thermo Fisher Scientific). cDNA synthesis was achieved with 1 μg of total RNA using the QuantiTect Reverse Transcription Kit (Qiagen NV) according to the manufacturer’s instructions. The mRNA transcripts of TLR-2, TLR-4, cytokines TGF-β and IL-4, and the house keeping gene β-actin (Table S1) were analyzed by real-time PCR using the iQ™ SYBR® Green Supermix (Bio-Rad Laboratories Inc., Hercules, CA, USA). Target gene transcripts were normalized to the β-actin housekeeping gene. Fold change from the reference was calculated by using the 2(Ct sample − housekeeping)/2(Ct reference − housekeeping) comparative Ct method, wherein Ct is the threshold cycle.
Bacteriology
Ceca (0.5 g) were homogenized in 2× concentration of peptone water and incubated for 12 hours at 37°C for initial enrichment of the bacteria. Subsequently, cecal homogenates were streaked onto naladixic acid antibiotic containing XLT plate and incubated for 24 hours at 37°C. The black colored S. enteritidis colonies in plates were qualitatively confirmed as Salmonella by following the standard methods.35
Statistical analyses
Experimental results were expressed as mean ± standard error mean (SEM) of 8–10 chickens in each group. Data were examined by nonparametric Kruskal–Wallis test and the P-value difference between the groups was determined by Mann–Whitney test36 using the GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). A P-value of <0.05 was considered statistically significant.
Results and discussion
Characterization of isolated Salmonella proteins’ extract and candidate OMPs-F-PNPs vaccine
The isolated OMPs and F-protein were confirmed by SDS-PAGE analyses. Proteins with approximate molecular weight 22, 23, 28, 34, 36, 45, 46, 55, 65, 68, and 70 kDa were detected in the OMP-enriched extract (Figure S1). The F-protein-enriched extract had subunit proteins with approximate molecular weight 21, 28, 35, 42, 50, and 58 kDa (Figure S1). Complex proteins are present in OMPs and the flagellin-enriched extract of S. enteritidis.37,38 SDS-PAGE analyses of isolated OMPs revealed a complex electrophoretic mobility profile containing at least 12 different proteins ranging from 14 to 70 kDa.37 Similarly, flagellin-enriched extract had proteins of 28–58 kDa size.38 Our OMPs and F-protein-enriched extracts of S. enteritidis were comparable to published reports.37,38
Understanding the physicochemical properties of nanoparticles is essential to develop an effective vaccine as well as to determine the fate of nanoparticles in vivo.39 Our OMPs-F-PNPs vaccine particles were spherical, uniform size distribution, and without aggregates as observed under FESEM analysis (Figure 1A). The average particle size distribution of OMPs-F-PNPs was 215±5 nm with the polydispersity index of 0.2 (Figure 1B). OMPs-F-PNPs had the negative average zeta potential charge of −38±4 mV (Figure 1C). PNPs loaded with ovalbumin (OVA) using the solvent displacement method are spherical in shape with uniform size.20 In an earlier study, antigen-loaded and surface flagellin-coated PNPs by solvent displacement method have 391 nm size particles with zeta potential −34 mV.20 OVA-loaded PNPs were found well dispersed and spherical in shape with the particle size distribution of 290 nm and the surface charge of −36 mV.40 Size of nanoparticles is critical for efficient uptake by APCs and for the induction of Th1 and Th2 immune responses. It is well established that particle sizes around 500 nm or less are optimum for uptake by dendritic cells (DCs) and macrophages.41 The 20–200 nm virus-like particles are internalized by APCs via clathrin-dependent endocytosis.42 Surface charge plays a major role in nanoparticle uptake by immune cells and immune activation.43 Nanoparticles with the zeta potential value of above −25 mV are essential to prevent large aggregates and improve their stability.44 PNPs’ negative charge helps to maintain high stability and homogeneity in physiological conditions.40 The higher the negative charge of nanoparticles, greater is the interaction between nanoparticles and mucus in the mucosal layer.26 Spherical particles were actively internalized by DCs and deliver loaded antigens.45 Based on the available reports, OMPs-F-PNPs constructed for this study were of the appropriate size, charge, and shape for uptake by APCs and subsequent induction of immune responses.
The entrapment efficiency of OMPs and F-protein was 78%, and the surface F-protein loading efficiency was around 25% in OMPs-F-PNPs. This was consistent with PNPs-loaded peanut proteins by solvent displacement method with 70%–80% encapsulation efficiency.30 Similarly, the encapsulation efficiency of OVA-loaded PNPs was 84%.40 PNPs’ surface coating with F-protein was analyzed by UV–vis spectroscopy. The empty PNPs had no detectable absorbance peak at 280 nm, whereas F-protein and PNPs-F had absorption at 280 nm (data not shown). The presence of F-protein absorbance peak in PNPs-F formulation confirms the occurrence of F-protein in formulated nanoparticles. Presence of antigen in PNPs was confirmed by regular UV–vis spectroscopy.40
The hemolysis assay was designed to identify if nanoparticles lyse RBCs.32 Initial screening for compatibility of the polymer was performed using whole blood, which determines the in vivo acute toxicity based on the lysis of RBCs.46 In vitro assay showing nanoparticle-induced hemolysis correlates with in vivo toxicity.47 PNPs did not induce the lysis of erythrocytes even with 1,000 μg concentration, and the absorbance value was comparable to control PBS-treated RBCs (Figure S2A). In contrast, Triton X-100 lysed 100% of RBCs. Even the morphology of RBCs was intact upon PNPs’ treatment as shown by microscopy (Figure S2B). Mice inoculated orally with PNPs did not induce the expression of inflammatory mediators or any functional biomarkers with the absence of any histological changes in major vital organs compared to control group,48 suggesting that PNPs are safe for oral delivery.
The PNPs’ stability in acidic and alkaline pH environments was studied to determine the PNPs’ ability to withstand stomach and intestinal conditions. Nanoparticles form turbidity in biological solution, and a reduction in turbidity indicates reduced stability.30 Increasing the time of incubation from 1 to 3 hours slightly increased the turbidity reduction values (Figure S2C). PNPs incubated at pH 3.5 for 3 hours had 10% reduction in turbidity. While the percentage turbidity reduction at 0 and 3 hours’ incubation at pH 6.5 and 7.4 was comparable (Figure S2C), indicating that PNPs were stable at both acidic and alkaline pH conditions. Processed PNPs were found stable in biological buffer over a period of time.30 Hemolysis and turbidity reduction assay results suggested that PNPs are suitable for the delivery of vaccine antigens orally in chickens.
In vitro uptake of PNPs-F by immune cells
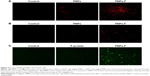
PBMCs and splenocytes harvested from healthy layer chickens were used in the assay. Through germ line-encoded pattern recognition receptor TLR5, the flagellin triggers both immune and nonimmune cells. The TLR-5 is present in a variety of immune cells, including DCs, monocytes, macrophages, and lymphocytes. Myeloid-derived DCs directly recognize F-protein through TLR5, and spleen DCs indirectly respond to F-protein by stimulating other TLR-5-expressing cells.49–52 Moreover, due to similarity in the size of pathogens to nanoparticles, they are identified and internalized by APCs39 such as DCs.45 To understand whether the F-protein surface-coated PNPs are more readily targeted to chicken immune cells, a preliminary in vitro study with PBMCs and splenocytes was performed. Cells treated with RITC-tagged PNPs or PNPs-F showed red or green fluorescence under a fluorescence microscope compared to control cells (Figure 2). PNPs-F-treated PBMCs and splenocytes had high fluorescence signal compared to PNPs’ treatment (Figure 2A and B). Furthermore, immunostaining of flagellin using the specific antibody showed a clear signal in PNPs-F treated immune cells, whereas soluble F-protein treatment showed very low signal. Medium only control did not have any background fluorescence signal in cells (Figure 2C). Uniformity of particle size and surface charge determines the fate of nanoparticles’ internalization in immune cells. Fluorescent-labeled PNPs were internalized in mouse macrophage J744 cells by the clathrin-mediated endocytosis pathway.53 The results of this in vitro study revealed F-protein coating on the PNPs facilitate better uptake of particles than those particles without F-protein.
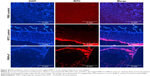
In vivo and ex vivo analyses of mucoadhesive nature of PNPs-F
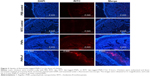
Follicle-associated epithelium of PPs is composed of microfold (M) cell. The M cells sample foreign antigens and initiate the specific adaptive immune response.54 Flagellin plays a major role in the colonization of Salmonella to the intestinal mucosa and invasion through M cells.23,55 Soluble antigens of Salmonella including flagellin if not delivered through nanoparticles are poorly internalized by M cells in PPs.56 The PNPs are natural TLR-4-specific adjuvants and recognize M cells and other lymphoid cells expressing pathogen recognition receptors.27,57 To confirm whether PNPs-F mimics live Salmonella in targeting to ileal mucosal immune cells, we performed in vivo and ex vivo studies in the ileum of chicken. PNPs-F were delivered orally and found adhered to mucosal surface, by ileal PPs and lamina propria immune cells in both in vivo and ex vivo studies. The PNPs without F-protein surface coating were poorly internalized by ileal immune cells (Figures 3 and S3). In the control group, RITC fluorescent dye was found nonspecifically adhered to the mucosal surface in ex vivo study, but such nonspecific adherence was absent in birds given orally (Figures 3 and S3). Thus, our fluorescent dye-based mucoadhesive PNPs’ study results suggested that F-protein surface labeling is essential for an improved uptake of nanoparticles by chicken ileal immune cells. In a related study, PNPs’ surface coated with F-protein delivered orally were found uptaken by M cells in rodent ileal PPs.23 Taken together with our in vitro, in vivo, and ex vivo results, we conclude that PNPs surface coated with F-protein specifically target chicken immune cells and also ileal mucosal M cells.
OMPs-F-PNPs enhanced OMPs-specific antibody response
Antibodies play a major role in clearing extracellular bacteria, whereas activated effector T lymphocytes clear intracellular bacteria; both are critical for protective response against Salmonella.58 OMPs-specific IgG antibodies were detectable in serum after prime-inoculation of OMPs-F-PNPs in chickens (Figure 4A), and after first and second booster vaccinations, the induced antibody titers were substantially higher (P<0.05) compared to soluble antigens received group (Figure 4B and C). As expected, S. enteritidis challenge significantly boosted OMPs-specific IgG and IgA antibody responses in all the treatment groups compared to mock control (Figure 5A–F). In particular, OMPs-F-PNPs vaccination significantly (P<0.001) increased OMPs-specific IgG antibody response in serum and bile samples compared to the mock group (Figure 5A and B). Similarly, OMPs-F-PNPs vaccination significantly (P<0.001) increased OMPs-specific IgA antibody response in bile, cloaca, and small intestine samples compared to the mock group (Figure 5C–E). But, OMPs-specific IgA antibody titers in OMPs-F-PNPs vaccinated chickens’ bile, cloacal swab, small intestine, and tracheal wash samples were numerically increased compared to mock-challenge and soluble antigens-vaccinated groups (Figure 5C–F). In mice, OMPs’ vaccination increases specific antibody response and provides cross-protective response against Salmonella infection.59 In chickens,60 parenterally administered soluble OMPs, challenged with S. enteritidis, induce antibody response. A report published two decades ago concluded that OMPs injected subcutaneously with an adjuvant increased the antibody response and deceased the bacterial shedding in chickens.16 Salmonella antigen-conjugated starch microparticles delivered orally in mice induced specific IgA response that was detected in feces.61 Spherical shaped nanoparticles-based antigen delivery improved antigen-specific antibody titers.45 Nanoparticles release antigens in a controlled manner and enhance the duration of availability of antigens to immune cells, thereby increasing the quality of immune response.62 OVA-loaded and surface flagellin-coated PNPs delivered orally in mice enhanced IgG and IgA antibody response compared to soluble antigen and surface unmodified PNPs.20
OMPs-F-PNPs improved T-cell responses
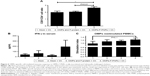
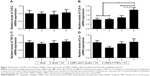
Stimulation of antigen-specific CD4+ and CD8+ T cells indicates the trigger of cellular immunity.40 OMPs-F-PNPs oral vaccination induced cell-mediated immune response in chickens was determined by estimating CD8+/CD4+ cell ratios, interferon gamma (IFN-γ) levels in serum, and OMPs-specific lymphocyte proliferation response in PBMCs. We immunostained chicken splenocytes and performed the flow cytometry, and the CD4+ and CD8+ T cells were gated as reported earlier (Figure S4).34 The CD8+ T-cell frequency was higher in all the treatment groups compared to CD4+ T cells, which correspond to an inverse CD4+/CD8+ cell ratio. OMPs-F-PNPs vaccinated birds splenocytes had significantly higher (P<0.05) CD8+/CD4+ cell ratio compared to mock-vaccinated and soluble antigen-vaccinated birds challenged with virulent Salmonella (Figure 6A). OVA-loaded PNPs vaccination in mice enhances CD4+ and CD8+ T cells in spleen compared to soluble OVA and PNPs.40 OVA-PNPs immunized mice splenocytes increase the frequency of OVA-specific IFN-γ-secreting CD8+ T cells.27
The OMPs-F-PNPs vaccinated and challenged chickens had slightly increased levels of IFN-γ in serum compared to other treatment groups (Figure 6B). In OMPs-F-PNPs vaccinated and challenged chickens, PBMCs restimulated with OMPs had slightly increased lymphocytes stimulation index compared to other treatment groups (Figure 6C). Due to rapid absorption and degradation of in vivo delivered soluble antigen, it did not stimulate specific immune response, whereas PNPs nanovaccine due to sustained antigen release stimulates long-lasting immunity and enhances antigen-specific lymphocytes’ proliferation.40 Antigen-specific proliferation of lymphocytes suggests the induction of cell-mediated immunity in poultry.12 Salmonella OMPs activate DCs by augmenting the expression of maturation markers CD80 and CD86.63 OMPs-treated DCs induce allogenic lymphocyte proliferation and increased IFN-γ production in mice.63 Mice immunized with porins and challenged with Salmonella had higher frequency of IFN-γ producing CD4+ T cells.64 PBMCs of birds inoculated with OMPs and co-restimulated with concanavalin A and OMPs induced OMP-specific cell proliferation.15 Killed Salmonella-injected birds had increased OMPs and flagella-specific lymphocyte proliferative response with enhanced serum IFN-γ levels.65 Nanoparticles (20–200 nm) enhance CD4 and CD8 cells’ frequency and Th1 immune response.45,62 Intraperitoneally administered PNPs increases IFN-γ production and CD8+ T-cell response in mice.27 Due to potent adjuvant property, antigen-loaded bioadhesive PNPs enhance antigen-specific Th1 immune response.5
OMPs-F-PNPs upregulated TLRs and cytokine mRNA expression
PNPs activate TLR-2, −4, and −5 to stimulate Th1 response in DCs.27 Immunostimulatory adjuvants trigger innate immunity via the engagement of pathogen-associated molecular patterns (PAMPs). TLRs recognize PAMPs and trigger cytokine production and initiate innate and adaptive immune responses.66,67 Salmonella sp. flagellin is a ligand for TLR-5, a PAMP-like receptor on DCs and macrophages.67 OMPs also activate the TLR-4 signaling pathway leading to the maturation of DCs.63 Specific TLRs control the function of APCs by upregulating the expression of activation markers CD80 and CD86 and adhesion molecules.27 In our study, the expression of TLR-2 mRNA in ceca lymphoid tissues was slightly upregulated in OMPs-F-PNPs-vaccinated chickens compared to control groups (Figure 7A). Remarkably, OMPs-F-PNPs significantly (P<0.05) upregulated TLR-4 mRNA expression in cecal tonsils compared to all other groups (Figure 7B). PNPs act as an adjuvant by stimulating the complement system and attract immature APCs through activating TLR-2 and TLR-4 signaling pathways28 and promoting the secretion of cytokine.27 In the Salmonella-challenged birds, the levels of expression of TGF-β and IL-4 cytokines mRNA were slightly upregulated in OMPs-F-PNPs-vaccinated birds compared to soluble antigens-vaccinated groups (Figure 7C and D), while soluble antigens downregulated IL-4 mRNA expression (Figure 7D). In splenocytes of mice vaccinated with porins, increased IL-4 and IFN-γ mRNA expressions were detected both at pre- and post-Salmonella challenges, respectively.64 OVA-loaded PNPs significantly enhance Th1 and Th2 mRNA expression levels in splenocytes of mice.40 Thus, our avian vaccination data presented here suggest that OMPs-F-PNPs-vaccinated chickens have the potential to modulate TLRs and cytokines’ expression in the cecal tonsils.
OMPs-F-PNPs reduced the Salmonella shedding
Oral vaccination of birds with OMPs-F-PNPs was shown to deliver the vaccine to intestinal immune cells with resultant induced OMPs-specific antibody and T-cell responses. To determine if this vaccination approach modulated the dynamics of anamnestic response in the intestines of birds; in a pilot study, vaccinated birds were challenged with a very high dose of S. eneritidis (1×109 CFU per bird). The resultant data showed that OMPs-F-PNPs vaccination still cleared the Salmonella from the cecum of 33% of birds (Figure S5). Empty PNPs induced potent adjuvant effect was shown to protect against lethal Salmonella challenge in mice.27 High levels of Salmonella-specific IgG and IgA antibodies clear the infection from the gut of chicken.68 Induction of intestinal secretory IgA and T-cell responses protects chickens from Salmonella.69
Conclusion
A mucoadhesive polyanhydride nanocarrier was formulated and designed to deliver Salmonella subunit vaccine antigens orally to poultry. The surface-engineered nanovaccine with Salmonella F-protein was designed to specifically target ileal immune cells. In OMPs-F-PNPs inoculated and challenged birds, the robust anamnestic immune response was indicated by augmented OMPs-specific IgG, mucosal IgA, T-cell, and cytokine responses. Further studies are required to improve the vaccine efficacy by adding secondary adjuvant in the vaccine formulation to mitigate Salmonella shedding in poultry.
Acknowledgments
We thank our animal care unit staff Mr Keith Patterson and Jack Sidle for help in chicken studies. Dr Tea Meulia and Leona Horst and her team at MCIC, OARDC, provided help in FESEM analysis. We sincerely thank Dr Steven Krakowka for his help in editing the manuscript. This work was partially supported by the USDA-NIFA grant and OSU Accelerator Award. Salaries and research support were provided by state and federal funds appropriated to OARDC, The Ohio State University.
Disclosure
The authors report no conflicts of interest in this work.
References
Hendriksen RS, Vieira AR, Karlsmose S, et al. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8(8):887–900. | ||
Santos AC, Roberts JA, Cook AJ, et al. Salmonella typhimurium and salmonella enteritidis in England: costs to patients, their families, and primary and community health services of the NHS. Epidemiol Infect. 2011;139(5):742–753. | ||
Lee SK, Chon JW, Song KY, Hyeon JY, Moon JS, Seo KH. Prevalence, characterization, and antimicrobial susceptibility of Salmonella Gallinarum isolated from eggs produced in conventional or organic farms in South Korea. Poult Sci. 2013;92(10):2789–2797. | ||
Humphrey T, Jørgensen F. Pathogens on meat and infection in animals – establishing a relationship using campylobacter and salmonella as examples. Meat Sci. 2006;74(1):89–97. | ||
Ochoa J, Irache JM, Tamayo I, Walz A, Delvecchio VG, Gamazo C. Protective immunity of biodegradable nanoparticle-based vaccine against an experimental challenge with Salmonella Enteritidis in mice. Vaccine. 2007;25(22):4410–4419. | ||
Varmuzova K, Faldynova M, Elsheimer-Matulova M, et al. Immune protection of chickens conferred by a vaccine consisting of attenuated strains of Salmonella Enteritidis, Typhimurium and Infantis. Vet Res. 2016;47(1):94. | ||
de Freitas Neto OC, Mesquita AL, de Paiva JB, Zotesso F, Berchieri Júnior A. Control of Salmonella enterica serovar Enteritidis in laying hens by inactivated Salmonella Enteritidis vaccines. Braz J Microbiol. 2008;39(2):390–396. | ||
Jawale CV, Chaudhari AA, Jeon BW, Nandre RM, Lee JH. Characterization of a novel inactivated Salmonella enterica serovar Enteritidis vaccine candidate generated using a modified cI857/λ PR/gene E expression system. Infect Immun. 2012;80(4):1502–1509. | ||
European Food Safety Authority (EFSA). Opinion of the Scientific Panel on biological hazards (BIOHAZ) related to the use of vaccines for the control of Salmonella in poultry. EFSA Journal. 2004;2(12):114. | ||
Arnon R, Shapira M, Jacob CO. Synthetic vaccines. J Immunol Methods. 1983;61(3):261–273. | ||
Barrow PA. Salmonella infections: immune and non-immune protection with vaccines. Avian Pathol. 2007;36(1):1–13. | ||
Okamura M, Lillehoj HS, Raybourne RB, Babu U, Heckert R. Antigen-specific lymphocyte proliferation and interleukin production in chickens immunized with killed Salmonella enteritidis vaccine or experimental subunit vaccines. Avian Dis. 2003;47(4):1331–1338. | ||
Sundara Baalaji N, Mathew MK, Krishnaswamy S. Functional assay of Salmonella typhi OmpC using reconstituted large unilamellar vesicles: a general method for characterization of outer membrane proteins. Biochimie. 2006;88(10):1419–1424. | ||
Matsui K, Arai T. Specificity of Salmonella porin as an eliciting antigen for cell-mediated immunity (CMI) reaction in murine salmonellosis. Microbiol Immunol. 1989;33(12):1063–1067. | ||
Prejit, Agarwal RK, Porteen K, et al. Evaluation of recombinant outer membrane protein based vaccine against Salmonella Typhimurium in birds. Biologicals. 2013;41(3):162–168. | ||
Meenakshi M, Bakshi CS, Butchaiah G, Bansal MP, Siddiqui MZ, Singh VP. Adjuvanted outer membrane protein vaccine protects poultry against infection with Salmonella enteritidis. Vet Res Commun. 1999;23(2):81–90. | ||
Pore D, Mahata N, Pal A, Chakrabarti MK. Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS One. 2011;6(7):e22663. | ||
Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303. | ||
Pan Z, Cong Q, Geng S, et al. Flagellin from recombinant attenuated Salmonella enterica serovar Typhimurium reveals a fundamental role in chicken innate immunity. Clin Vaccine Immunol. 2012;19(3):304–312. | ||
Salman HH, Irache JM, Gamazo C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine. 2009;27(35):4784–4790. | ||
Vasserman Y, Pitcovski J. Genetic detoxification and adjuvant-activity retention of Escherichia coli enterotoxin LT. Avian Pathol. 2006;35(2):134–140. | ||
Kim SY, Doh HJ, Jang MH, Ha YJ, Chung SI, Park HJ. Oral immunization with Helicobacter pylori-loaded poly(D, L-lactide-co-glycolide) nanoparticles. Helicobacter. 1999;4(1):33–39. | ||
Salman HH, Gamazo C, Campanero MA, Irache JM. Salmonella-like bioadhesive nanoparticles. J Control Release. 2005;106(1–2):1–13. | ||
Agüeros M, Areses P, Campanero MA, et al. Bioadhesive properties and biodistribution of cyclodextrin-poly(anhydride) nanoparticles. Eur J Pharm Sci. 2009;37(3–4):231–240. | ||
Garland MJ, Singh TR, Woolfson AD, Donnelly RF. Electrically enhanced solute permeation across poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels: effect of hydrogel crosslink density and ionic conductivity. Int J Pharm. 2011;406(1–2):91–98. | ||
Karolewicz B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm J. 2016;24(5):525–536. | ||
Tamayo I, Irache JM, Mansilla C, Ochoa-Repáraz J, Lasarte JJ, Gamazo C, Poly GC. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17(9):1356–1362. | ||
Camacho AI, da Costa Martins R, Tamayo I, et al. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine. 2011;29(41):7130–7135. | ||
Lopac SK, Torres MP, Wilson-Welder JH, Wannemuehler MJ, Narasimhan B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J Biomed Mater Res B Appl Biomater. 2009;91(2):938–947. | ||
Rebouças JS, Irache JM, Camacho AI, et al. Development of poly(anhydride) nanoparticles loaded with peanut proteins: the influence of preparation method on the immunogenic properties. Eur J Pharm Biopharm. 2012;82(2):241–249. | ||
Ochoa-Repáraz J, Sesma B, Alvarez M, Jesús Renedo M, Irache JM, Gamazo C. Humoral immune response in hens naturally infected with Salmonella Enteritidis against outer membrane proteins and other surface structural antigens. Vet Res. 2004;35(3):291–298. | ||
Sankar R, Ravikumar V. Biocompatibility and biodistribution of suberoylanilide hydroxamic acid loaded poly (DL-lactide-co-glycolide) nanoparticles for targeted drug delivery in cancer. Biomed Pharmacother. 2014;68(7):865–871. | ||
Dhakal S, Renu S, Ghimire S, et al. Mucosal immunity and protective efficacy of intranasal inactivated influenza vaccine is improved by chitosan nanoparticle delivery in pigs. Front Immunol. 2018;9:934. | ||
Shanmugasundaram R, Selvaraj RK. Regulatory T cell properties of chicken CD4+CD25+ cells. J Immunol. 2011;186(4):1997–2002. | ||
Ferreira AJ, Ferreira CS, Knobl T, et al. Comparison of three commercial competitive-exclusion products for controlling Salmonella colonization of broilers in Brazil. J Food Prot. 2003;66(3):490–492. | ||
Ross K, Adams J, Loyd H, et al. Combination nanovaccine demonstrates synergistic enhancement in efficacy against influenza. ACS Biomater Sci Eng. 2016;2(3):368–374. | ||
Hamid N, Jain SK. Characterization of an outer membrane protein of Salmonella enterica serovar typhimurium that confers protection against typhoid. Clin Vaccine Immunol. 2008;15(9):1461–1471. | ||
Komoriya K, Shibano N, Higano T, Azuma N, Yamaguchi S, Aizawa SI. Flagellar proteins and type III-exported virulence factors are the predominant proteins secreted into the culture media of Salmonella typhimurium. Mol Microbiol. 1999;34(4):767–779. | ||
Zhao L, Seth A, Wibowo N, et al. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. | ||
Liu C, Shen Q, Zheng W, et al. Poly(anhydride) nanoparticles act as effective adjuvants to elicit a persistent immune response. RSC Adv. 2017;7(87):55459–55470. | ||
Mottram PL, Leong D, Crimeen-Irwin B, et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4(1):73–84. | ||
Xiang SD, Scholzen A, Minigo G, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40(1):1–9. | ||
Gutjahr A, Phelip C, Coolen AL, et al. Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines (Basel). 2016;4(4):E34:34. | ||
Sankar R, Karthik A, Prabu A, Karthik S, Shivashangari KS, Ravikumar V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B Biointerfaces. 2013;108:80–84. | ||
Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release. 2015;220(Pt A):141–148. | ||
Dobrovolskaia MA, Mcneil SE. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J Control Release. 2013;172(2):456–466. | ||
Lu S, Duffin R, Poland C, et al. Efficacy of simple short-term in vitro assays for predicting the potential of metal oxide nanoparticles to cause pulmonary inflammation. Environ Health Perspect. 2009;117(2):241–247. | ||
Vela-Ramirez JE, Goodman JT, Boggiatto PM, et al. Safety and biocompatibility of carbohydrate-functionalized polyanhydride nanoparticles. Aaps J. 2015;17(1):256–267. | ||
Hajam IA, Dar PA, Shahnawaz I, Jaume JC, Lee JH. Bacterial flagellin-a potent immunomodulatory agent. Exp Mol Med. 2017;49(9):e373. | ||
Salazar-Gonzalez RM, Srinivasan A, Griffin A, et al. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol. 2007;179(9):6169–6175. | ||
Vicente-Suarez I, Brayer J, Villagra A, Cheng F, Sotomayor EM. TLR5 ligation by flagellin converts tolerogenic dendritic cells into activating antigen-presenting cells that preferentially induce T-helper 1 responses. Immunol Lett. 2009;125(2):114–118. | ||
Honko AN, Mizel SB. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun. 2004;72(11):6676–6679. | ||
Gamazo C, Bussmann H, Giemsa S, et al. Interactions of poly (anhydride) nanoparticles with macrophages in light of their vaccine adjuvant properties. Int J Pharm. 2015;496(2):922–930. | ||
Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008;52(1):2–12. | ||
Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect. 2001;3(14–15):1183–1190. | ||
Kim S-H, Jang Y-S. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med. 2014;46(3):e85. | ||
Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010;6(11):e1001147. | ||
Maclennan CA. Antibodies and protection against invasive salmonella disease. Front Immunol. 2014;5(5):635. | ||
Liu Q, Liu Q, Zhao X, Liu T, et al. Immunogenicity and cross-protective efficacy induced by outer membrane proteins from salmonella typhimurium mutants with truncated LPS in mice. Int J Mol Sci. 2016;17(3):416. | ||
Okamura M, Ueda M, Noda Y, et al. Immunization with outer membrane protein A from Salmonella enterica serovar Enteritidis induces humoral immune response but no protection against homologous challenge in chickens. Poult Sci. 2012;91(10):2444–2449. | ||
Strindelius L, Degling Wikingsson L, Sjoholm I. Extracellular antigens from Salmonella enteritidis induce effective immune response in mice after oral vaccination. Infect Immun. 2002;70(3):1434–1442. | ||
Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines. 2010;9(9):1095–1107. | ||
Lee JS, Jung ID, Lee CM, et al. Outer membrane protein a of Salmonella enterica serovar Typhimurium activates dendritic cells and enhances Th1 polarization. BMC Microbiol. 2010;10(1):263. | ||
Galdiero M, de Martino L, Marcatili A, Nuzzo I, Vitiello M, Cipollaro de L’Ero G. Th1 and Th2 cell involvement in immune response to Salmonella typhimurium porins. Immunology. 1998;94(1):5–13. | ||
Okamura M, Lillehoj HS, Raybourne RB, Babu US, Heckert RA. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-gamma production. Comp Immunol Microbiol Infect Dis. 2004;27(4):255–272. | ||
Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. | ||
Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–1103. | ||
Beal RK, Powers C, Wigley P, Barrow PA, Smith AL. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathology. 2004;33(1):25–33. | ||
Penha Filho RAC, Moura BS, de Almeida AM, Montassier HJ, Barrow PA, Berchieri Junior A. Humoral and cellular immune response generated by different vaccine programs before and after Salmonella Enteritidis challenge in chickens. Vaccine. 2012;30(52):7637–7643. |
Supplementary materials
  | Table S1 Sequence of the primers used in qRT-PCR analyses |
  | Figure S4 Flow cytometry gating pattern of control, IgG isotype, CD4+, CD8+, and CD4+CD8+ cells. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.